Abstract
Although leptin and its receptor (ObR) have emerged as important cancer biomarkers, the role of the leptin system in brain tumor development remains unknown. We screened 87 human brain tumor biopsies using immunohistochemistry and detected leptin and ObR in 55.2% and 60.9% cases, respectively. In contrast, leptin and ObR were absent in 14 samples of normal brain tissue. The presence of leptin correlated with ObR with overall concordance 80.5%. The leptin/ObR system was highly expressed in glioblastomas and anaplastic astrocytomas, while lower expression of both markers was noted in low‐grade astrocytomas and gangliogliomas. The association between leptin/ObR and the degree of tumor malignancy was highly significant (P < 0.001). Using double immunofluorescence of glioblastoma tissues, we found co‐expression of leptin with ObR and with the proliferation marker Ki‐67 in 87% and 64% of cells, respectively. The leptin/ObR‐positive tissues also expressed activated forms of STAT3 and Akt. In line with this finding, ObR‐positive glioblastoma cells responded to leptin with cell growth and induction of the STAT3 and Akt pathways as well as inactivation of the cell cycle suppressor Rb. In summary, our data demonstrate that the leptin/ObR system is expressed in malignant brain tumors and might be involved in tumor progression.
Keywords: glioblastoma, leptin, leptin receptor, malignant progression, novel biomarker
INTRODUCTION
Leptin is a hormone produced mainly by the adipose tissue (79). Initially discovered as a cytokine controlling food intake and energy balance, recently, leptin has emerged as a potent regulator of different physiological and pathological processes, including cancer progression 28, 71, 76. Indeed, breast, colorectal, endometrial and prostate cancer tissues (but not their corresponding normal tissues), express leptin receptors (ObR) and leptin has been shown to induce cancer cell growth and survival 28, 29, 30, 31, 32, 33, 34, 35, 41, 42, 43, 54, 56, 69, 71, 74. Furthermore, leptin can activate migration and invasion, and is a potent angiogenic factor acting independently or through up‐regulation of vascular endothelial growth factor (VEGF) 2, 12, 25, 57, 67, 68. In addition, leptin can amplify some oncogenic pathways via transactivation of receptors for epidermal growth factor (EGF) 24, 70 and insulin‐like growth factor I (IGF‐I) 24, 59, 65. Importantly, in all studied human cancers, the expression of ObR is highly correlated with the presence of leptin, suggesting that leptin‐dependent malignancy might be regulated via autocrine/paracrine mechanisms 24, 30, 35, 41, 43.
In the normal adult brain, leptin acts as an appetite regulator and appears to be involved in hippocampal‐dependent learning and memory (52). In addition, leptin is required for neuronal and glial maturation in the developing brain 1, 72. Expression of the leptin receptor, ObR, has been described in the hypothalamus, the hippocampus, the cerebellum and brain microvessels 6, 22, 23. Although it is accepted that ObR in the brain is activated by leptin produced by the peripheral adipose tissue, some reports suggested that leptin can be synthesized within the central nervous system 48, 49, 50. Indeed, expression of leptin and ObR mRNA and protein has been shown in rat pituitary gland (9).
Yet, the potential role of leptin in human brain tumors has never been explored. A single report demonstrated leptin and ObR mRNA expression in various intracranial tumors (40); however, the detection of corresponding proteins has not been attempted.
Despite significant advances in surgical procedures (eg, introduction of gamma knife surgery), radiotherapy and advanced chemotherapeutic regimens, glial tumors remain a major cause or morbidity and mortality, especially in highly developed, industrialized countries 20, 55. Poorly circumscribed tumor margins, micro‐invasion, the diffuse infiltrating nature of neoplastic astrocytes, high rates of recurrence and tumor progression as well as the notorious aggressiveness of glioblastomas are all contributing factors for failed brain cancer therapy. In this context, we explored the value of leptin, an angiogenic, mitogenic and motogenic cytokine, as a potential pharmaceutical target in brain cancers.
MATERIALS AND METHODS
Tissue samples
Twenty‐three cases of formalin‐fixed, paraffin‐embedded brain tumor biopsies (2 astrocytoma, 5 anaplastic astrocytoma and 16 glioblastoma) were obtained from the archives collection of the Neuropathology Core, Department of Neurosciences, Temple University. Seventy‐eight biopsies (25 astrocytoma, 17 anaplastic astrocytoma, 5 ependymoma, 2 oligodendroglioma, 15 glioblastoma and 14 normal brains) were included in a brain glioma tissue array (US Biomax, Rockville, MD, USA). All samples were anonimized. All biopsies were characterized for tumor type and grade, according to the parameters established by the latest World Health Organization classification of brain tumors (44).
Immunohistochemistry (IHC)
The standard procedure for IHC detection of leptin and ObR has been described in detail before 24, 30, 41, 42, 43. We used a modified avidin–biotin–peroxidase methodology according to the manufacturer's instructions (Vector Laboratories, Burlingame, CA, USA). The modified protocol includes deparaffination of sections in xylene, re‐hydration through descending grades of alcohol up to water, non‐enzymatic antigen retrieval in citrate buffer, pH 6.0 at 95°C for 1 h, quenching of endogenous peroxidase with 6% H2O2 in methanol for 20 minutes and blocking with normal horse serum (for mouse monoclonal antibodies, mAbs) or normal goat serum (for rabbit polyclonal antibodies, pAbs) and incubation with primary Abs at room temperature for 2–16 h in a humidified chamber. After rinsing with phosphate buffered saline (PBS), the sections were incubated with secondary biotinilated anti‐mouse or anti‐rabbit Abs for 1 h, followed by avidin–biotin–peroxidase complexes for 1 h. The peroxidase was developed with diaminobenzydine tablets for 3 minutes, and the slides were counterstained with hematoxylin, dehydrated, cleared in xylene and mounted with Permount (Fisher Scientific, Pittsburgh, PA, USA).
The following primary Abs were used: for leptin detection, A‐20 rabbit pAb 1:100 dilution (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and for ObR, H‐300 rabbit polyclonal, 1:50 (Santa Cruz Biotechnology). The H‐300 ObR antibody recognizes the intracellular portion of ObR and is able to detect all ObR isoforms. For detection of phospho‐Akt (Ser473), we used D9E rabbit pAb, 1:50 (Cell Signaling, Danvers, MA, USA) and for phospho‐STAT3 (Tyr705), D3A7 mouse mAb, 1:500 (Cell Signaling). For the proliferation marker Ki‐67, mouse mAb Ki‐S5, 1:100 (DAKO, Glostrup, Denmark) was employed. To control Ab specificity, slides were processed with the omission of the primary antibody. The IHC staining was evaluated and photographed using an Olympus AX70 microscope equipped with a DP70 digital camera at a 400× magnification. Two independent investigators scored the expression of leptin and ObR. A scale of 0, + (weak inmmunoreactivity), ++ (moderate immunoreactivity) and +++ (robust immunoreactivity) was applied, as detailed by us before for leptin/ObR system 24, 30, 41, 42 according to the intensity of immunolabeling and number of positive cells. Tissues scoring at least 1 plus (+) was considered positive, while that below 1+ was considered negative. In the above IHC analysis, there was no discrepancy between the two observers regarding the patterns of biomarker expression and the scores assigned to analyzed sections.
Double labeling immunofluorescence (IF)
For all tissue sections analyzed, deparaffination, antigen retrieval and blocking steps were performed as described above. Then, the sections were incubated with the first primary Abs overnight. After washing with PBS, the sections were incubated with fluoresceine‐conjugated secondary Abs for 1 h. After rinsing thoroughly, the slides were incubated with second primary Abs overnight, followed by incubation with rhodamine‐conjugated secondary Abs. Finally, the slides were washed and mounted with VectaShield mounting medium with or without 4′,6‐diamidino‐2‐phenylindole (DAPI) (Vector Laboratories) and visualized at 1000× magnification using a Nikon inverted fluorescent microscope equipped with deconvolution software (Slidebook 4.0, Intelligent Imaging, Denver, CO, USA). The number of cells expressing analyzed marker and/or co‐expressing two markers was evaluated visually by two observers in 10 random fields containing at least 200 cells each. The fraction of positive cells was calculated as % of stained cells/total cells in the field ± standard error of the mean.
Cell lines
Glioblastoma cell lines LN229, LN18, U138 and U118 were purchased from ATCC (Manassas, VA, USA) and cultured as recommended by the supplier.
Cell proliferation experiments
The cells were grown in 24 well plates in normal medium (Dulbecco/Vogt modified Eagle's minimal essential medium (DMEM) low glucose plus 10% Fetal bovine serum (FBS)) and then synchronized for 24–48 h in serum‐free medium (SFM: DMEM plus 0.5 mg/mL bovine serum albumin, 1 µM FeSO4 and 2 mM L‐glutamine). The cells were next stimulated with 200 ng/mL leptin for 1, 2, 3 and 5 days, or left untreated. After that, the cells were counted with trypan‐blue exclusion. Each experiment was repeated at least three times.
Western blotting (WB)
Glioblastoma cell cultures synchronized in SFM were treated with 200 ng/mL leptin for 15, 30 and 60 minutes, or left untreated. The proteins extracted from astrocyte culture (growing primary cell line was obtained from Dr. Kris Reiss, Temple University). The expression of ObR and downstream signaling molecules was evaluated by WB in 100–200 µg of total protein lysates. The following primary Abs from cell signaling were used: for phospho‐Akt, Akt Ser473 pAb, 1:500; for total Akt, Akt pAb, 1:1000; for phospho‐STAT3, STAT3 Tyr705, D3A7 mAb, 1: 500; for total STAT3, STAT3 79D7 mAb, 1:1000; for phospho‐ERK1/2, p44/42 mitogen‐activated protein kinase (MAPK; ERK1/2) pAb Thr202/Tyr204, 1:1000; for total ERK1/2, p44/42 MAPK pAb, 1:1000; for phospho‐Rb, Rb Ser795 pAb, 1:250. For ObR, we used H‐300 pAb, 1:500 (Santa Cruz); and for glyceraldehyde‐3‐phosphate dehydrogenase, RDI‐TRK5G4‐6C5 mAb, 1:1000 (Research Diagnostic Inc., Concord, MA, USA). The intensity of bands corresponding to studied proteins was measured as described before (51).
Statistical analysis
In the analysis of tissue biopsies, relationships among variables were evaluated using Fisher's exact test for count data with a significance level of 0.05. Concordance in the expression was quantified, both calculating the overall agreement and the Cohen's kappa. Statistical analyses were performed using R for Windows software. Cell growth experiments were analyzed using one‐way analysis of variance.
RESULTS
Leptin and ObR are overexpressed in brain cancer and correlate with the degree of malignancy
Using IHC with specific Abs, we examined the expression of leptin and ObR in glial tumors (87 cases) and in matched or unmatched normal brain sections (14 samples) (Figure 1).
Figure 1.
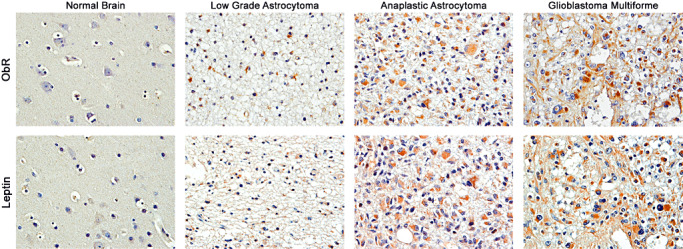
Expression of leptin and ObR in brain tumors. The expression of leptin and ObR was detected by immunohistochemistry, as described in Materials and Methods. Immunohistochemical detection of leptin and ObR was negative in normal control brain samples. In low‐grade glial tumors, leptin and ObR were weakly expressed in the cytoplasm of tumoral astrocytes. In anaplastic tumors, the expression of leptin/ObR was significantly increased, and in glioblastoma multiformes, leptin and ObR were robustly expressed in the cytoplasm of neoplastic cells. The magnification in all panels is 400×.
The expression of both leptin and ObR was weakly positive in low‐grade diffuse fibrillary astrocytomas, but was considerably increased in anaplastic astrocytomas and glioblastomas, where the labeling was the most intense. Both proteins were localized in the cytoplasm of neoplastic cells. The cellular localization of leptin and ObR was similar to that described previously in other tumor types 30, 35, 41, 42, 43. In addition, the expression of leptin and ObR was most prominent in perivascular areas and in groups of cells that were invading the adjacent brain parenchyma. In contrast, all normal brain tissues were negative for both leptin and ObR (Figure 1).
In effect of IHC assessment, the tissues were classified as either positive or negative for each marker, as detailed in Materials and Methods. Of 87 tumor cases, 48 biopsies (55.2%) were positive for leptin and 53 biopsies (60.9%) were positive for ObR. When both markers were considered jointly in the tumor group, 42 cases (48.2%) were positive for leptin/ObR, while 28 cases (32%) were negative for leptin and ObR. Other 17 cases were discordant for leptin and ObR expression. In tumors, the overall degree of concordance between leptin and ObR was 80.5% (95% confidence interval: 74–89.9%, kappa coefficient 0.600).
Figure 2 shows the percentage of cases positive for leptin and ObR, according to the degree of malignancy. We found a significant association (P < 0.001) between both markers and tumor grade. Specifically, using grade 2 tumors (astrocytoma, ependymoma, oligodendroglioma; 34 cases) as reference = 1, the odds of being positive for leptin were 3.5 for grade 3 tumors (anaplastic astrocytoma; 22 cases) and 10 for grade 4 tumors [glioblastoma multiforme (GBM); 31 cases]. In the case of ObR, the odds were 2.7 and 3.6, in grade 3 and 4, respectively.
Figure 2.
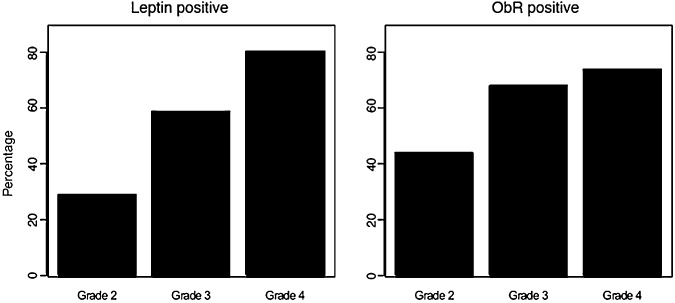
Expression of leptin/ObR in relation to tumor malignancy. The expression of leptin and ObR was detected by immunohistochemistry and scored, as described in Materials and Methods. Percentage of cases positive for leptin (Ob) (left panel) and ObR (right panel) according to the degree of malignancy is shown. The association between the tumor grade and the leptin system expression was statistically evaluated, as described in Materials and Methods. Abbreviations: Grade 2 = astrocytoma, ependymoma, oligodendroglioma; Grade 3 = anaplastic astrocytoma; Grade 4 = glioblastoma multiforme.
The high degree of co‐expression of leptin and ObR in GBM was verified by IF. Several cases of GBM identified as leptin and ObR‐positive in the initial IHC screening were analyzed using double IF and deconvoluted microscopy (Figure 3A). Leptin and ObR were detected in all cases and both markers displayed mostly cytoplasmic localization, as described before in other cancer models analyzed by IHC and IF 3, 14, 24, 30, 41, 42, 43. Specifically, the expression of leptin and ObR was observed in ∼90% and 87% of cells, respectively, while co‐expression of the markers was seen in ∼87% of cells (Figure 3A).
Figure 3.
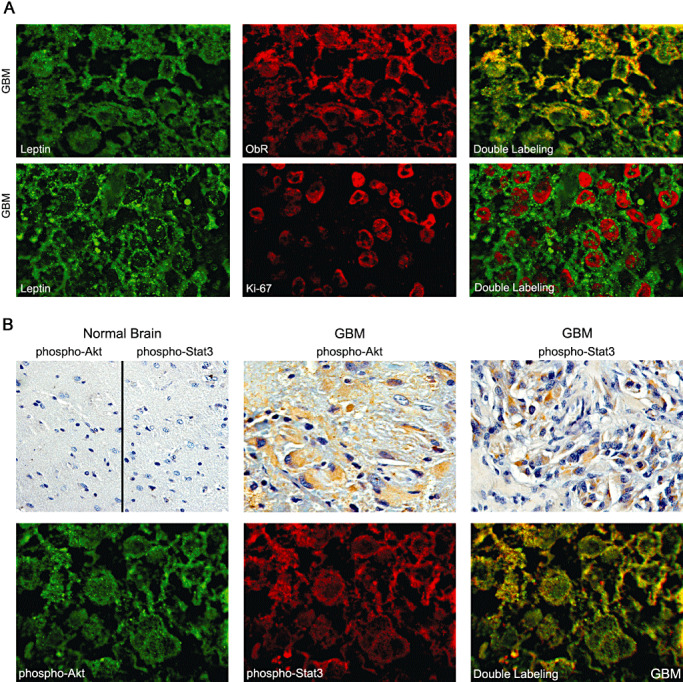
Coexpression of leptin, ObR and other tumor markers in GBM tissues. A. The co‐expression of leptin and ObR and leptin and Ki‐67 was studied in GBM by double immunofluorescence (IF), as described in Materials and Methods. Upper panel: leptin, green fluorescence; ObR, red fluorescence; yellow, double leptin/ObR labeling showing physical co‐localization of the markers. Lower panel: leptin, green fluorescence; Ki‐67, red fluorescence. No colocalization of leptin and Ki‐67 is seen in double labeling. B. The co‐expression of phospho‐Akt and phospho‐STAT3 was studied in the leptin/ObR GBM cases. Upper panel: phospho‐Akt and phospho‐STAT3 were studied in glioblastoma multiforme (GBM) cases and in normal brain by immunohistochemistry, as described in Materials and Methods. Lowe panel: the same markers were assessed in the same GMB cases by double IF. Phospho‐Akt, green fluorescence; phospho‐STAT3, red fluorescence; yellow, co‐localization of activated Akt and STAT3. The images presented are from consecutive sections of one representative GBM case. 1000× magnification.
The same GBM cases were used to assess if the presence of leptin is associated with the expression of the proliferation marker Ki‐67 (Figure 3A). As expected, GBM tissues expressed high levels of the proliferation antigen, specifically, nuclear Ki‐67 was found in ∼ 64% of cells. Notably, all leptin‐positive cells were also positive for Ki‐67 expression (Figure 3A).
Next, we analyzed if selected GBM cases expressing a high level of leptin/ObR and Ki‐67 also co‐express other cancer markers characteristic for ObR signaling, namely, phosphorylated/activated forms of Akt and STAT3. Using IHC, we found that the selected leptin/ObR‐positive GBM cases expressed phospho‐Akt and phospho‐STAT3 at high levels (++ and higher). By IHC, the activated Akt displayed mostly cytoplasmatic and perinuclear pattern of expression, while activated STAT3 was cytoplasmatic, perinuclear and nuclear. Neither marker was detectable in normal brain sections (Figure 3B).
When the above tissue sections were analyzed by double IF, activated forms of Akt and STAT3 were found in ∼80% of cells. The staining revealed predominatly cytoplasmatic and moderate nuclear expression of both proteins. Interestingly, in ∼58% of cells, some degree of co‐localization of activated STAT3 and Akt was detected, suggesting that the proteins might occupy the same intracellular compartments (Figure 3B).
Glioblastoma cell lines express ObR and respond to leptin with cell growth
To identify proper cellular models to study leptin effects on brain tumor cells, we evaluated ObR expression in different cell lines and in the primary culture of normal astrocytes (Figure 4). The highest expression of the long signaling forms of ObR (∼190, 130 kDa) was noted in LN229 and LN18 cells, low levels of ObR were expressed in U138 cells and ObR was not detectable in U118 cells as well as in the primary culture of normal astrocytes.
Figure 4.
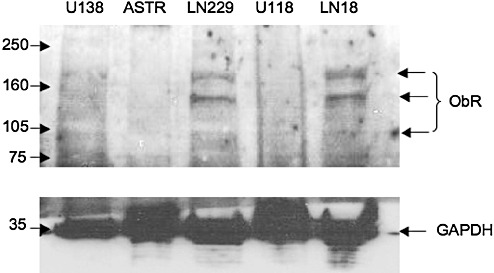
Expression of ObR in malignant and normal brain cell lines. ObR expression was studied in U138, LN229, U118, LN18 GBM cell lines and in normal astrocytes (ASTR) by western blotting, as described in Materials and Methods. The ObR isoforms ∼95, 130 and 185 kDa are indicated by arrows. The expression of a control protein glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) in different cells in the same blot is also depicted. The positions of molecular weight markers (kDa) are indicated on the left.
The effects of leptin on GBM cell growth were tested with ObR‐positive (LN18 and LN229) and ObR‐negative (U118) cell lines (Figure 5). ObR‐positive cells responded to 200 ng/mL leptin with cell growth, while ObR‐negative cells did not proliferate (or even died) in SFM. The greatest response to leptin in LN18 and LN229 cell lines was observed at 3 days of treatment (42% and 51%, respectively, vs. untreated controls). The dynamics of growth response to leptin in GBM cells was similar to that in other cancer cells (29).
Figure 5.
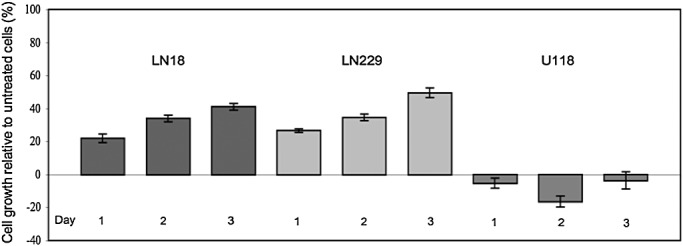
Leptin stimulates proliferation of ObR‐positive GBM cells. Cells were synchronized in serum‐free medium and then stimulated with 200 ng/mL leptin for 1, 2 and 3 days. The graph represents relative % change in cell number, with untreated cells taken as reference (0%). Each point represents at least four independent repeats. Standard errors of the mean bars are shown.
Leptin induces intracellular signaling in glioblastoma cells
In LN18 and LN229 cells, leptin induced the activation of the STAT3 and Akt pathways (Figure 6). The maximum induction of STAT3 phosphorylation on Tyr 705 was seen at 60 minutes (∼5‐fold and 2.4‐fold, in LN18 and LN229 cells, respectively); however, lower levels of activation were noticeable at 30 minutes in both cell lines. The phosphorylation of Akt on Ser 573 followed a similar pattern showing the maximal response at 60 minutes (∼2.0‐fold in LN18 cells and ∼2.3‐fold in LN229 cells), while lower levels of stimulation were observed at 15 and 30 minutes. This relatively delayed ObR signaling represents typical dynamics of leptin response in cancer cells, as described by us previously (29). Leptin also induced phosphorylation of Rb on Ser 795 at 15, 30 and 60 minutes in LN18 and at 60 minutes in LN229 cells. The treatment, however, failed to induce ERK1/2 phopsphorylation in both tested cell lines.
Figure 6.
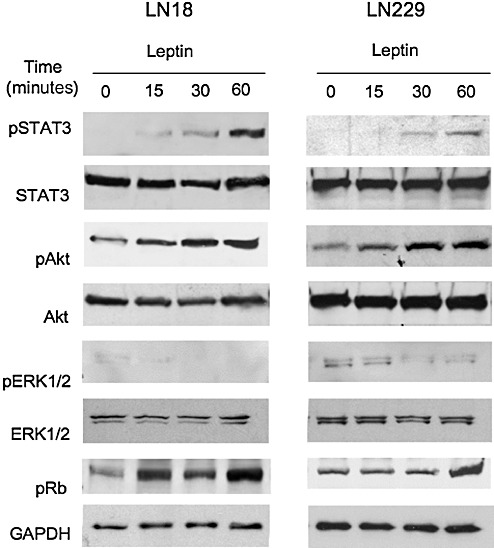
Effects of leptin on STAT3 and Akt activation and Rb phosphorylation in GBM cells. LN229 and LN18 cells were grown in normal medium and then shifted to serum‐free medium for 24 h (LN18) or 48 h (LN229). Then, the cells were left untreated or were stimulated with 200 ng/mL leptin for 15, 30 and 60 minutes. The expression of total and activated forms of STAT3 (79 kDa), Akt (60 kDa), ERK1/2 (42/44 kDa) as well as pRb (110 kDa), and a control protein glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH; 35 kDa) was assessed by western blotting, as described in Materials and Methods.
DISCUSSION
Recent evidence suggests that leptin can be involved in neoplastic processes, especially in cancers of the breast, colon, prostate and endometrium 13, 16, 28, 66, 74, 76, 78. Many different cancer cell types express the signaling form of ObR and respond to leptin with proliferation, survival and/or transformation. In some cancer models, leptin has been shown to induce cell migration and invasion. In addition, leptin can interfere with the efficacy of anti‐cancer drugs such as antiestrogens and HER2 targeting Abs 28, 46.
However, the role of the leptin/ObR system in human brain cancer remains unclear. The potential involvement of the system in glioblastoma has been suggested by data of Brown et al, who demonstrated that C6 rat glioblastoma cells express leptin and that downregulation of leptin mRNA in these cells induced cell death (9). In addition, Knerr et al documented leptin and ObR mRNA expression in various human intracranial tumors, including two cases of GBM and five cases of astrocytoma (40); however, the presence of corresponding proteins and their function has never been addressed. Our study is the first to demonstrate leptin and ObR expression on the protein level in a large series of brain cancer specimens. We also documented the existence of the functional ObR in GBM cell lines.
In our IHC analysis, leptin was detected in 55.2% cases and ObR in 60.9% cases. In brain cancer tissues, the presence of leptin correlated with ObR with overall degree of concordance between the markers 80.5%. In contrast, leptin and ObR were undetectable in all samples of normal brain tissue. These data are reminiscent of the observations in breast, colorectal and endometrial cancer, where leptin and ObR were co‐expressed in neoplastic, but not in relevant non‐malignant tissues 24, 30, 35, 42, 43. We confirmed the coexistence of leptin and ObR proteins in GBM by double IF staining, which revealed coexpression of the markers in 87% of cells. Interestingly, at variance with our study, Knerr et al (40) described similar expression levels of leptin and ObR mRNAs in unaffected temporal lobe tissue, GBMs and astrocytomas. This discrepancy could be related to the use of different methodology and study end point (ie, RNA vs. protein) as well as potential misestimation of mRNA results caused by the very limited number of tumors examined and contamination with endothelial cells within tumor specimens.
The mechanism of leptin/ObR expression in glial tumors is yet to be characterized. We have shown that in breast and colorectal cancer models leptin and/or ObR can be upregulated by hypoxic conditions or under normoxia by insulin, IGF‐1 or EGF 3, 7, 14, 30.
The present study also suggested a correlation between the expression of leptin/ObR and the degree of tumor malignancy. Both markers were highly expressed in glioblastomas and anaplastic astrocytomas, while lower expression of leptin/ObR was noted in low‐grade astrocytomas and gangliogliomas. The association between leptin/ObR and the degree of tumor malignancy was statistically significant (P < 0.001). Similarly, in breast cancer, expression of leptin/ObR was found to correlate with greater tumor size, higher tumor grade and/or worse prognosis 24, 30, 35. In gastric cancers, overexpression of leptin was significantly associated with tumor stage, lymph node metastasis and poor prognosis (80).
Our in vitro studies confirmed that several cell lines derived from glial tumors can express ObR and respond to leptin with induction of the transcription factor STAT3 and stimulation of the Akt growth/survival pathway. In addition, leptin treatment resulted in phosphorylation and deactivation of the tumor suppressor Rb, which was followed by the stimulation of cell growth. Similar effects of leptin on intracellular signaling and proliferation were noted in other tumor cell models 15, 16, 19, 21, 29. In line with these results, our double IF analysis of GBM tissues demonstrated high expression of activated forms of Akt, STAT3 and Ki‐67 in cases expressing high levels of leptin and ObR.
Malignant astrocytic gliomas, especially GBMs, are characterized by poor prognosis and low patient survival rates, despite advanced treatments with surgery, radiotherapy and chemotherapy 8, 10, 61, 73. The search for potential new therapeutic options for GBM resulted in the identification of several biomarkers and potential pharmaceutical targets, among them: VEGF, VEGF receptor (VEGFR), EGF receptor (EGFR), EGFR variant III, phosphatase and tensin homolog (PTEN) phosphatase, PI‐3 kinase, mTOR kinase, IGF I receptor (IGF‐IR) and HIF‐1alpha (HIF‐1a, the regulatory subunit of HIF‐1) 4, 5, 17, 18, 26, 27, 36, 37, 38, 39, 45, 53, 58, 62, 63, 64, 75, 77, pRb and cyclin D (11), and cyclin‐dependant kinase CDK4/6 4, 60.
In this context, it is important to note that leptin can influence several signaling systems involved in brain cancer development or progression. For instance, leptin can up‐regulate VEGF expression in different cell types 25, 32. In addition, leptin can transactivate estrogen receptor, EGFR and HER2 and activate downstream signaling pathways 24, 70. Furthermore, bidirectional crosstalk between ObR and IGF‐IR has been described (65). Leptin can also activate mammalian target for rapamycin (mTOR) kinase via PI‐3 kinase, and mTOR has been described to induce leptin (47).
In different cancer cell models, leptin has been shown to interfere with various anti‐neoplastic treatments, including targeted therapies 24, 29, 70. Consequently, the relationships between the leptin system and other major brain cancer molecular markers should be assessed in brain tumors. If warranted, anti‐leptin compounds could be developed as drugs in mono‐ or combined therapies for brain cancer.
ACKNOWLEDGMENTS
This work was supported by the funds from the Sbarro Health Research Organization (ES) and by NIH grant P01NS036466 (LDV).
REFERENCES
- 1. Ahima RS, Bjorbaek C, Osei S, Flier JS (1999) Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology 140:2755–2762. [DOI] [PubMed] [Google Scholar]
- 2. Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M et al (2000) Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3‐kinase‐, rho‐, and rac‐dependent signaling pathways. FASEB J 14:2329–2338. [DOI] [PubMed] [Google Scholar]
- 3. Bartella V, Cascio S, Auriemma A, Fiorio E, Russo A, Surmacz E (2008) Insulin‐dependent leptin expression in breast cancer cells. Cancer Res 68:4919–4927. [DOI] [PubMed] [Google Scholar]
- 4. Biernat W, Debiec‐Rychter M, Liberski PP (1998) Mutations of TP53, amplification of EGFR, MDM2 and CDK4, and deletions of CDKN2A in malignant astrocytomas. Pol J Pathol 49:267–271. [PubMed] [Google Scholar]
- 5. Biernat W, Huang H, Yokoo H, Kleihues P, Ohgaki H (2004) Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol 14:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjorbaek C, Elmquist JK, Michl P, Ahima RS, Van Bueren A, McCall AL, Flier JS (1998) Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology 139:3485–3491. [DOI] [PubMed] [Google Scholar]
- 7. Bouloumie A, Drexler HC, Lafontan M, Busse R (1998) Leptin, the product of Ob gene, promotes angiogenesis. Circ Res 83:1059–1066. [DOI] [PubMed] [Google Scholar]
- 8. Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C (2008) Glioblastoma in adults. Crit Rev Oncol Hematol 67:139–152. [DOI] [PubMed] [Google Scholar]
- 9. Brown R, Morash B, Ur E, Wilkinson M (2005) RNAi‐mediated silencing of leptin gene expression increases cell death in C6 glioblastoma cells. Brain Res Mol Brain Res 139:357–360. [DOI] [PubMed] [Google Scholar]
- 10. Buatti J, Ryken TC, Smith MC, Sneed P, Suh JH, Mehta M, Olson JJ (2008) Radiation therapy of pathologically confirmed newly diagnosed glioblastoma in adults. J Neurooncol 89:313–337. [DOI] [PubMed] [Google Scholar]
- 11. Buschges R, Weber RG, Actor B, Lichter P, Collins VP, Reifenberger G (1999) Amplification and expression of cyclin D genes (CCND1, CCND2 and CCND3) in human malignant gliomas. Brain Pathol 9:435–442; discussion 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y (2001) Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF‐2 and VEGF. Proc Natl Acad Sci USA 98:6390–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, Gonzalez RR (2008) Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer 123:2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cascio S, Bartella V, Auriemma A, Johannes GJ, Russo A, Giordano A, Surmacz E (2008) Mechanism of leptin expression in breast cancer cells: role of hypoxia‐inducible factor‐1alpha. Oncogene 27:540–547. [DOI] [PubMed] [Google Scholar]
- 15. Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V et al (2004) Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF‐7 cells. J Biol Chem 279:19908–19915. [DOI] [PubMed] [Google Scholar]
- 16. Catalano S, Giordano C, Rizza P, Gu G, Barone I, Bonofiglio D et al (2009) Evidence that leptin through STAT and CREB signaling enhances cyclin D1 expression and promotes human endometrial cancer proliferation. J Cell Physiol 218:490–500. [DOI] [PubMed] [Google Scholar]
- 17. Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, Loeffler JS (2004) The prognostic significance of phosphatidylinositol 3‐kinase pathway activation in human gliomas. J Clin Oncol 22:1926–1933. [DOI] [PubMed] [Google Scholar]
- 18. Chan AS, Leung SY, Wong MP, Yuen ST, Cheung N, Fan YW, Chung LP (1998) Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol 22:816–826. [DOI] [PubMed] [Google Scholar]
- 19. Choi JH, Park SH, Leung PC, Choi KC (2005) Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen‐activated protein kinases in ovarian cancer cells. J Clin Endocrinol Metab 90:207–210. [DOI] [PubMed] [Google Scholar]
- 20. Davis FG, Kupelian V, Freels S, McCarthy B, Surawicz T (2001) Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro Oncol 3:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dieudonne MN, Machinal‐Quelin F, Serazin‐Leroy V, Leneveu MC, Pecquery R, Giudicelli Y (2002) Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun 293:622–628. [DOI] [PubMed] [Google Scholar]
- 22. Elmquist JK (2000) Anatomic basis of leptin action in the hypothalamus. Front Horm Res 26:21–41. [DOI] [PubMed] [Google Scholar]
- 23. Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB (1998) Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547. [PubMed] [Google Scholar]
- 24. Fiorio E, Mercanti A, Terrasi M, Micciolo R, Remo A, Auriemma A et al (2008) Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer 8:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frankenberry KA, Somasundar P, McFadden DW, Vona‐Davis LC (2004) Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am J Surg 188:560–565. [DOI] [PubMed] [Google Scholar]
- 26. Frederick L, Wang XY, Eley G, James CD (2000) Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 60:1383–1387. [PubMed] [Google Scholar]
- 27. Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A et al (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21:2683–2710. [DOI] [PubMed] [Google Scholar]
- 28. Garofalo C, Surmacz E (2006) Leptin and cancer. J Cell Physiol 207:12–22. [DOI] [PubMed] [Google Scholar]
- 29. Garofalo C, Sisci D, Surmacz E (2004) Leptin interferes with the effects of the antiestrogen ICI 182 780 in MCF‐7 breast cancer cells. Clin Cancer Res 10:6466–6475. [DOI] [PubMed] [Google Scholar]
- 30. Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga‐Koda L, Golaszewska J et al (2006) Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity‐related stimuli. Clin Cancer Res 12:1447–1453. [DOI] [PubMed] [Google Scholar]
- 31. Gonzalez RR, Leavis PC (2003) A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine 21:185–195. [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK et al (2006) Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF‐R2). J Biol Chem 281:26320–26328. [DOI] [PubMed] [Google Scholar]
- 33. Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP (2001) Leptin is a growth factor for colonic epithelial cells. Gastroenterology 121:79–90. [DOI] [PubMed] [Google Scholar]
- 34. Hu X, Juneja SC, Maihle NJ, Cleary MP (2002) Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 94:1704–1711. [DOI] [PubMed] [Google Scholar]
- 35. Ishikawa M, Kitayama J, Nagawa H (2004) Enhanced expression of leptin and leptin receptor (OB‐R) in human breast cancer. Clin Cancer Res 10:4325–4331. [DOI] [PubMed] [Google Scholar]
- 36. Jensen RL, Ragel BT, Whang K, Gillespie D (2006) Inhibition of hypoxia inducible factor‐1alpha (HIF‐1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol 78:233–247. [DOI] [PubMed] [Google Scholar]
- 37. Ke LD, Fueyo J, Chen X, Steck PA, Shi YX, Im SA, Yung WK (1998) A novel approach to glioma gene therapy: down‐regulation of the vascular endothelial growth factor in glioma cells using ribozymes. Int J Oncol 12:1391–1396. [DOI] [PubMed] [Google Scholar]
- 38. Ke LD, Shi YX, Im SA, Chen X, Yung WK (2000) The relevance of cell proliferation, vascular endothelial growth factor, and basic fibroblast growth factor production to angiogenesis and tumorigenicity in human glioma cell lines. Clin Cancer Res 6:2562–2572. [PubMed] [Google Scholar]
- 39. Ke LD, Shi YX, Yung WK (2002) VEGF(121), VEGF(165) overexpression enhances tumorigenicity in U251 MG but not in NG‐1 glioma cells. Cancer Res 62:1854–1861. [PubMed] [Google Scholar]
- 40. Knerr I, Schuster S, Nomikos P, Buchfelder M, Dotsch J, Schoof E et al (2001) Gene expression of adrenomedullin, leptin, their receptors and neuropeptide Y in hormone‐secreting and non‐functioning pituitary adenomas, meningiomas and malignant intracranial tumours in humans. Neuropathol Appl Neurobiol 27:215–222. [DOI] [PubMed] [Google Scholar]
- 41. Koda M, Sulkowska M, Kanczuga‐Koda L, Cascio S, Colucci G, Russo A et al (2007) Expression of the obesity hormone leptin and its receptor correlates with hypoxia‐inducible factor‐1alpha in human colorectal cancer. Ann Oncol 18(Suppl. 6):vi116–vi119. [DOI] [PubMed] [Google Scholar]
- 42. Koda M, Sulkowska M, Kanczuga‐Koda L, Surmacz E, Sulkowski S (2007) Overexpression of the obesity hormone leptin in human colorectal cancer. J Clin Pathol 60:902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koda M, Sulkowska M, Wincewicz A, Kanczuga‐Koda L, Musiatowicz B, Szymanska M, Sulkowski S (2007) Expression of leptin, leptin receptor, and hypoxia‐inducible factor 1 alpha in human endometrial cancer. Ann N Y Acad Sci 1095:90–98. [DOI] [PubMed] [Google Scholar]
- 44. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15:1311–1333. [DOI] [PubMed] [Google Scholar]
- 46. Mauro L, Catalano S, Bossi G, Pellegrino M, Barone I, Morales S et al (2007) Evidences that leptin up‐regulates E‐cadherin expression in breast cancer: effects on tumor growth and progression. Cancer Res 67:3412–3421. [DOI] [PubMed] [Google Scholar]
- 47. Maya‐Monteiro CM, Bozza PT (2008) Leptin and mTOR: partners in metabolism and inflammation. Cell Cycle 7:1713–1717. [DOI] [PubMed] [Google Scholar]
- 48. Morash B, Li A, Murphy PR, Wilkinson M, Ur E (1999) Leptin gene expression in the brain and pituitary gland. Endocrinology 140:5995–5998. [DOI] [PubMed] [Google Scholar]
- 49. Morash B, Wilkinson D, Murphy P, Ur E, Wilkinson M (2001) Developmental regulation of leptin gene expression in rat brain and pituitary. Mol Cell Endocrinol 185:151–159. [DOI] [PubMed] [Google Scholar]
- 50. Morash BA, Imran A, Wilkinson D, Ur E, Wilkinson M (2003) Leptin receptors are developmentally regulated in rat pituitary and hypothalamus. Mol Cell Endocrinol 210:1–8. [DOI] [PubMed] [Google Scholar]
- 51. Morelli C, Garofalo C, Sisci D, Del Rincon S, Cascio S, Tu X et al (2004) Nuclear insulin receptor substrate 1 interacts with estrogen receptor alpha at ERE promoters. Oncogene 23:7517–7526. [DOI] [PubMed] [Google Scholar]
- 52. Morrison CD (2009) Leptin signaling in brain: a link between nutrition and cognition? Biochim Biophys Acta 1792:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Narita Y, Nagane M, Mishima K, Huang HJ, Furnari FB, Cavenee WK (2002) Mutant epidermal growth factor receptor signaling down‐regulates p27 through activation of the phosphatidylinositol 3‐kinase/Akt pathway in glioblastomas. Cancer Res 62:6764–6769. [PubMed] [Google Scholar]
- 54. O'Brien SN, Welter BH, Price TM (1999) Presence of leptin in breast cell lines and breast tumors. Biochem Biophys Res Commun 259:695–698. [DOI] [PubMed] [Google Scholar]
- 55. Ohgaki H (2009) Epidemiology of brain tumors. Methods Mol Biol 472:323–342. [DOI] [PubMed] [Google Scholar]
- 56. Okumura M, Yamamoto M, Sakuma H, Kojima T, Maruyama T, Jamali M et al (2002) Leptin and high glucose stimulate cell proliferation in MCF‐7 human breast cancer cells: reciprocal involvement of PKC‐alpha and PPAR expression. Biochim Biophys Acta 1592:107–116. [DOI] [PubMed] [Google Scholar]
- 57. Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE et al (2001) Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med 33:95–102. [DOI] [PubMed] [Google Scholar]
- 58. Ragel BT, Couldwell WT, Gillespie DL, Jensen RL (2007) Identification of hypoxia‐induced genes in a malignant glioma cell line (U‐251) by cDNA microarray analysis. Neurosurg Rev 30:181–187; discussion 7. [DOI] [PubMed] [Google Scholar]
- 59. Ray A, Nkhata KJ, Cleary MP (2007) Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol 30:1499–1509. [PubMed] [Google Scholar]
- 60. Reifenberger G, Ichimura K, Reifenberger J, Elkahloun AG, Meltzer PS, Collins VP (1996) Refined mapping of 12q13‐q15 amplicons in human malignant gliomas suggests CDK4/SAS and MDM2 as independent amplification targets. Cancer Res 56:5141–5145. [PubMed] [Google Scholar]
- 61. Ryken TC, Frankel B, Julien T, Olson JJ (2008) Surgical management of newly diagnosed glioblastoma in adults: role of cytoreductive surgery. J Neurooncol 89:271–286. [DOI] [PubMed] [Google Scholar]
- 62. Sathornsumetee S, Rich JN (2006) New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther 6:1087–1104. [DOI] [PubMed] [Google Scholar]
- 63. Sathornsumetee S, Hjelmeland AB, Keir ST, McLendon RE, Batt D, Ramsey T et al (2006) AAL881, a novel small molecule inhibitor of RAF and vascular endothelial growth factor receptor activities, blocks the growth of malignant glioma. Cancer Res 66:8722–8730. [DOI] [PubMed] [Google Scholar]
- 64. Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN (2007) Molecularly targeted therapy for malignant glioma. Cancer 110:13–24. [DOI] [PubMed] [Google Scholar]
- 65. Saxena NK, Taliaferro‐Smith L, Knight BB, Merlin D, Anania FA, O'Regan RM, Sharma D (2008) Bidirectional crosstalk between leptin and insulin‐like growth factor‐I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res 68:9712–9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schaffler A, Scholmerich J, Buechler C (2007) Mechanisms of disease: adipokines and breast cancer—endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab 3:345–354. [DOI] [PubMed] [Google Scholar]
- 67. Sharma D, Saxena NK, Vertino PM, Anania FA (2006) Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal‐transduction pathways. Endocr Relat Cancer 13:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sierra‐Honigmann MR, Nath AK, Murakami C, Garcia‐Cardena G, Papapetropoulos A, Sessa WC et al (1998) Biological action of leptin as an angiogenic factor. Science 281:1683–1686. [DOI] [PubMed] [Google Scholar]
- 69. Smith‐Kirwin SM, O'Connor DM, De Johnston J, Lancey ED, Hassink SG, Funanage VL (1998) Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab 83:1810–1813. [DOI] [PubMed] [Google Scholar]
- 70. Soma D, Kitayama J, Yamashita H, Miyato H, Ishikawa M, Nagawa H (2008) Leptin augments proliferation of breast cancer cells via transactivation of HER2. J Surg Res 149:9–14. [DOI] [PubMed] [Google Scholar]
- 71. Somasundar P, McFadden DW, Hileman SM, Vona‐Davis L (2004) Leptin is a growth factor in cancer. J Surg Res 116:337–349. [DOI] [PubMed] [Google Scholar]
- 72. Steppan CM, Swick AG (1999) A role for leptin in brain development. Biochem Biophys Res Commun 256:600–602. [DOI] [PubMed] [Google Scholar]
- 73. Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. [DOI] [PubMed] [Google Scholar]
- 74. Surmacz E (2007) Obesity hormone leptin: a new target in breast cancer? Breast Cancer Res 9:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Trojan J, Cloix JF, Ardourel MY, Chatel M, Anthony DD (2007) Insulin‐like growth factor type I biology and targeting in malignant gliomas. Neuroscience 145:795–811. [DOI] [PubMed] [Google Scholar]
- 76. Vona‐Davis L, Rose DP (2007) Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14:189–206. [DOI] [PubMed] [Google Scholar]
- 77. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259. [DOI] [PubMed] [Google Scholar]
- 78. Wincewicz A, Koda M, Sulkowska M, Kanczuga‐Koda L, Sulkowski S (2008) Comparison of STAT3 with HIF‐1alpha, Ob and ObR expressions in human endometrioid adenocarcinomas. Tissue Cell 40:405–410. [DOI] [PubMed] [Google Scholar]
- 79. Zhang F, Chen Y, Heiman M, Dimarchi R (2005) Leptin: structure, function and biology. Vitam Horm 71:345–372. [DOI] [PubMed] [Google Scholar]
- 80. Zhao X, Huang K, Zhu Z, Chen S, Hu R (2007) Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol 22:1317–1321. [DOI] [PubMed] [Google Scholar]


