Abstract
Sporadic juvenile muscular atrophy of the distal upper extremity or Hirayama's Disease (HD) and autosomal dominant motor distal neuronopathy/axonopathy (CMT2D/dSMA-V), produced by glycyl-tRNA synthetase (GARS) gene mutations, share some clinical features including: young age of onset, predilection for the distal upper extremity, asymmetry, sparing of proximal muscles and unusual cold sensitivity. However, incomplete penetrance of GARS gene mutations may account for apparently non-familial cases. In order to inquire whether GARS gene mutations are associated with HD we studied seven patients fulfilling the clinical and electrodiagnostic criteria for HD. All patients underwent MRI of cervical spine that excluded compressive myelopathy in neutral position and intramedullary pathology. Each patient was tested for the presence of mutations in GARS by sequencing all coding exons amplified from genomic DNA. No pathogenic mutations were found, excluding the role of GARS gene as a possible factor in the etiology of HD in this cohort.
Keywords: Hirayama's disease, GARS gene, distal spinal muscular atrophy, dSMA-V
Introduction
Juvenile muscular atrophy of the distal upper extremity or Hirayama's Disease (HD) is characterized by a typical pattern of weakness and amyotrophy of hand and forearm muscles sparing the brachioradialis (1). When both upper limbs are affected, the involvement is always asymmetric. The disease affects mainly young males and after progressing for a few years it generally stabilizes and remains confined to the upper limbs (1). In many cases HD is associated with lower cervical cord atrophy and asymmetric cord flattening (2, 3). Concerning its etiology, some authors suggested mechanical compression of the lower cervical spine during flexion, but this pathogenic mechanism is not unanimously accepted (1–5). Others consider HD as a segmental form of spinal muscular atrophy (6).
Several features raised the possibility of a genetic contribution in the etiology of HD: strong predilection for certain ethnic groups (Japanese, Chinese, and Indians), relatively uniform age of onset and natural history. Although typically HD occurs sporadically, familial cases have been reported (2, 7).
Recently glycyl-tRNA synthetase (GARS) gene mutations have been associated with the autosomal dominant motor distal neuronopathy/axonopathy (CMT2D/dSMA-V) characterized by adolescent onset of weakness and atrophy of thenar and first dorsal interosseus muscles (8). However, it has been demonstrated that incomplete penetrance of these mutations may account for apparently non-familial cases (9). HD shares with dSMA-V some clinical features including young age of onset, predilection for the distal upper extremity muscles (especially thenar and first dorsal interossei), asymmetry, sparing of proximal muscles and unusual cold sensitivity (8).
In order to assess the contribution of the GARS gene to the etiology of HD we studied seven patients fulfilling the clinical and electrodiagnostic criteria for HD. They were followed up for at least six years (range 6–11), therefore diffuse degenerative anterior horn disorders could be ruled out with a relatively high degree of certainty.
Patients and methods
We studied seven Israeli males of European (N=4) or North African (N=3) ancestry without family history of neuromuscular diseases, followed in four medical centers in Israel. The inclusion criteria were: a: distal weakness and amyotrophy of one or two hands, b: affecting at least two distal myotomes and two peripheral nerve territories, c: lasting more than 12 months, d: neuropathic pattern on EMG, e: no trauma to the limb or any other clinical explanation for the symptoms.
The exclusion criteria were: a: involvement of lower limbs, b: bulbar features. c: pyramidal signs, d: cerebellar features, e: significant sensory disturbances or pain, f: history of poliomyelitis, g: history of radiotherapy, h: conduction blocks, abnormal conduction velocities or abnormal sensory responses on nerve conduction studies.
The follow-up ranged from six to eleven years.
Electrophysiological examinations were performed for diagnostic purposes using routine motor and sensory nerve conduction (NCS) techniques in the median, ulnar, radial and axillary nerves bilaterally. Electromyography (EMG) was performed in proximal and distal muscles of the upper limbs with concentric needle electrodes. Cervical MRI imaging was performed in neutral position with standard techniques and analyzed in both sagittal and axial planes by an experienced neuro-radiologist. The screening of GARS genes on chromosome 7p15 was performed by one of the authors (LGG) by sequencing all coding exons amplified from genomic DNA using intronic primers as previously reported (8).
Results
The clinical features are presented in Table 1. In all cases the disease started unilaterally between the ages of 15–25 years and extended to the contralateral upper limb after 12 to 30 months (Table 1). Three to five years after onset, the progression of illness arrested remaining confined to the upper extremities. Six patients showed a low-amplitude positional and action tremor, usually bilateral, worse in the weaker and more atrophic hand. In three patients the tremor was associated with minipolymyoclonus of fingers and contributed significantly to the clinical disability. In six out of seven patients exposure to cold temperature aggravated the symptoms.
Table 1. Clinical features in seven patients with Hirayama disease.
M=male, bilat. = bilateral involvement, f-u. = follow-up, + = mild, ++ = moderate, +++ = severe
| Patient | Gender | Onset age (years) |
Time to bilat. (months) |
Time to arrest (months) |
Length of f-u. (years) |
Tremor | Cold effect |
|---|---|---|---|---|---|---|---|
| 1 | M | 15 | 12 | 36 | 6 | + + + | + + + |
| 2 | M | 18 | 24 | 42 | 9 | + + + | + + |
| 3 | M | 16 | 30 | 36 | 6 | + + | − |
| 4 | M | 17 | 24 | 60 | 11 | + + + | + + + |
| 5 | M | 25 | 24 | 48 | 6 | + + | + + |
| 6 | M | 18 | 12 | 42 | 8 | − | + + + |
| 7 | M | 20 | 24 | 36 | 6 | + + + | + + |
Electrodiagnostic examinations were performed in all patients. In all the nerve conduction studies were normal in motor and sensory fibers of upper and lower limbs, except for a low compound muscle action potential amplitude in the ulnar nerve on the affected side in most patients (bilaterally, but asymmetrically in two). Fibrillations were found relatively rarely, mainly in very distal muscles (abductor pollicis brevis, first dorsal interosseus, abductor digiti minimi), while signs of chronic reinnervation were more extensive and could be found in both hand and forearm muscles (flexor carpi radialis, extensor indicis, and others).
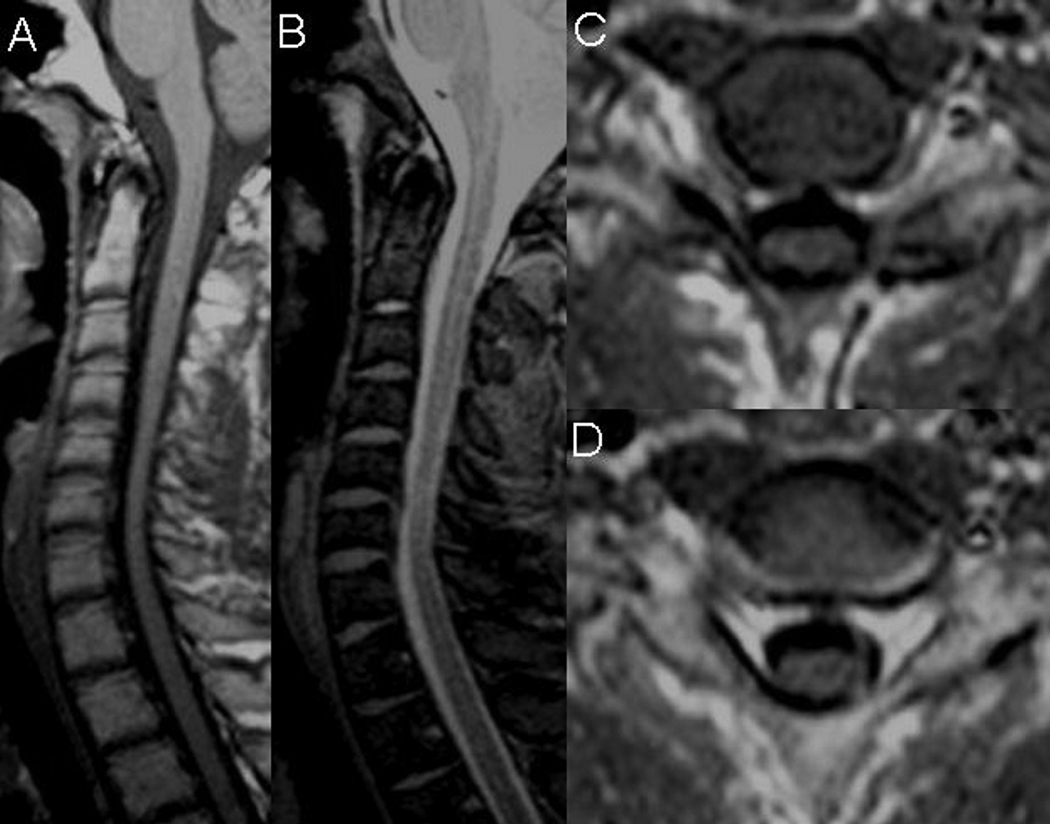
All patients underwent MRI of cervical spine that excluded compressive myelopathy in neutral position and intramedullary pathology. Minimal, clinically non-significant, discogenic changes at C6–7 level were seen in two patients. Mild atrophy and flattening of the spinal cord in the lower cervical area was seen in three patients (Figure 1).
Figure 1. Cervical MRI of patient 4 in neutral neck position.
Mild cord atrophy at the levels of C5–C7 seen on sagittal T1-weighted (A) and T2-weighted (B) modalities. Mild cord flattening at the C6(C) and C7 (D) levels seen on axial T1-weighted images.
The diagnosis of HD was confirmed by lack of progression beyond the upper limbs and no upper motor neuron features after a follow-up ranging from six to eleven years.
After informed consent, blood was drawn and each patient was tested for the presence of mutations in GARS by sequencing all coding exons amplified from genomic DNA using intronic primers. No pathogenic mutations were found.
Discussion
In all seven cases the clinical features and evolution are typical for HD; hand tremor, minipolymyoclonus of fingers and bilateral involvement have been previously described in many patients from different populations (5, 10–12).
Previous genetic studies of Indian and European HD patients have excluded deletions and haploinsufficiency in survival motor neuron (SMN1 and SMN2) genes and mutations in the superoxide dismutase 1 (SOD1) gene as the cause of this disease (2, 7, 13). There is significant similarity between HD and the recently described distal spinal muscular atrophy type V syndrome (dSMA-V) associated with mutations in GARS gene on chromosome 7p15. However, this analysis did not detect GARS mutations in seven Israeli patients of European and North African ancestry with HD. The phenotype in our cases is identical to that described in Japanese, Chinese and Indian patients; it is therefore likely that GARS gene is not involved in the etiology of HD in these populations as well.
Acknowledgements
The authors thank the patients and their families. The authors are indebted to Rafi Koren, Nava Blumen and Lihi Blumen for technical support.
References
- 1.Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology. 2000;54:1922–1926. doi: 10.1212/wnl.54.10.1922. [DOI] [PubMed] [Google Scholar]
- 2.Misra UK, Kalita J, Mishra VN, Kesari A, Mittal B. A clinical, magnetic resonance imaging and survival motor neuron gene deletion study of Hirayama Disease. Arch Neurol. 2005;62:120–123. doi: 10.1001/archneur.62.1.120. [DOI] [PubMed] [Google Scholar]
- 3.Fu I, Pei X, Zhang J, Kang D, Han H, Fan D. Morphological changes of the lower cervical spinal cord under neutral and fully flexed position by MRI in Chinese patients with Hirayama's disease. Amyotroph Lateral Scler. 2008;9:156–162. doi: 10.1080/17482960701726123. [DOI] [PubMed] [Google Scholar]
- 4.Schroder R, Keller E, Flacke S, Schmidt S, Pohl C, Klockgether T, Schlegel R. MRI findings in Hirayama's disease: flexion-induced cervical myelopathy or intrinsic motor neuron disease ? J Neurol. 1999;246:1069–1074. doi: 10.1007/s004150050514. [DOI] [PubMed] [Google Scholar]
- 5.Willeit J, Kiechl S, Kiechl-Kohlendorfer U, Golaszewski S, Peer S, Poewe W. Juvenile asymmetric segmental spinal muscular atrophy (Hirayama's disease) Three cases without evidence of "flexion myelopathy". Acta Neurol Scand. 2008;104(5):320–322. doi: 10.1034/j.1600-0404.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- 6.Murray B, Mitsumoto H. Disorders of upper and lower motor neurons. In: Bradley WG, Daroff RB, Fenichel GM, Jankovic J, editors. Neurology in Clinical Practice. 5th edn. Philadelphia: Butterworth-Heinemann Elsevier Co.; 2008. pp. 2183–2220. [Google Scholar]
- 7.Roberrecht W, Aguirre T, Van den Bosch L, Theys P, Nees H, Cassiman JJ, et al. Familial juvenile focal amyotrophy of the upper extremity (Hirayama disease). Superoxide dismutase 1 genotype and activity. Arch Neurol. 1997;54:46–50. doi: 10.1001/archneur.1997.00550130032012. [DOI] [PubMed] [Google Scholar]
- 8.Sivakumar K, Kyriakides T, Puls I, Nicholson GA, Funalot B, Antonellis A, et al. Phenotypic spectrum of disorders associated with glycyl-tRNA synthetase mutations. Brain. 2005;128:2304–2314. doi: 10.1093/brain/awh590. [DOI] [PubMed] [Google Scholar]
- 9.Dubourg O, Azzedine H, Yaou RB, Pouget J, Barois A, Meininger V, et al. The G526R glycyl-tRNA synthetase gene mutation in distal hereditary motor neuropathy type V. Neurology. 2006;66:1721–1726. doi: 10.1212/01.wnl.0000218304.02715.04. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama K. Juvenile muscular atrophy of distal upper extremity (Hirayama disease) Internal Medicine. 2000;39:283–290. doi: 10.2169/internalmedicine.39.283. [DOI] [PubMed] [Google Scholar]
- 11.Gourie-Devi M, Nalini A. Long-term follow-up of 44 patients with brachial monomelic amyotrophy. Acta Neurol Scand. 2003;107:215–220. doi: 10.1034/j.1600-0404.2003.02142.x. [DOI] [PubMed] [Google Scholar]
- 12.Peiris JB, Seneviratne KN, Wickremasinghel HR, Gunatilake SB, Gamage R. Non familial juvenile distal spinal muscular atrophy of upper extremity. J Neurol Neurosurg Psychiatry. 1989;52:314–319. doi: 10.1136/jnnp.52.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamez J, Also E, Alias A, Corbera-Bellalta M, Barcelo MJ, Centuno M, et al. Investigation of the role of SMN1 and SMN2 haploinsufficiency as a risk factor for Hirayama's disease: Clinical, neurophysiological and genetic characteristics in a Spanish series of 13 patients. Clin Neurol Neurosurg. 2007;109:844–848. doi: 10.1016/j.clineuro.2007.07.019. [DOI] [PubMed] [Google Scholar]