Summary
Primary familial and congenital polycythemia (PFCP) is an autosomal-dominant proliferative disorder characterized by erythrocytosis and hypersensitivity of erythroid progenitors to erythropoietin (Epo). Several lines of evidence suggest a causal role of truncated erythropoietin receptor (EpoR) in this disease. In this review, we discuss PFCP in the context of erythrocytosis and EpoR signaling. We focus on recent studies describing mechanisms underlying Epo-dependent EpoR down-regulation. One mechanism depends on internalization mediated through the p85 regulatory subunit of the Phosphoinositide 3-Kinase, and the other utilizes ubiquitin-based proteasomal degradation. Truncated PFCP EpoRs are not properly down-regulated upon stimulation, underscoring the importance of these mechanisms in the pathogenesis of PFCP.
Keywords: Erythrocytosis, primary familial and congenital polycythemia, erythropoietin receptor, endocytosis, receptor down-regulation
Erythrocytosis
Erythropoiesis is the process through which multi-potential hematopoietic stem cells differentiate into mature red blood cells. The principle growth factor that regulates erythropoiesis is erythropoietin (Epo), which is made mainly by the kidney. Epo production is increased under conditions of reduced red blood cell mass (anemia) or decreased cellular oxygen tension (hypoxia).
Erythrocytosis, often used interchangeably with polycythemia (“many cells”), comprises a heterogeneous group of disorders characterized by the expansion of the erythrocyte compartment in the peripheral blood, reflected by an increase in hematocrit, hemoglobin content, or number of red blood cells (Cario 2005). Erythrocytosis is classified as congenital or acquired and may be primary or secondary (Table 1). Primary erythrocytosis indicates that the erythrocytosis results from a molecular defect in the hematopoietic progenitor cells, whereas secondary erythrocytosis, although also a hematologic disorder, is due to aberrant regulation of erythropoiesis-promoting substances, mainly Epo, that act on these progenitors (Gordeuk et al., 2005). Once the stimulus for increased Epo production is removed, the erythroid proliferation should return to the physiologic norm.
Table 1.
| Primary (Epo low) |
Secondary (Epo normal or high) |
|
|---|---|---|
| Congenital | Primary familial and congenital polycythemia (EpoR mutations, others) |
Disorders of defective oxygen sensing mechanism (mutations in VHL, PHD, HIF) |
| Disorders of altered affinity of hemoglobin for oxygen (hemoglobin with increased oxygen affinity, 2,3-DPG deficiency, methemoglobinemia) | ||
| Congenital cyanotic heart or lung disease | ||
| Acquired | Polycythemia vera (JAK2 mutations) |
Increased Epo production (chronic hypoxia due to lung or heart disease, high altitude residence, tobacco abuse, CO poisoning, Epo secreting tumors, post-renal transplant, renal artery stenosis) |
| Androgen use/abusec, Epo use/abuse |
Abbreviations: Epo: erythropoietin; EpoR erythropoietin eceptor; VHL von Hippel Lindau tumor suppressor; PHD HIF prolyl hydroxylase; HIF hypoxia-inducible factor; 2,3-DPG: 2,3 diphosphoglycerate; CO: carbon monoxide.
Conflicting data regarding Epo level in androgen use.
Secondary erythrocytosis can be driven by various causes of hypoxia resulting in physiologically increased Epo production (Robert and Means 2004). Tissue hypoxia can be caused by congenital defects such as altered oxygen affinity of hemoglobin from mutations in the α or β globin genes (Charache et al., 1966, Gonzalez Fernandez et al., 2009, Robert and Means 2004, Rumi et al., 2009), defective bisphosphoglycerate mutase leading to 2,3-diphosphoglycerate (DPG) deficiency (Cartier et al., 1972, Galacteros et al., 1984, Hoyer et al., 2004), or methemoglobinemia, which in rare cases can be due to congenital deficiencies of NADH- and NADPH-methemoglobin reductases (Davis et al., 2004, Fermo et al., 2008, Yilmaz et al., 2005). Secondary erythrocytosis can also result from defects in components of the oxygen sensing mechanism, such as the hypoxia-inducible factor (HIF), EGLN/HPH/PHD family of HIF-prolyl hydroxylases, and von Hippel-Lindau tumor suppressor protein (VHL) (Al-Sheikh et al., 2008b, Gordeuk et al., 2005, Ladroue et al., 2008, Martini et al., 2008, Percy et al., 2007, Percy et al., 2008, Percy et al., 2006).
Most patients with primary erythrocytosis have polycythemia vera, an acquired myeloproliferative neoplasm. In 2005, several groups identified somatic gain-of-function mutations in the JAK2 tyrosine kinase (V617F and exon 12), which functions downstream of Epo, in the vast majority of polycythemia vera patients (reviewed in (Levine and Gilliland 2008, Vainchenker et al., 2008)). This prompted the World Health Organization to include JAK2 mutations in the diagnostic criteria for this disease (Patnaik and Tefferi 2009).
A small minority of patients with primary erythrocytosis have a congenital proliferative disorder of erythroid progenitor cells referred to as primary familial and congenital polycythemia (PFCP). PFCP is an autosomal-dominant disorder, although sporadic cases are also reported. PFCP is diagnosed when kindreds have isolated erythrocytosis without splenomegaly, low serum Epo levels, normal hemoglobin oxygen affinities, and bone marrow erythroid progenitors that exhibit Epo hypersensitivity (Prchal 2001). The natural history of PFCP shows no propensity to leukemic transformation nor is development of other myeloproliferative neoplasms observed (Prchal 2005). Clinically, PFCP patients may present with symptoms ranging from headaches, dizziness, epistaxis, exertional dyspnea to pruritis after bathing (Bourantas et al., 2006). Thrombotic and hemorrhagic events with premature morbidity and mortality have been reported (Prchal et al., 1995, Queisser et al., 1988), but many appear to have a benign clinical course (Emanuel et al., 1992, Juvonen et al., 1991, Prchal et al., 1985). Clinical symptoms are effectively relieved by phlebotomy; however, the increased risk of cardiovascular morbidity is not ameliorated by maintaining a normal hematocrit (Cario 2005, Van Maerken et al., 2004). Recent discoveries of the molecular defects involving the receptor for Epo that underlie this rare disorder are the subject of this review.
Signaling from the EpoR
Epo, essential for terminal differentiation of erythroid cells, is the primary cytokine that controls definitive erythropoiesis and its function is mediated through the Epo receptor (EpoR) (Constantinescu et al., 1999, Richmond et al., 2005). EpoR belongs to the cytokine receptor super-family, and consists of an extracellular domain, a single transmembrane domain, and a cytoplasmic domain. EpoR lacks intrinsic enzymatic activities and relies on the cytosolic tyrosine kinase JAK2 for signal transduction. JAK2 binding to the EpoR in the endoplasmic reticulum promotes its maturation and appearance on the plasma membrane (Huang et al., 2001). At the cell surface, binding of Epo to the EpoR extracellular domain activates JAK2 kinase activity. Subsequently, activated JAK2 phosphorylates many of the tyrosine residues in the cytoplasmic domain of EpoR, thereby providing a platform for the recruitment and activation of signaling mediators through SH2 domain-mediated interactions. Consistent with their essential roles in erythropoiesis, Epo, EpoR, and JAK2 deficient mice die embryonically due to severe anemia (Neubauer et al., 1998, Parganas et al., 1998, Wu et al., 1995). On the other hand, as indicated earlier, pathological activation of JAK2 is the major molecular lesion in patients with BCR/ABL-negative myeloproliferative neoplasms, including the majority of patients with polycythemia vera.
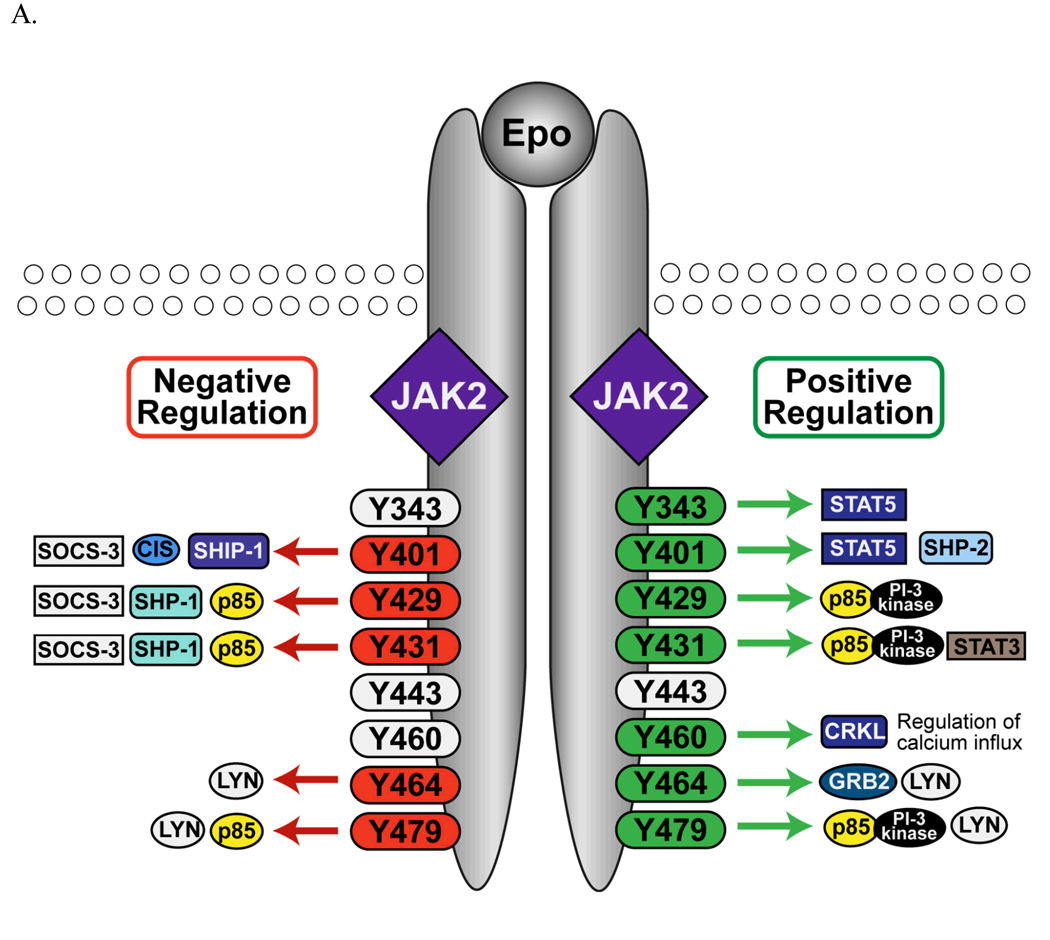
Much is known of the signaling pathways activated by Epo through signaling mediators recruited to the EpoR cytoplasmic phosphorylated tyrosines (Figure 1A). Because the majority of these studies have been performed on murine EpoR, we adopted the murine numbering system in this review. The signal transducer and activator of transcription 5 (STAT5) is recruited to Tyr343 and Tyr401 and is subsequently phosphorylated by JAK2 (Gobert et al., 1996, Klingmuller et al., 1996). Upon phosphorylation, STAT5 translocates into the nucleus to initiate gene transcription of proteins such as the anti-apoptotic proteins Bcl-x (Socolovsky et al., 1999), Pim-1, and Oncostatin M (Menon et al., 2006a). The adaptor molecule growth factor receptor protein 2 (GRB2) binds to the EpoR upon stimulation, presumably via a predicted GRB2 binding site at Tyr464, and through the guanine nucleotide exchange factor for Ras, SOS, activates the Ras/MAPK pathway to promote cell proliferation (Barber et al., 1997). The GRB2/SOS complex can also be recruited to Tyr401 via SH2-containing inositol-5-phosphatase (SHIP-1) (Mason et al., 2000) or protein tyrosine phosphatase SHP2 (Tauchi et al., 1996) to activate the Ras/MAPK pathway. The p85 regulatory subunit of Phosphoinositide 3-Kinase (PI3K) binds to phosphorylated Tyr429/Tyr431 or Tyr479 and recruits and activates the catalytic subunit of PI3K (Klingmuller et al., 1997, Sulahian et al., 2009). PI3K stimulates AKT-associated survival pathways (Bouscary et al., 2003), inhibits pro-apoptotic activities of the Forkhead transcription factor Foxo3 (Kashii et al., 2000), and also promotes erythroid proliferation and maturation (Klingmuller et al., 1997, Zhao et al., 2006). In addition, phosphorylated Tyr464 and/or Tyr479 binds to the SH2 domain of the Src tyrosine kinase Lyn (Chin et al., 1998), which regulates the expansion and differentiation of erythroid progenitors (Harder et al., 2004, Ingley et al., 2005, Karur et al., 2006, Tilbrook et al., 2001). The CRKL adapter protein, recruited to phosphorylated Tyr460 upon stimulation, is phosphorylated by Lyn to augment MAPK activation (Arai et al., 2001). Moreover, Epo stimulates a rise in intracellular calcium, possibly through an interaction with Tyr460 in the EpoR and the transient receptor potential channels, which contributes to differentiation (Chu et al., 2002, Gillo et al., 1993, Hensold et al., 1991, Hirschler-Laszkiewicz et al., 2009). In human EpoR, an additional juxtamembrane tyrosine, Tyr309, exists that modulates STAT1 activation (Arcasoy and Karayal 2005).
Figure 1.
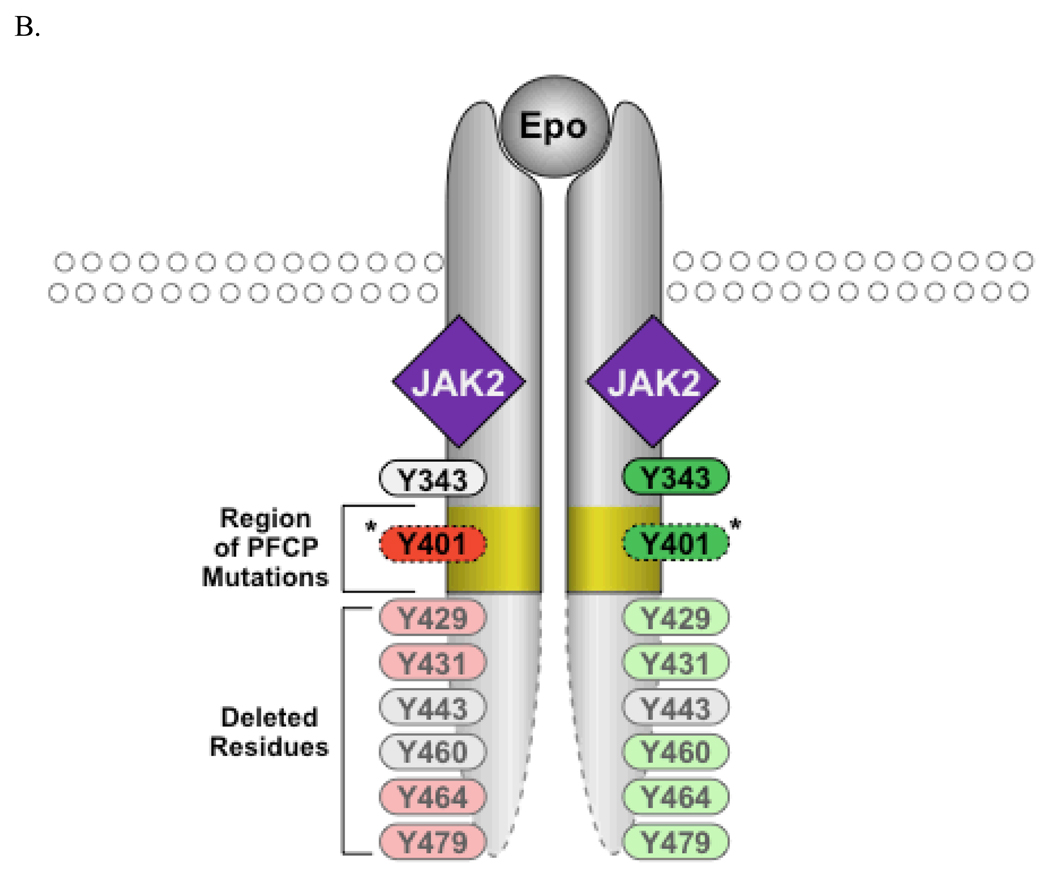
Signaling from the EpoR. A. Proteins recruited to EpoR cytoplasmic tyrosine residues that positively or negatively regulate EpoR signaling are depicted. B. Truncated EpoR from PFCP patients lack parts of the EpoR cytoplasmic domain. Y401 is retained in some but not all of the PFCP receptors.
Somewhat unexpectedly, mice expressing a minimal tyrosine-null EpoR allele are able to support steady-state erythropoiesis but fail to efficiently support stress erythropoiesis (Menon et al., 2006b, Zang et al., 2001). Therefore, signaling independent of EpoR cytoplasmic tyrosines plays essential roles for steady-state erythropoiesis (Wojchowski et al., 2006). Although not entirely understood, the MAPK signaling axis seems to play an important role in these pathways (Menon et al., 2006a). Much remains to be uncovered of the protein components and pathways underlying Epo-induced tyrosine-independent signaling. Together, EpoR tyrosine dependent and independent signaling cascades ultimately result in the survival, proliferation, and differentiation of erythroid progenitor cells.
In order to tightly regulate and to prevent uncontrolled erythropoiesis, negative regulators are also elicited upon activation to attenuate EpoR signal transduction (Figure 1A) (Wormald and Hilton 2004). For example, the tyrosine phosphatase SHP-1 (also known as SHPTP-1 or HCP) is recruited to phosphorylated Tyr429 and de-phosphorylates JAK2 (Klingmuller et al., 1995, Yi et al., 1995). The numbers of erythroid progenitors CFU-Es from SHP1-deficient (viable motheaten) mice are dramatically increased in the spleen and are hypersensitive to Epo (Van Zant and Shultz 1989). The SH2-containing inositol-5-phosphatase (SHIP-1) is recruited to Tyr401 of the EpoR (Mason et al., 2000) and SHIP-1 null mice show elevated formation of BFU-Es and CFU-Es in the bone marrow (Helgason et al., 1998). In addition, members of the suppressor of cytokine signaling (SOCS) family proteins and the cytokine-inducible SH2-domain-containing protein (CIS) can bind to cytokine receptors or the associated JAK kinases and attenuate signal transduction both by direct interference with signaling and by targeting the receptor complex for ubiquitin-mediated proteasomal degradation (Wormald and Hilton 2004). Upon Epo stimulation, SOCS-3 binds to Tyr401 or Tyr429 and Tyr431 (Hortner et al., 2002, Sasaki et al., 1999) and CIS binds to Tyr401 (Verdier et al., 1998), blocking access of STAT5 to the EpoR. SOCS1 interacts with the critical phospho-tyrosine residue Tyr1007 within the JAK2 catalytic loop and reduces its tyrosine-kinase activity and suppresses the tyrosine-phosphorylation and activation of STAT5 (Endo et al., 1997, Yasukawa et al., 1999). Transcription of SOCS-1, SOCS-3, and CIS is induced by Epo, thus forming a negative feedback circuit (Starr et al., 1997). Moreover, over-expression of lymphocyte linker protein (Lnk), an adaptor protein that becomes tyrosine-phosphorylated following Epo induction, inhibits survival and differentiation of erythroid progenitors. Lnk-deficient mice display enhanced Epo-mediated signaling and erythropoiesis (Tong et al., 2005).
EpoR mutations in PFCP
The molecular basis of PFCP was not known until the study of a large family from Finland who had autosomal dominant erythrocytosis caused by excessive sensitivity of erythroid progenitors to Epo (Juvonen et al., 1991). Linkage analysis and later sequencing identified a mutation in the EpoR gene that was likely the cause of the erythrocytosis (de la Chapelle et al., 1993a, de la Chapelle et al., 1993b). This mutation (G6002A) leads to a premature stop codon at Trp439, resulting in a truncated EpoR. To date, 16 different EpoR alleles have been found in PFCP patients (Table 2). All these mutations reside in exon VIII, which encodes the C-terminal portion of the EpoR cytoplasmic domain. Except for two missense mutations that may not be linked to the polycythemic phenotype (Le Couedic et al., 1996, Sokol et al., 1994), all other mutations result in premature termination and, consequently, these mutant receptors lack portions of the C-terminal EpoR cytoplasmic domain (Figure 1B). Because these truncated EpoRs lack Tyr429 and/or Tyr401, loss of negative regulation by SHP-1, SOCS-3, CIS, or SHIP1 was assumed to be the underlying cause for PFCP. Consistent with this notion, prolonged JAK2 and STAT5 activation and STAT5 DNA binding activity were observed in cells expressing PFCP EpoR mutants (Arcasoy et al., 1999, Arcasoy and Karayal 2005).
Table 2.
EpoR mutations in Primary Familial and Congenital Polycythemia.a
| EpoR mutation | Consequences of the Mutation | Reference |
|---|---|---|
| del5828-5829 | Frameshift: P381Q-* | (Al-Sheikh et al., 2008a) |
| G5881T | E399* | (Arcasoy et al., 2002) |
| del5938-5941 | Frameshift: G418P- LLPALSTLSWTPAPSSCVHGHCALSCPLPHPT-* |
(Petersen et al., 2004) |
| del5957-5958 | F424* | (Al-Sheikh et al., 2008a) |
| G5959T | E425* | (Kralovics and Prchal 2001) |
| C5964G | Y426* | (Kralovics et al., 1998) |
| 5967insT | Frameshift: I428Y-PGPQLPALASMDTVP-* | (Kralovics et al., 1997a) |
| del5971C | Frameshift: L429W- TPAPSSCVHGHCALSCPLPHPT-* |
(Al-Sheikh et al., 2008a) |
| dup5968-5975 | Frameshift: D430E- SWTPAPSSCVHGHCALSCPLPHPT-* |
(Watowich et al., 1999) |
| 5974insG | Frameshift: D430G-PQLPALASMDTVP-* | (Sokol et al., 1995) |
| del5985-5991 | Frameshift: Q434C-VHGHCALSCPLPHPT-* | (Arcasoy et al., 1997, Kralovics et al., 1997a) |
| C5986T | Q434* | (Furukawa et al., 1997) |
| G6002A | W439* | (de la Chapelle et al., 1993b, Percy et al., 1998) |
| G6003A | W439* | (Rives et al., 2007) |
| A6146G | N487S (Missense Mutation) | (Le Couedic et al., 1996) |
| C6148T | P488S (Missense Mutation) | (Kralovics et al., 1997b, Sokol et al., 1994) |
Abbreviations: Ins, insertion; del, deletion; dup, duplication;
termination codon. In the shortest deletion, P381 corresponds to C356 in the murine EpoR, and in the longest deletion, W439 corresponds to R414 in the murine EpoR.
A mouse model of PFCP was generated by replacing the murine EpoR with a truncated human EpoR identified in PFCP patients. To mimic the heterozygous state of truncated EpoR in patients, the murine EpoR gene was replaced with a wild-type and a mutant EpoR. These mice develop polycythemia within 3–6 weeks of age and have increased hematocrit and elevated hemoglobin concentrations, mimicking the human disorder (Divoky et al., 2001). Homozygous mice of the mutant allele are also polycythemic (Divoky et al., 2001).
Down-regulation of the EpoR and PFCP
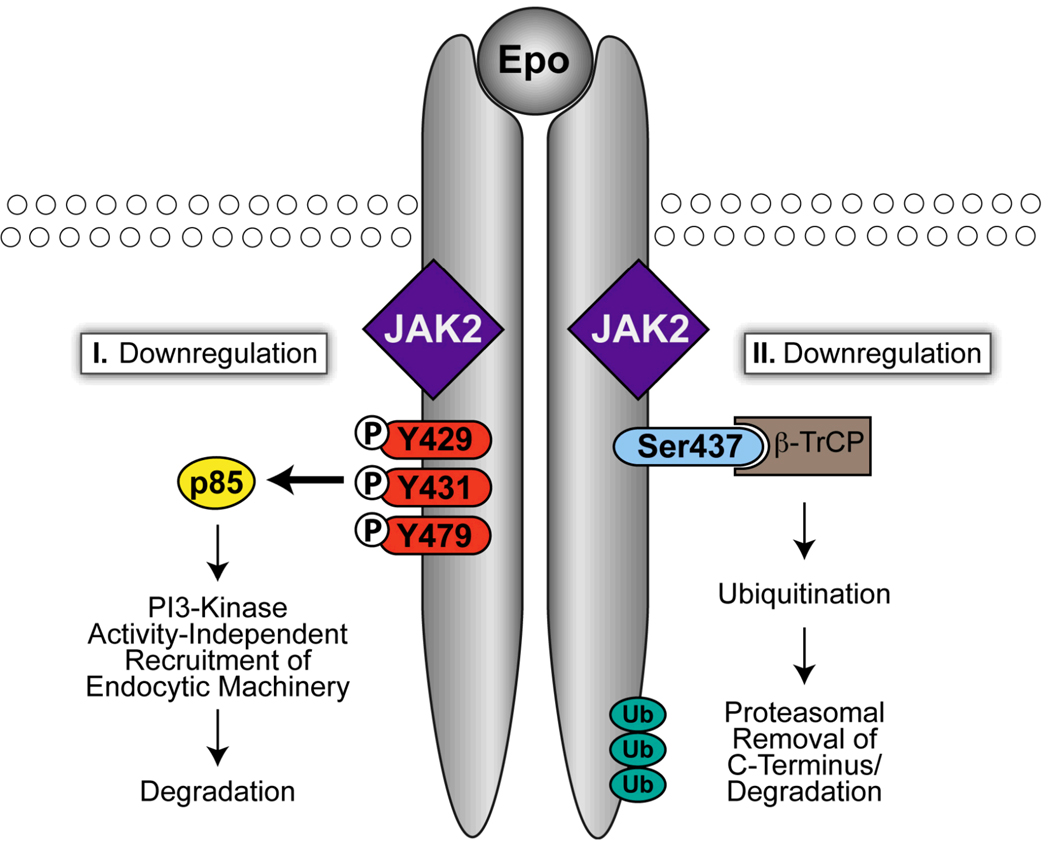
Recent advances in the knowledge of mechanisms underlying EpoR down-regulation lead to the hypothesis that dysregulation of EpoR down-regulation may also contribute to PFCP (Figure 2). Upon Epo stimulation, cell-surface EpoR is internalized and degraded; few, if any, EpoR molecules recycle back to the cell surface (Beckman et al., 1999, Levin et al., 1998, Walrafen et al., 2005). Because cell-surface levels of EpoR control cellular Epo sensitivity (Suzuki et al., 2002), Epo-induced internalization is an efficient way to rapidly decrease Epo responsiveness. It also may bring about destruction of activated protein complexes and terminate signaling. We reported that upon Epo stimulation, cell-surface EpoR is internalized via clathrin-mediated endocytosis, and this process requires both JAK2 kinase activity and EpoR cytoplasmic tyrosines (Sulahian et al., 2009). Phosphorylated Tyr429, Tyr431, and Tyr479 in the EpoR cytoplasmic domain share redundant functions in mediating Epo-dependent EpoR internalization via binding to the p85α or p85β subunit of PI3K (Sulahian et al., 2009). Interestingly, although binding of p85 to the EpoR recruits and activates the catalytic subunit of PI3K, inhibition of the PI3K activity with wortmannin does not impair EpoR internalization (Sulahian et al., 2009). Therefore, p85 promotes EpoR internalization via a PI3K kinase activity-independent mechanism, possibly by acting as a scaffold that also recruits proteins of the endocytic machinery. These results support the emerging concept that p85 has functions besides regulating PI3K kinase activity.
Figure 2.
Epo-induced EpoR down-regulation. Upon Epo stimulation, EpoR is down-regulated through two mechanisms, one dependent on p85 and the other on β-TrCP-mediated proteasomal degradation.
All truncated EpoR alleles from PFCP patients lack Tyr429, Tyr431, and Tyr479 (Figure 1B). None of the mutants tested bound p85β and none were internalized or degraded upon Epo stimulation ((Sulahian et al., 2009) and our unpublished results). Consistent with these results, erythroid progenitors expressing PFCP receptors are hypersensitive to Epo (Sulahian et al., 2009). Importantly, fusion of KY429LY431L but not KFLFL (where Y429 and Y431 were replaced with Phe), residues encompassing Tyr429 and Tyr431, to a PFCP receptor restores both p85β binding and ligand-induced internalization and reverses Epo hypersensitivity in primary erythroid progenitors (Sulahian et al., 2009). Therefore, failure to recruit p85 and internalize may contribute to the prolonged signaling of the truncated EpoRs in PFCP.
Besides phospho-tyrosine-based down-regulation, several lines of evidence indicate that ubiquitination also plays an important role in EpoR down-regulation. Epo binding rapidly stimulates EpoR ubiquitination at the plasma membrane and JAK2 kinase activity is required for EpoR ubiquitination (Walrafen et al., 2005). Epo-induced degradation of the EpoR is sensitive to inhibitors of both proteasomal and lysosomal function (Walrafen et al., 2005). Because an EpoR fragment containing the EpoR C-terminus was detected upon stimulation in the presence of both proteasomal and lysosomal inhibitors but not with lysosomal inhibitors alone (Walrafen et al., 2005), part of the EpoR cytoplasmic domain may be removed by the proteasome upon Epo stimulation and the remaining part of the receptor may be sorted to the lysosome for degradation (Walrafen et al., 2005). Although the exact boundary is not clear, it was proposed that this would remove the tyrosine residues and terminate tyrosine-based signaling. Later work demonstrated that β-Transducin repeat-containing protein (β-TrCP), the substrate-binding subunit of a cullin–RING ubiquitin ligase complex (Petroski and Deshaies 2005), is responsible for Epo-induced EpoR ubiquitination (Meyer et al., 2007). Mutation of Ser437 in the β-TrCP binding motif in the EpoR cytoplasmic domain abolishes inducible binding of β-TrCP to the EpoR and also blocks EpoR ubiquitination and degradation (Meyer et al., 2007). In addition, cells expressing EpoR with a Ser437 mutation are hypersensitive to Epo and show prolonged presence of phosphorylated EpoR (Meyer et al., 2007). Because PFCP EpoRs lack the β-TrCP binding motif, these truncated receptors are not expected to recruit β-TrCP upon stimulation nor be degraded by the proteasome and later lysosome upon stimulation.
Thus, two novel pathways of Epo-induced EpoR down-regulation have been identified. One pathway depends on internalization mediated through p85 and the other utilizes ubiquitin-based proteasomal degradation. We observed an internalization mechanism at high Epo concentration that depends on JAK2 but not cytoplasmic EpoR tyrosines. We thus surmised that at low Epo concentrations, EpoR is internalized via the p85-dependent pathway and subsequently degraded in the lysosome, whereas at high Epo concentrations, EpoR is down-regulated through β-TrCP-mediated ubiquitination and degradation. This proposal is consistent with findings that endocytosis pathway of EGF receptors varies with EGF concentrations (Sigismund et al., 2005). Truncated PFCP receptors lack essential determinants for both pathways. Therefore, PFCP EpoRs not only cannot recruit negative regulators such as phosphatases to inactivate JAK2, these mutant receptors also are defective in Epo-induced receptor down-regulation. Consequently, these defects result in hypersensitivity to Epo and prolonged signaling.
Other unidentified mutations in PFCP
There is evidence suggesting that mutations in genes other than the EpoR may cause PFCP. For example, in a study of 43 unrelated PFCP subjects, only five (12%) of the patients had EpoR mutations (Kralovics and Prchal 2001). In another study, no mutation in the EpoR cytoplasmic domain was detected in PFCP patients from eight families of Greek origin (Bourantas et al., 2006). A caveat on the prevalence of EpoR mutations in PFCP was raised by Skoda and Prchal in 2005. They cautioned that measurement of serum Epo levels, which is a major diagnostic criterion of primary vs. secondary erythrocytosis (Table 1), is not necessarily sensitive and accurate, and stated “some of our previous reports on the absence of association of EpoR mutations and PFCP need to be re-evaluated… some of the families we previously classified as PFCP had inappropriately normal or even slightly increased Epo levels” (Skoda and Prchal 2005). Therefore, the true prevalence of EpoR mutations in PFCP remains unclear.
In addition, there seems to exist yet unidentified environmental or genetic factors that may influence the clinical manifestation of PFCP. For example, a child who inherited the same PFCP EpoR allele as affected family members has not shown laboratory evidence of polycythemia, despite the fact that the child’s erythroid progenitors are hypersensitive to Epo (Kralovics et al., 1998). These environmental and genetic factors remain to be identified.
Diagnostic evaluation of erythrocytosis
The diagnostic evaluation of patients with erythrocytosis is not always straightforward, and has changed significantly with the discovery of the molecular defects underlying myeloproliferative neoplasms and congenital polycythemia (Patnaik and Tefferi 2009). Given the high prevalence of JAK2 V617F mutation in polycythemia vera, analysis for JAK2 V617F would quickly identify patients with this disorder. For those with no JAK2 V617F mutation, serum Epo level should be determined. A decreased serum Epo level should lead to a search for other JAK2 mutations. Presence of other JAK2 mutations would establish the diagnosis of polycythemia vera; if no JAK2 mutation is found and erythrocytosis is lifelong, mutational analysis for EpoR and associated defects as outlined in this review would establish the diagnosis of PFCP. If serum Epo level is normal or increased and associated with acquired erythrocytosis, the diagnostic possibilities would include those listed in the bottom right panel of Table 1. If Epo is normal or elevated, but with a lifelong history of erythrocytosis, then determination of P50 (oxygen tension at which hemoglobin saturation is 50%) would help to distinguish between patients with various causes of increased oxygen affinity (decreased P50), and those with defective oxygen sensing (normal P50) (top right panel of Table 1).
Future directions
Although the knowledge of mechanisms underlying Epo-induced receptor down-regulation has furthered our understanding of the molecular pathogenesis of PFCP, many key issues are unresolved. For example, upon Epo stimulation, we do not know how and where activation and termination of different signaling cascades are compartmentalized, nor how PFCP receptors and wild-type receptors differ. The relative importance of the various mechanisms of receptor down-regulation and the recruitment of phosphatases in controlling EpoR signaling duration are not known. The molecular mechanisms and protein partners in p85-mediated EpoR internalization have not been identified and it is not known whether mutations in p85 or β-TrCP exist in erythrocytosis patients.
Combining molecular testing for JAK2 mutations and better assays to accurately determine serum Epo level, we should be able to more correctly categorize patients with erythrocytosis. Molecular elucidation of the mechanisms underlying the etiology and clinical manifestation of PFCP may aid in a rational translation of bench-top knowledge to the bedside for this disease and other erythrocytoses. As discussed in this review, therapies targeting the endocytosis and down-regulation of the EpoR may be beneficial to PFCP patients. In addition, because JAK2 kinase inhibitors are already undergoing clinical trials at various centers around the world to evaluate their efficacy in treatment of myeloproliferative neoplasms, if proven effective and safe, these inhibitors may represent a potential targeted therapy for patients with PFCP as well.
Acknowledgements
We thank Drs. Eugene Frenkel and Nai-wen Chi for their insightful comments of this manuscript, and apologize to many colleagues whose contributions we were unable to list because of reference citation limitations. Our research on the EpoR was supported by a grant from the National Institute of Health (R01 HL089966).
References
- Al-Sheikh M, Mazurier E, Gardie B, Casadevall N, Galacteros F, Goossens M, Wajcman H, Prehu C, Ugo V. A study of 36 unrelated cases with pure erythrocytosis revealed three new mutations in the erythropoietin receptor gene. Haematologica. 2008a;93:1072–1075. doi: 10.3324/haematol.12260. [DOI] [PubMed] [Google Scholar]
- Al-Sheikh M, Moradkhani K, Lopez M, Wajcman H, Prehu C. Disturbance in the HIF-1alpha pathway associated with erythrocytosis: further evidences brought by frameshift and nonsense mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Blood Cells Mol Dis. 2008b;40:160–165. doi: 10.1016/j.bcmd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Arai A, Kanda E, Nosaka Y, Miyasaka N, Miura O. CrkL is recruited through its SH2 domain to the erythropoietin receptor and plays a role in Lyn-mediated receptor signaling. J Biol Chem. 2001;276:33282–33290. doi: 10.1074/jbc.M102924200. [DOI] [PubMed] [Google Scholar]
- Arcasoy MO, Degar BA, Harris KW, Forget BG. Familial erythrocytosis associated with a short deletion in the erythropoietin receptor gene. Blood. 1997;89:4628–4635. [PubMed] [Google Scholar]
- Arcasoy MO, Harris KW, Forget BG. A human erythropoietin receptor gene mutant causing familial erythrocytosis is associated with deregulation of the rates of Jak2 and Stat5 inactivation. Exp Hematol. 1999;27:63–74. doi: 10.1016/s0301-472x(98)00003-4. [DOI] [PubMed] [Google Scholar]
- Arcasoy MO, Karayal AF. Erythropoietin hypersensitivity in primary familial and congenital polycythemia: role of tyrosines Y285 and Y344 in erythropoietin receptor cytoplasmic domain. Biochim Biophys Acta. 2005;1740:17–28. doi: 10.1016/j.bbadis.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Arcasoy MO, Karayal AF, Segal HM, Sinning JG, Forget BG. A novel mutation in the erythropoietin receptor gene is associated with familial erythrocytosis. Blood. 2002;99:3066–3069. doi: 10.1182/blood.v99.8.3066. [DOI] [PubMed] [Google Scholar]
- Barber DL, Corless CN, Xia K, Roberts TM, D'Andrea AD. Erythropoietin activates Raf1 by an Shc-independent pathway in CTLL-EPO-R cells. Blood. 1997;89:55–64. [PubMed] [Google Scholar]
- Beckman DL, Lin LL, Quinones ME, Longmore GD. Activation of the erythropoietin receptor is not required for internalization of bound erythropoietin. Blood. 1999;94:2667–2675. [PubMed] [Google Scholar]
- Bourantas LK, Chatzikyriakidou A, Dasoula A, Syrrou M, Bournatas KL, Georgiou I. Absence of mutations of the EPO-receptor gene in Greek patients with familial polycythemia. Eur J Haematol. 2006;76:537–538. doi: 10.1111/j.1600-0609.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- Bouscary D, Pene F, Claessens YE, Muller O, Chretien S, Fontenay-Roupie M, Gisselbrecht S, Mayeux P, Lacombe C. Critical role for PI 3-kinase in the control of erythropoietin-induced erythroid progenitor proliferation. Blood. 2003;101:3436–3443. doi: 10.1182/blood-2002-07-2332. [DOI] [PubMed] [Google Scholar]
- Cario H. Childhood polycythemias/erythrocytoses: classification, diagnosis, clinical presentation, and treatment. Ann Hematol. 2005;84:137–145. doi: 10.1007/s00277-004-0985-1. [DOI] [PubMed] [Google Scholar]
- Cartier P, Labie D, Leroux JP, Najman A, Demaugre F. [Familial diphosphoglycerate mutase deficiency: hematological and biochemical study] Nouv Rev Fr Hematol. 1972;12:269–287. [PubMed] [Google Scholar]
- Charache S, Weatherall DJ, Clegg JB. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966;45:813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin H, Arai A, Wakao H, Kamiyama R, Miyasaka N, Miura O. Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway. Blood. 1998;91:3734–3745. [PubMed] [Google Scholar]
- Chu X, Cheung JY, Barber DL, Birnbaumer L, Rothblum LI, Conrad K, Abrasonis V, Chan YM, Stahl R, Carey DJ, Miller BA. Erythropoietin modulates calcium influx through TRPC2. J Biol Chem. 2002;277:34375–34382. doi: 10.1074/jbc.M205541200. [DOI] [PubMed] [Google Scholar]
- Constantinescu SN, Ghaffari S, Lodish HF. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends Endocrinol Metab. 1999;10:18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- Davis CA, Crowley LJ, Barber MJ. Cytochrome b5 reductase: the roles of the recessive congenital methemoglobinemia mutants P144L, L148P, and R159*. Arch Biochem Biophys. 2004;431:233–244. doi: 10.1016/j.abb.2004.08.005. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A, Sistonen P, Lehvaslaiho H, Ikkala E, Juvonen E. Familial erythrocytosis genetically linked to erythropoietin receptor gene. Lancet. 1993a;341:82–84. doi: 10.1016/0140-6736(93)92558-b. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A, Traskelin AL, Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A. 1993b;90:4495–4499. doi: 10.1073/pnas.90.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divoky V, Liu Z, Ryan TM, Prchal JF, Townes TM, Prchal JT. Mouse model of congenital polycythemia: Homologous replacement of murine gene by mutant human erythropoietin receptor gene. Proc Natl Acad Sci U S A. 2001;98:986–991. doi: 10.1073/pnas.98.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel PD, Eaves CJ, Broudy VC, Papayannopoulou T, Moore MR, D'Andrea AD, Prchal JF, Eaves AC, Prchal JT. Familial and congenital polycythemia in three unrelated families. Blood. 1992;79:3019–3030. [PubMed] [Google Scholar]
- Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- Fermo E, Bianchi P, Vercellati C, Marcello AP, Garatti M, Marangoni O, Barcellini W, Zanella A. Recessive hereditary methemoglobinemia: two novel mutations in the NADH-cytochrome b5 reductase gene. Blood Cells Mol Dis. 2008;41:50–55. doi: 10.1016/j.bcmd.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Narita M, Sakaue M, Otsuka T, Kuroha T, Masuko M, Azegami T, Kishi K, Takahashi M, Utsumi J, Koike T, Aizawa Y. Primary familial polycythaemia associated with a novel point mutation in the erythropoietin receptor. Br J Haematol. 1997;99:222–227. doi: 10.1046/j.1365-2141.1997.3583172.x. [DOI] [PubMed] [Google Scholar]
- Galacteros F, Rosa R, Prehu MO, Najean Y, Calvin MC. [Diphosphoglyceromutase deficiency: new cases associated with erythrocytosis] Nouv Rev Fr Hematol. 1984;26:69–74. [PubMed] [Google Scholar]
- Gillo B, Ma YS, Marks AR. Calcium influx in induced differentiation of murine erythroleukemia cells. Blood. 1993;81:783–792. [PubMed] [Google Scholar]
- Gobert S, Chretien S, Gouilleux F, Muller O, Pallard C, Dusanter-Fourt I, Groner B, Lacombe C, Gisselbrecht S, Mayeux P. Identification of tyrosine residues within the intracellular domain of the erythropoietin receptor crucial for STAT5 activation. Embo J. 1996;15:2434–2441. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Fernandez FA, Villegas A, Ropero P, Carreno MD, Anguita E, Polo M, Pascual A, Henandez A Experience of Erythropathology Cooperative Spanish Group. Haemoglobinopathies with high oxygen affinity. Ann Hematol. 2009;88:235–238. doi: 10.1007/s00277-008-0581-x. [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Stockton DW, Prchal JT. Congenital polycythemias/erythrocytoses. Haematologica. 2005;90:109–116. [PubMed] [Google Scholar]
- Harder KW, Quilici C, Naik E, Inglese M, Kountouri N, Turner A, Zlatic K, Tarlinton DM, Hibbs ML. Perturbed myelo/erythropoiesis in Lyn-deficient mice is similar to that in mice lacking the inhibitory phosphatases SHP-1 and SHIP-1. Blood. 2004;104:3901–3910. doi: 10.1182/blood-2003-12-4396. [DOI] [PubMed] [Google Scholar]
- Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensold JO, Dubyak G, Housman DE. Calcium ionophore, A23187, induces commitment to differentiation but inhibits the subsequent expression of erythroid genes in murine erythroleukemia cells. Blood. 1991;77:1362–1370. [PubMed] [Google Scholar]
- Hirschler-Laszkiewicz I, Tong Q, Conrad K, Zhang W, Flint WW, Barber AJ, Barber DL, Cheung JY, Miller BA. TRPC3 activation by erythropoietin is modulated by TRPC6. J Biol Chem. 2009;284:4567–4581. doi: 10.1074/jbc.M804734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortner M, Nielsch U, Mayr LM, Heinrich PC, Haan S. A new high affinity binding site for suppressor of cytokine signaling-3 on the erythropoietin receptor. Eur J Biochem. 2002;269:2516–2526. doi: 10.1046/j.1432-1033.2002.02916.x. [DOI] [PubMed] [Google Scholar]
- Hoyer JD, Allen SL, Beutler E, Kubik K, West C, Fairbanks VF. Erythrocytosis due to bisphosphoglycerate mutase deficiency with concurrent glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. Am J Hematol. 2004;75:205–208. doi: 10.1002/ajh.20014. [DOI] [PubMed] [Google Scholar]
- Huang LJ, Constantinescu SN, Lodish HF. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol Cell. 2001;8:1327–1338. doi: 10.1016/s1097-2765(01)00401-4. [DOI] [PubMed] [Google Scholar]
- Ingley E, McCarthy DJ, Pore JR, Sarna MK, Adenan AS, Wright MJ, Erber W, Tilbrook PA, Klinken SP. Lyn deficiency reduces GATA-1, EKLF and STAT5, and induces extramedullary stress erythropoiesis. Oncogene. 2005;24:336–343. doi: 10.1038/sj.onc.1208199. [DOI] [PubMed] [Google Scholar]
- Juvonen E, Ikkala E, Fyhrquist F, Ruutu T. Autosomal dominant erythrocytosis caused by increased sensitivity to erythropoietin. Blood. 1991;78:3066–3069. [PubMed] [Google Scholar]
- Karur VG, Lowell CA, Besmer P, Agosti V, Wojchowski DM. Lyn kinase promotes erythroblast expansion and late-stage development. Blood. 2006;108:1524–1532. doi: 10.1182/blood-2005-09-008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashii Y, Uchida M, Kirito K, Tanaka M, Nishijima K, Toshima M, Ando T, Koizumi K, Endoh T, Sawada K, Momoi M, Miura Y, Ozawa K, Komatsu N. A member of Forkhead family transcription factor, FKHRL1, is one of the downstream molecules of phosphatidylinositol 3-kinase-Akt activation pathway in erythropoietin signal transduction. Blood. 2000;96:941–949. [PubMed] [Google Scholar]
- Klingmuller U, Bergelson S, Hsiao JG, Lodish HF. Multiple tyrosine residues in the cytosolic domain of the erythropoietin receptor promote activation of STAT5. Proc Natl Acad Sci U S A. 1996;93:8324–8328. doi: 10.1073/pnas.93.16.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Klingmuller U, Wu H, Hsiao JG, Toker A, Duckworth BC, Cantley LC, Lodish HF. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci U S A. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralovics R, Indrak K, Stopka T, Berman BW, Prchal JF, Prchal JT. Two new EPO receptor mutations: truncated EPO receptors are most frequently associated with primary familial and congenital polycythemias. Blood. 1997a;90:2057–2061. [PubMed] [Google Scholar]
- Kralovics R, Prchal JT. Genetic heterogeneity of primary familial and congenital polycythemia. Am J Hematol. 2001;68:115–121. doi: 10.1002/ajh.1162. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Sokol L, Broxson EH, Jr, Prchal JT. The erythropoietin receptor gene is not linked with the polycythemia phenotype in a family with autosomal dominant primary polycythemia. Proc Assoc Am Physicians. 1997b;109:580–585. [PubMed] [Google Scholar]
- Kralovics R, Sokol L, Prchal JT. Absence of polycythemia in a child with a unique erythropoietin receptor mutation in a family with autosomal dominant primary polycythemia. J Clin Invest. 1998;102:124–129. doi: 10.1172/JCI2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, Feunteun J, Pouyssegur J, Richard S, Gardie B. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359:2685–2692. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- Le Couedic JP, Mitjavila MT, Villeval JL, Feger F, Gobert S, Mayeux P, Casadevall N, Vainchenker W. Missense mutation of the erythropoietin receptor is a rare event in human erythroid malignancies. Blood. 1996;87:1502–1511. [PubMed] [Google Scholar]
- Levin I, Cohen J, Supino-Rosin L, Yoshimura A, Watowich SS, Neumann D. Identification of a cytoplasmic motif in the erythropoietin receptor required for receptor internalization. FEBS Lett. 1998;427:164–170. doi: 10.1016/s0014-5793(98)00414-1. [DOI] [PubMed] [Google Scholar]
- Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Teofili L, Cenci T, Giona F, Torti L, Rea M, Foa R, Leone G, Larocca LM. A novel heterozygous HIF2AM535I mutation reinforces the role of oxygen sensing pathway disturbances in the pathogenesis of familial erythrocytosis. Haematologica. 2008;93:1068–1071. doi: 10.3324/haematol.13210. [DOI] [PubMed] [Google Scholar]
- Mason JM, Beattie BK, Liu Q, Dumont DJ, Barber DL. The SH2 inositol 5-phosphatase Ship1 is recruited in an SH2-dependent manner to the erythropoietin receptor. J Biol Chem. 2000;275:4398–4406. doi: 10.1074/jbc.275.6.4398. [DOI] [PubMed] [Google Scholar]
- Menon MP, Fang J, Wojchowski DM. Core erythropoietin receptor signals for late erythroblast development. Blood. 2006a;107:2662–2672. doi: 10.1182/blood-2005-02-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B, Wojchowski DM. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J Clin Invest. 2006b;116:683–694. doi: 10.1172/JCI25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L, Deau B, Forejtnikova H, Dumenil D, Margottin-Goguet F, Lacombe C, Mayeux P, Verdier F. beta-Trcp mediates ubiquitination and degradation of the erythropoietin receptor and controls cell proliferation. Blood. 2007;109:5215–5222. doi: 10.1182/blood-2006-10-055350. [DOI] [PubMed] [Google Scholar]
- Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- Pardanani A. JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia. 2008;22:23–30. doi: 10.1038/sj.leu.2404948. [DOI] [PubMed] [Google Scholar]
- Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, Grosveld G, Ihle JN. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- Patnaik MM, Tefferi A. The complete evaluation of erythrocytosis: congenital and acquired. Leukemia. 2009;23:834–844. doi: 10.1038/leu.2009.54. [DOI] [PubMed] [Google Scholar]
- Percy MJ, Furlow PW, Beer PA, Lappin TR, McMullin MF, Lee FS. A novel erythrocytosis-associated PHD2 mutation suggests the location of a HIF binding groove. Blood. 2007;110:2193–2196. doi: 10.1182/blood-2007-04-084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, Lee FS. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–168. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, McMullin MF, Roques AW, Westwood NB, Acharya J, Hughes AE, Lappin TR, Pearson TC. Erythrocytosis due to a mutation in the erythropoietin receptor gene. Br J Haematol. 1998;100:407–410. doi: 10.1046/j.1365-2141.1998.00550.x. [DOI] [PubMed] [Google Scholar]
- Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, McMullin MF, Lee FS. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KB, Hokland P, Petersen GB, Nyvold CG. Erythropoietin receptor defect: a cause of primary polycythaemia. Br J Haematol. 2004;125:537–538. doi: 10.1111/j.1365-2141.2004.04931.x. [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- Prchal JT. Pathogenetic mechanisms of polycythemia vera and congenital polycythemic disorders. Semin Hematol. 2001;38:10–20. doi: 10.1016/s0037-1963(01)90135-0. [DOI] [PubMed] [Google Scholar]
- Prchal JT. Polycythemia vera and other primary polycythemias. Curr Opin Hematol. 2005;12:112–116. doi: 10.1097/01.moh.0000154029.05396.d2. [DOI] [PubMed] [Google Scholar]
- Prchal JT, Crist WM, Goldwasser E, Perrine G, Prchal JF. Autosomal dominant polycythemia. Blood. 1985;66:1208–1214. [PubMed] [Google Scholar]
- Prchal JT, Semenza GL, Prchal J, Sokol L. Familial polycythemia. Science. 1995;268:1831–1832. doi: 10.1126/science.7604250. [DOI] [PubMed] [Google Scholar]
- Queisser W, Heim ME, Schmitz JM, Worst P. [Idiopathic familial erythrocytosis. Report on a family with autosomal dominant inheritance] Dtsch Med Wochenschr. 1988;113:851–856. doi: 10.1055/s-2008-1067733. [DOI] [PubMed] [Google Scholar]
- Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Rives S, Pahl HL, Florensa L, Bellosillo B, Neusuess A, Estella J, Debatin KM, Kohne E, Schwarz K, Cario H. Molecular genetic analyses in familial and sporadic congenital primary erythrocytosis. Haematologica. 2007;92:674–677. doi: 10.3324/haematol.10787. [DOI] [PubMed] [Google Scholar]
- Robert T, Means J. Erythrocytosis. In: Greer JF JNLJohnP, Rodgers GeorgeM, Paraskevas Frixos, Glader Bertil., editors. Wintrobe's Clinical Hematology 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1495–1508. [Google Scholar]
- Rumi E, Passamonti F, Pagano L, Ammirabile M, Arcaini L, Elena C, Flagiello A, Tedesco R, Vercellati C, Marcello AP, Pietra D, Moratti R, Cazzola M, Lazzarino M. Blood p50 evaluation enhances diagnostic definition of isolated erythrocytosis. J Intern Med. 2009;265:266–274. doi: 10.1111/j.1365-2796.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, Sasaki M, Johnston JA, Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999;4:339–351. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoda R, Prchal JT. Lessons from familial myeloproliferative disorders. Semin Hematol. 2005;42:266–273. doi: 10.1053/j.seminhematol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Sokol L, Luhovy M, Guan Y, Prchal JF, Semenza GL, Prchal JT. Primary familial polycythemia: a frameshift mutation in the erythropoietin receptor gene and increased sensitivity of erythroid progenitors to erythropoietin. Blood. 1995;86:15–22. [PubMed] [Google Scholar]
- Sokol L, Prchal JF, D'Andrea A, Rado TA, Prchal JT. Mutation in the negative regulatory element of the erythropoietin receptor gene in a case of sporadic primary polycythemia. Exp Hematol. 1994;22:447–453. [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Sulahian R, Cleaver O, Huang LJ. Ligand-induced EpoR internalization is mediated by JAK2 and p85 and is impaired by mutations responsible for primary familial and congenital polycythemia. Blood. 2009;113:5287–5297. doi: 10.1182/blood-2008-09-179572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Ohneda O, Takahashi S, Higuchi M, Mukai HY, Nakahata T, Imagawa S, Yamamoto M. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100:2279–2288. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- Tauchi T, Damen JE, Toyama K, Feng GS, Broxmeyer HE, Krystal G. Tyrosine 425 within the activated erythropoietin receptor binds Syp, reduces the erythropoietin required for Syp tyrosine phosphorylation, and promotes mitogenesis. Blood. 1996;87:4495–4501. [PubMed] [Google Scholar]
- Tilbrook PA, Palmer GA, Bittorf T, McCarthy DJ, Wright MJ, Sarna MK, Linnekin D, Cull VS, Williams JH, Ingley E, Schneider-Mergener J, Krystal G, Klinken SP. Maturation of erythroid cells and erythroleukemia development are affected by the kinase activity of Lyn. Cancer Res. 2001;61:2453–2458. [PubMed] [Google Scholar]
- Tong W, Zhang J, Lodish HF. Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood. 2005;105:4604–4612. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchenker W, Dusa A, Constantinescu SN. JAKs in pathology: role of Janus kinases in hematopoietic malignancies and immunodeficiencies. Semin Cell Dev Biol. 2008;19:385–393. doi: 10.1016/j.semcdb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Van Maerken T, Hunninck K, Callewaert L, Benoit Y, Laureys G, Verlooy J. Familial and congenital polycythemias: a diagnostic approach. J Pediatr Hematol Oncol. 2004;26:407–416. doi: 10.1097/00043426-200407000-00002. [DOI] [PubMed] [Google Scholar]
- Van Zant G, Shultz L. Hematologic abnormalities of the immunodeficient mouse mutant, viable motheaten (mev) Exp Hematol. 1989;17:81–87. [PubMed] [Google Scholar]
- Verdier F, Chretien S, Muller O, Varlet P, Yoshimura A, Gisselbrecht S, Lacombe C, Mayeux P. Proteasomes regulate erythropoietin receptor and signal transducer and activator of transcription 5 (STAT5) activation. Possible involvement of the ubiquitinated Cis protein. J Biol Chem. 1998;273:28185–28190. doi: 10.1074/jbc.273.43.28185. [DOI] [PubMed] [Google Scholar]
- Walrafen P, Verdier F, Kadri Z, Chretien S, Lacombe C, Mayeux P. Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood. 2005;105:600–608. doi: 10.1182/blood-2004-03-1216. [DOI] [PubMed] [Google Scholar]
- Watowich SS, Xie X, Klingmuller U, Kere J, Lindlof M, Berglund S, de la Chapelle A. Erythropoietin receptor mutations associated with familial erythrocytosis cause hypersensitivity to erythropoietin in the heterozygous state. Blood. 1999;94:2530–2532. [PubMed] [Google Scholar]
- Wojchowski DM, Menon MP, Sathyanarayana P, Fang J, Karur V, Houde E, Kapelle W, Bogachev O. Erythropoietin-dependent erythropoiesis: New insights and questions. Blood Cells Mol Dis. 2006;36:232–238. doi: 10.1016/j.bcmd.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;279:821–824. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. Embo J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T, Zhang J, Miura O, Ihle JN. Hematopoietic cell phosphatase associates with erythropoietin (Epo) receptor after Epo-induced receptor tyrosine phosphorylation: identification of potential binding sites. Blood. 1995;85:87–95. [PubMed] [Google Scholar]
- Yilmaz D, Cogulu O, Ozkinay F, Kavakli K, Roos D. A novel mutation in the DIA1 gene in a patient with methemoglobinemia type II. Am J Med Genet A. 2005;133A:101–102. doi: 10.1002/ajmg.a.30467. [DOI] [PubMed] [Google Scholar]
- Zang H, Sato K, Nakajima H, McKay C, Ney PA, Ihle JN. The distal region and receptor tyrosines of the Epo receptor are non-essential for in vivo erythropoiesis. Embo J. 2001;20:3156–3166. doi: 10.1093/emboj/20.12.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Kitidis C, Fleming MD, Lodish HF, Ghaffari S. Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood. 2006;107:907–915. doi: 10.1182/blood-2005-06-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]