Abstract
Objectives
The incidence of metastatic renal cell cancer (mRCC) is rising. To date, Interleukin-2 (IL-2) is the only treatment offering a complete response rate for mRCC. We wish to test the hypothesis that the combination of restricted availability and expense associated with IL-2 administration results in differential access to the medication based on race and gender, despite similar clinical indications for its use.
Methods
We used data from the Surveillance, Epidemiology and End Results program and the Centers for Medicare Services (CMS) to clinically characterize subjects with mRCC diagnosed from 1992 through 2002. We linked these subjects to claims identified in the CMS databases. We then assigned subjects to cohorts receiving radical nephrectomy, IL-2, both, or neither. A logistic model was created to identify factors that had significant independent effects on the receipt of IL-2.
Results
3,730 individuals were identified with mRCC. After controlling for other variables, female subjects were less likely to receive IL-2 (O.R. 0.80). African American subjects were also less likely to receive IL-2 (O.R 0.55). Married individuals were much more likely to receive IL-2 (O.R 1.9).
Conclusions
African Americans and women were much less likely to be treated with IL-2 after controlling for relevant clinical variables. These data document that the only therapy offering a complete response to patients with mRCC is less frequently given to those who are African American or female. It is possible that the racial and gender-based disparities in treatment with IL-2 will be replicated with newer, expensive treatment options for mRCC. Further prospective investigation into mitigating barriers to receipt of effective care for mRCC is urgently needed.
Objectives
The burden of renal cell carcinoma is rising nationally. The increase in any-stage incidence is steeper for African Americans than Caucasians (1). Although increased use of abdominal imaging may partially explain the rise in detection of localized tumors(2–4), the incidence of metastatic renal cell cancer is also rising, as is mortality from the disease (again, especially among African Americans.) (1). Encouragingly, treatment options to address the growing burden of metastatic disease are proliferating. However, most of these new treatments offer a reduction or halt in the rate of disease progression, as opposed to a complete tumor response. While much attention has focused on novel oral agents (such as sunitinib, a tyrosine kinase inhibitor), the only Food and Drug Administration (FDA)-approved treatment with a measurable complete response rate in patients with metastatic renal cell cancer is high dose interleukin-2 (IL-2). The objective response rate to IL-2 in one large retrospective series was 20% (5). Administration of IL-2, approved by the FDA in 1992, can be associated with significant toxicity. Because of its toxicity profile, its use is limited to selected centers nationwide with experience in administering IL-2 (61 centers are listed on the manufacturer’s website). Many of these centers are affiliated with academic medical centers in urban settings.
Physicians employ several clinical factors when deciding whether to administer IL-2 to patients with metastatic renal cell carcinoma. Among these are the patient’s performance status, renal function, histologic type of cancer, the presence of brain metastases, and patient preferences for side effects. However, the combination of restricted availability and substantial expense associated with IL-2 administration raises the possibility that receipt of IL-2 is related to non-clinical factors. Lower socioeconomic status or an inability to obtain transportation to an academic medical center are examples of possible non-clinical factors that would restrict availability to IL-2. In light of the potential structural barriers to IL-2 therapy, we hypothesized that individuals of lower socioeconomic status, those that are African American, and those living in rural settings are less likely to receive IL-2 as compared with those that are wealthier, Caucasian or live in urban areas. To test our hypotheses, we examined clinical and administrative data available through the SEER-Medicare linked database.
Methods
Data source
We used linked data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program and the Centers for Medicare and Medicaid Services (CMS) to identify and characterize a population-based cohort of patients with incident metastatic kidney cancer diagnosed from 1992 through 2002. SEER is a population-based cancer registry that collects data regarding incidence, treatment and mortality. The demographic composition, cancer incidence, and mortality trends in the SEER registries are representative of the entire United States population.
From 1992 through 1999, 11 SEER-affiliated registries (San Francisco, San Jose, Los Angeles, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta) provided incident cases for linkage with health care claims covered by the CMS. In 2000, the SEER-Medicare dataset expanded to include cases from the Greater California, Louisiana, New Jersey and Kentucky tumor registries. The Medicare program provides primary health insurance for 97% of the United States population 65 years and older(6). Successful linkage with CMS claims is achieved for more than 90% of Medicare patients whose cancer-specific data are tracked by SEER (6)
Cohort identification
We identified a cohort of Medicare beneficiaries diagnosed between 1992 and 2002 with metastatic kidney cancer. For each case in the cohort, we then searched both inpatient (Medicare Provider Analysis and Review [MEDPAR] file, based on International Classification of Diseases, 9th revision, Clinical Modification (ICD-9) codes) and physician claims (Carrier Claims file, based on American Medical Association Current Procedural Terminology (CPT) and ICD-9 codes) for kidney cancer–specific diagnosis and procedure codes.
Assignment of treatment
Next, we defined and applied an administrative code-based algorithm to determine the type of therapy received by each patient in the analytic cohort. CPT and ICD-9 codes for radical nephrectomy and delivery of immunotherapy were identified in Medicare claims linked to each new diagnosis of metastatic kidney cancer from the SEER data file.
Patient-level covariates
We used SEER variables to ascertain demographic and cancer-specific information (i.e., age at diagnosis, sex, race/ethnicity, marital status, SEER registry, tumor grade) for each case in the analytic cohort. We assigned median Census-tract income and Census-tract percentage of non-high school graduates as patient-level measures of income and education, respectively (7).
We measured preexisting comorbidity using a modification of the Charlson Index (8) by identifying comorbid conditions (which include diabetes, renal insufficiency and cardiovascular disease) from inpatient and physician claims submitted during the 12-month period before the index diagnosis (8).
Statistical analysis
Prior to fitting multilevel models, we performed several univariate analyses. We used chi-square tests to evaluate the level of association between receipt of IL-2 and various patient-level covariates. Kaplan Meier estimates were also used to evaluate univariate effects of IL-2 treatment on overall survival.
For our multilevel analyses, we defined the following primary outcomes: (1) receipt of IL-2 and (2) overall survival. A logistic model was created to determine factors that had significant independent effects on the receipt of IL-2. We also assessed relationships between key factors and overall survival using a Cox regression model. All statistical testing was two-sided, completed using computerized software (SAS v9.1, SAS Institute, Cary, NC), and carried out at the 5% significance level. We obtained approval for this study from the Institutional Review Board at the University of California, Los Angeles.
Results
3,730 individuals were identified with metastatic renal cell carcinoma between 1992 and 2002. Demographic characteristics of this sample are displayed in Table 1. 58% of the sample were male, 81% were between the ages of 65 and 85 years, and 54% were married. 82% of the sample were Caucasian, 9% were African American, and 4% were Hispanic. Approximately 50% of the sample was diagnosed in 1999 or later. Approximately 26% of subjects lived in a zip code with a median income of less than $35,000; 10% of subjects lived in a zip code where over 35% of residents had only a high school education.
Table 1.
Demographics
| % | |
|---|---|
| Sex | |
| Male | 58 |
| Female | 42 |
| Age | |
| Under 65 | 9 |
| 65–75 | 45 |
| 75–85 | 36 |
| Over 85 | 10 |
| Race | |
| White | 82 |
| Black | 9 |
| Hispanic | 5 |
| Other | 4 |
| Marital Status | |
| Single | 8 |
| Married | 54 |
| Divorced/Widowed | 35 |
| Unknown | 3 |
| KID Grade | |
| Grade 1 | 2 |
| Grade 2 | 7 |
| Grade 3 | 14 |
| Grade 4 | 5 |
| Grade Unknown | 72 |
| KID Year of Diagnosis | |
| 1992–1998 | 50 |
| 1999–2002 | 50 |
| Charlson | |
| 0 | 81 |
| 1–2 | 15 |
| 3 plus | 4 |
| Zip Code Median Income | |
| < $35,000 | 26 |
| $35,000–$45,000 | 27 |
| $45,000–$60,000 | 26 |
| >$60,000 | 21 |
| % Non-High School Graduates by Zip code | |
| <10% only high school education | 24 |
| 10–20% only high school education | 42 |
| 20–35% only high school education | 25 |
| >35% only high school education | 10 |
In univariate analysis, we examined factors associated with the receipt of radical nephrectomy only, receipt of IL-2 only, receipt of both or receipt of no active treatment for metastatic renal cell carcinoma. These data are presented under Table 2. Overall, 15% of subjects received IL-2 monotherapy and an additional 11% received IL-2 therapy subsequent to debulking nephrectomy. 74% of female subjects received no active treatment, as compared to 68% of men (p=0.0002). Subjects over 85 years old were unlikely to get any form of active treatment (94%) as compared with subjects 65–75 years old (61%, p<0.001). African American subjects were more likely to receive no treatment as compared with Caucasians (81% vs 70%, p=0.003). Single subjects were more likely to receive no active treatment as compared to married subjects (75% vs 63%, p<0.001). Subject median zip code income was not associated with treatment. There were regional variations in receipt of active treatment.
Table 2.
Factors associated with treatment choice
| Treatment | p-value | ||||
|---|---|---|---|---|---|
| Radical Nephrectomy % | IL-2 % | Both % | None % | ||
| Sex | p=0.0002 | ||||
| Male | 17 | 9 | 6 | 68 | |
| Female | 15 | 6 | 5 | 74 | |
| Age | p<0.0001 | ||||
| Under 65 | 18 | 11 | 10 | 61 | |
| 65–75 | 21 | 11 | 8 | 61 | |
| 75–85 | 13 | 6 | 3 | 78 | |
| Over 85 | 5 | 1 | 0 | 94 | |
| Race | p=0.0003 | ||||
| White | 16 | 8 | 7 | 70 | |
| Black | 10 | 6 | 4 | 81 | |
| Hispanic | 25 | 6 | 4 | 64 | |
| Other | 17 | 9 | 6 | 68 | |
| Marital Status | p<0.0001 | ||||
| Single | 16 | 6 | 74 | ||
| Married | 19 | 10 | 7 | 63 | |
| Divorced/Widowed | 13 | 5 | 3 | 79 | |
| Unknown | 11 | 9 | 2 | 78 | |
| Zip Code Median Income | p=0.3373 | ||||
| < $35,000 | 16 | 7 | 4 | 73 | |
| $35,000–$45,000 | 16 | 7 | 6 | 70 | |
| $45,000–$60,000 | 16 | 9 | 5 | 70 | |
| >$60,000 | 17 | 9 | 5 | 69 | |
| Unknown | 13 | 8 | 9 | 71 | |
| % Non-High School Graduates by Zip | p=0.0394 | ||||
| <10% only high school education | 17 | 8 | 6 | 69 | |
| 10–20% only high school education | 15 | 9 | 6 | 71 | |
| 20–35% only high school education | 16 | 7 | 5 | 72 | |
| >35% only high school education | 22 | 6 | 4 | 69 | |
| Unknown | 13 | 8 | 9 | 71 | |
| Charlson | p=0.0003 | ||||
| 0 | 17 | 9 | 6 | 69 | |
| 1–2 | 16 | 5 | 4 | 75 | |
| 3 plus | 9 | 4 | 3 | 84 | |
| KID Grade | p<0.0001 | ||||
| Grade 1 | 26 | 15 | 12 | 47 | |
| Grade 2 | 40 | 6 | 16 | 38 | |
| Grade 3 | 37 | 7 | 12 | 44 | |
| Grade 4 | 42 | 6 | 13 | 39 | |
| Grade Unknown | 8 | 8 | 3 | 81 | |
| Region | p=0.0036 | ||||
| Midwest | 14 | 9 | 5 | 72 | |
| Northeast | 14 | 7 | 5 | 74 | |
| South | 16 | 9 | 5 | 70 | |
| West | 19 | 7 | 6 | 68 | |
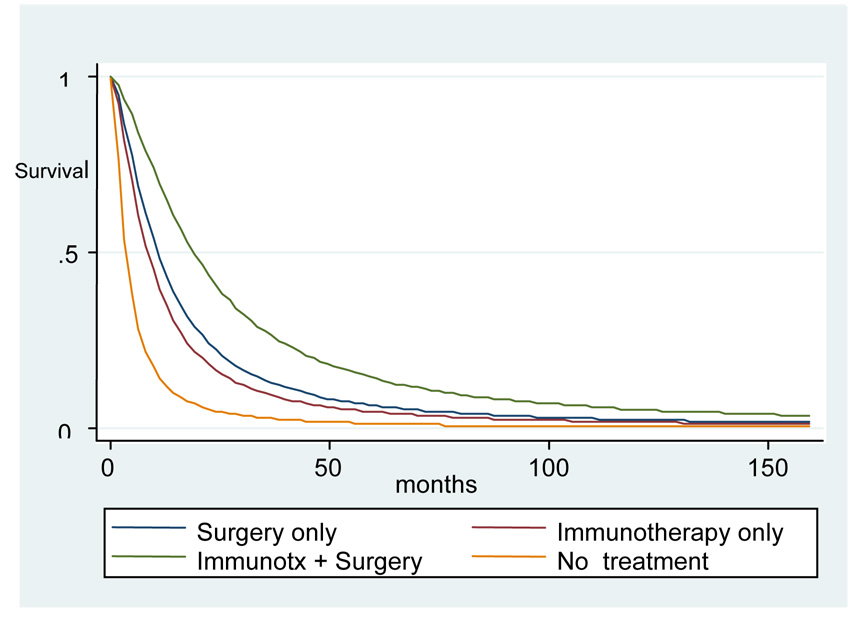
We fit a logistic regression model to predict receipt of IL-2, incorporating the significant factors identified on univariate analysis (Table 3). After controlling for other variables, female subjects had 20% lower odds of receiving IL-2 (O.R. 0.80) compared to males. African American subjects had 45% lower odds of receiving IL-2 (O.R 0.55), compared with Caucasians. Married individuals were much more likely than single individuals to receive IL-2 (O.R 1.9). Increased comorbidity as measured by Charlson score and increased histologic grade were associated with decreased odds of receiving Il-2. Residents of the Detroit and Seattle SEER registry areas were more likely to receive IL-2 (OR 2.0 and 2.2). We also examined survival in this non-randomized dataset. We created a Cox proportional hazards model to evaluate relative risks for overall mortality in our cohort by treatment type. However, assumptions of the model were violated when we evaluated the Schoenfeld residuals. Thus, we tested various data distributions to better fit our data. We created a model using a log-logistic distribution. In this model, receipt of surgery, IL-2, or both were all associated with decreased risk of mortality as compared with no treatment (Figure 1). Median survival for those who underwent both nephrectomy and immunotherapy (517 days, 95% CI 588–765 days was significantly higher than that for those who underwent surgery or immunotherapy alone, although those groups’ mean survival did not differ significantly from each other. (249 days, 95% CI 413–517 days and 283 days, 95% CI 346–436 days respectively). Subjects who received no therapy had the lowest median survival (97 days, 95% CI 185–207 days). Increased comorbidity and cancer grade were associated with increased mortality (data not shown).
Table 3.
Multivariate model results: Predictors of receipt of IL-2
| Effect | referent | Point Estimate |
95% Wald Confidence Limits |
P value | |
|---|---|---|---|---|---|
| RECEIPT OF NEPHRECTOMY | No nephrectomy | 2.011 | 1.580 | 2.561 | <.0001 |
| FEMALE | male | 0.795 | 0.644 | 0.982 | 0.0334 |
| AGE | 65 (continuous) | 0.949 | 0.937 | 0.961 | <.0001 |
| CHARLSON 1/2 | Charslon 0 | 0.567 | 0.403 | 0.797 | 0.0011 |
| CHARLSON ≥3 | Charlson 0 | 0.461 | 0.229 | 0.930 | 0.0305 |
| AFRICAN AMERICAN | Caucasian | 0.545 | 0.343 | 0.867 | 0.0103 |
| GRADE 2 | Grade 1 | 0.500 | 0.257 | 0.974 | 0.0416 |
| GRADE 3 | Grade 1 | 0.460 | 0.244 | 0.866 | 0.0161 |
| GRADE 4 | Grade 1 | 0.404 | 0.197 | 0.828 | 0.0133 |
| GRADE UNKNOWN | Grade 1 | 0.346 | 0.188 | 0.635 | 0.0006 |
| DETROIT | Utah | 1.988 | 1.001 | 3.945 | 0.0495 |
| SEATTLE | Utah | 2.232 | 1.114 | 4.475 | 0.0236 |
| MARRIED | Single | 1.863 | 1.179 | 2.946 | 0.0077 |
| YEAR | 1990 (continuous) | 1.045 | 1.008 | 1.084 | 0.0177 |
Figure 1.
Survival associated with different therapies for metastatic kidney cancer
Conclusions
Our study has several important findings. First, African Americans were much less likely to be treated with IL-2, whether as monotherapy or part of combined treatment, after controlling for age, tumor grade, comorbidity and other relevant clinical variables. While our study does not offer direct insight into the reason for this disparity, several possible explanations may underlie this finding. IL-2 is typically delivered at academically-affiliated medical centers; African Americans might have reduced access these centers if they are not located near their communities. Although we attempted to control for economic status, our metric, median income in the patient’s zip code, may be insufficiently specific to identify patients with limited financial resources. Thus, if African Americans in our study had fewer financial resources than other groups, this disparity may explain reduced access to an expensive therapy. Those patients might have difficulty affording Medicare co-payments for inpatient hospitalizations during which IL-2 is administered. Alternatively, cultural or personal preferences of African Americans may reduce their desire to receive IL-2 due to its perceived risk/benefit ratio.
Second, women were less likely to receive IL-2 therapy than men were. Again, the reasons for this discrepancy are unclear. If women have less social support than men in this population, they may be more hesitant to undergo an aggressive and potentially debilitating therapy. Gender-specific preferences for aggressive care may underlie this finding. Little is known about gender-specific preferences or differences in treatment choice in urologic cancers. Clearly, further prospective work in this area is indicated to illuminate this finding.
Third, married individuals were more likely to receive IL-2 as compared to single individuals. This finding may be explained by potential increased emotional and social support enjoyed by married individuals, which may make them more likely to undergo a treatment with significant potential health impact. Others have found that marital status can impact cancer treatment choice. Married status was positively associated with choice of potentially curative treatment in men with prostate cancer, versus observation only (8). Similarly, one population-based study reported that married individuals were 66% more likely to choose aggressive therapy (i.e. surgery) for lung cancer (9). Another large population-based study found that unmarried women with stage I or II breast cancer were less likely to receive definitive treatment (10).
Some of our findings were expected. Patients with increased comorbidity were less likely to receive IL-2. Patients with an increased number of comorbid conditions may have worsened performance status; IL-2 is typically administered only to patients with good performance status. We observed regional variation in use of IL-2. This may reflect differential judgments by physicians and/or patients in regards to the relative risks and benefits of IL-2 therapy. Our survival model serves as a validity check for our treatment assignment algorithm; as expected, those who received IL-2, nephrectomy, or both had superior survival as compared with those subjects who did not receive such treatment. These non-randomized data cannot be interpreted to support a survival benefit in favor of treatment. However, they do correspond to randomized clinical data which demonstrated such a benefit(11,12). As such, the data support the treatment assignment algorithm we created.
Our study has limitations. Although we attempted to control for relevant clinical variables that influence treatment with IL-2, unmeasured co-variates may have influenced our results. We attempted to control for variables we could measure in our multivariable model, but some of our variables may have been insufficiently sensitive or specific (e.g. using median values for census tract to impute socioeconomic status). We could not measure patient preferences for IL-2 therapy. Our data demonstrating survival of various treatment cohorts is biased by the non-random administration of treatments to subjects with varying prognoses. Finally, our findings in the Medicare population may not represent the experience of younger individuals with private insurance or no insurance.
Others have found evidence of racial disparity in patients with advanced renal cell cancers. Vaishampayan et al examined survival rates for African Americans with advanced kidney cancer in the SEER database. They reported that the median survival for African Americans versus Caucasians over 60 years old was 6 versus 11 months (p <0.001) (13). They suggest that the differences in survival may have a biologic basis, because the rates of complete response to IL-2 therapy are low. They further examined this issue in a retrospective review of patients with metastatic disease involved in clinical trials at their institution and found that the disparity in survival seen in SEER was duplicated in the clinical trial setting(14). The removal of the ‘access to care’ effect on survival may strengthen the argument for a biological basis for this difference, even though this study was small, retrospective, and had unbalanced risk factors between groups.
Regardless of whether the observed reduced survival in elderly African Americans with advanced kidney cancer is biologically driven, our study highlights the fact that the only therapy which has offered a complete response to patients with the disease is less frequently given to those who are African American. The arrival of new oral therapies for metastatic kidney cancer, such as sorafenib and sunitinib, has offered new hope to patients faced with this dire diagnosis. These medications may be more acceptable to patients who do not want to incur the risks of therapy with high dose IL-2 (15). They may also allow assessment of tumor response in poor-risk patients prior to subjecting them to consolidative nephrectomy, potentially sparing these individuals needless surgery (16). Availability of these medications may potentially ease access to effective treatment for individuals with metastatic kidney cancer, as they do not need to be administered at specialized facilities. However, monthly costs of these drugs are in the range of $6,000 (17), making the economic barriers to access for the uninsured and underinsured formidable. It is possible that the racial and gender-based disparities in treatment seen with IL-2 will be replicated with these new treatment options. Further prospective investigation into mitigating barriers to receipt of effective cancer care in this setting is urgently needed.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
This research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through the Urologic Diseases in America Project
References
- 1.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. Jama. 1999;281(17):1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Bretheau D, Lechevallier E, Eghazarian C, Grisoni V, Coulange C. Prognostic significance of incidental renal cell carcinoma. Eur Urol. 1995;27(4):319–323. doi: 10.1159/000475189. [DOI] [PubMed] [Google Scholar]
- 3.Homma Y, Kawabe K, Kitamura T, Nishimura Y, Shinohara M, Kondo Y, Saito I, Minowada S, Asakage Y. Increased incidental detection and reduced mortality in renal cancer--recent retrospective analysis at eight institutions. Int J Urol. 1995;2(2):77–80. doi: 10.1111/j.1442-2042.1995.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 4.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51(2):203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 5.Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS, Kammula US, Sherry RM, Royal RE, Steinberg SM, Rosenberg S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113(2):293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 7.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40(8 Suppl):IV-19–IV-25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 8.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg ER, Chute CG, Stukel T, Baron JA, Freeman DH, Yates J, Korson R. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. N Engl J Med. 1988;318(10):612–617. doi: 10.1056/NEJM198803103181006. [DOI] [PubMed] [Google Scholar]
- 10.Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 11.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23(1):133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 12.Atkins MB, Dutcher J, Weiss G, Margolin K, Clark J, Sosman J, Logan T, Aronson F, Mier J. Kidney cancer: the Cytokine Working Group experience (1986–2001): part I. IL-2-based clinical trials. Med Oncol. 2001;18(3):197–207. doi: 10.1385/MO:18:3:197. [DOI] [PubMed] [Google Scholar]
- 13.Vaishampayan UN, Do H, Hussain M, Schwartz K. Racial disparity in incidence patterns and outcome of kidney cancer. Urology. 2003;62(6):1012–1017. doi: 10.1016/j.urology.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi RT, Heilbrun LK, Jain V, Vaishampayan UN. Racial disparity in outcomes of a clinical trial population with metastatic renal cell carcinoma. Urology. 2006;68(2):296–301. doi: 10.1016/j.urology.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 15.Wong AS, Chong KT, Heng CT, Consigliere DT, Esuvaranathan K, Toh KL, Chuah B, Lim R, Tan J. Debulking nephrectomy followed by a "watch and wait" approach in metastatic renal cell carcinoma. Urol Oncol. 2008 doi: 10.1016/j.urolonc.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Neill MG, Jewett MA. The once and future role of cytoreductive nephrectomy. Urol Oncol. 2008;26(4):346–352. doi: 10.1016/j.urolonc.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Remak E, Charbonneau C, Negrier S, Kim ST, Motzer RJ. Economic evaluation of sunitinib malate for the first-line treatment of metastatic renal cell carcinoma. J Clin Oncol. 2008;26(24):3995–4000. doi: 10.1200/JCO.2007.13.2662. [DOI] [PubMed] [Google Scholar]