Abstract
The microtubule-associated protein tau forms insoluble filaments that deposit as neurofibrillary tangles (NFTs) in the brains of those with Alzheimer’s disease (AD) and other related neurodegenerative disorders. The presence of both NFTs and amyloid β (Aβ)-containing senile plaques within the brain is required to confirm the diagnosis of AD. However, the demonstration that familial AD can be caused by mutations that result in increased Aβ production has resulted in AD drug discovery strategies that are largely focused on reducing brain Aβ levels, with substantially less emphasis on tau-directed approaches. This trend may be changing, as there are an increasing number of research programs that are exploring ways to reduce NFTs in AD and related tauopathies. We briefly review recent advances in tau-based drug discovery, with an emphasis on the identification of compounds that inhibit the assembly of tau into multimers and fibrils.
INTRODUCTION
A definitive diagnosis of Alzheimer’s disease (AD) requires the identification of two hallmark pathological features within autopsied brain; senile plaques composed of insoluble amyloid β (Aβ) peptides and neurofibrillary tangles (NFTs) comprised of tau protein aggregates (Hyman, 1997; Ball et al., 1997). The discovery nearly two decades ago that familial AD can result from mutations in the amyloid precursor protein (APP) (George-Hyslop and Petit, 2005) from which Aβ peptides are proteolytically cleaved led to the formulation of the “amyloid hypothesis” of AD onset (Hardy and Selkoe, 2002). Subsequent research which demonstrated that mutations in presenilin-1 and -2 also cause early-onset AD, and the discovery that these proteins play a role in APP cleavage and Aβ genesis (Selkoe and Wolfe, 2007) lent further credence to the amyloid theory of AD. As a consequence, the majority of AD drug discovery and development activities have been focused on identifying therapeutic agents that can reduce the levels of Aβ peptides and senile plaques. Several Aβ-directed drug candidates have now progressed to AD clinical testing, but to date none have been successful in pivotal phase III trials.
Relative to studies of Aβ involvement in AD, substantially less effort has been directed to understanding the role of tau-containing NFTs. For many years, some believed that the somatodendritic accumulations of tau in AD might simply be markers of dying neurons and not causal in disease onset. However, this viewpoint changed dramatically when it was discovered that inherited forms of frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17) resulted from mutations within the tau gene (Goedert, 2005; Goedert and Jakes, 2005; Hutton et al., 1998). This provided evidence that alterations of tau could directly result in neurodegenerative disease, and suggested that the tau accumulations in AD brain could indeed be involved in disease onset and progression. Nonetheless, AD drug discovery programs aimed at minimizing the effects of pathologic tau still lag behind those focused on Aβ modulation. This is in part because the enzymes which cleave APP to yield Aβ have been identified (Hardy and Selkoe, 2002), and these provide attractive drug targets to the pharmaceutical sector which has substantial experience in the development of enzyme inhibitors. Although pharmaceutical strategies to lower tau levels may arguably be more difficult than those directed to Aβ, there appears to be increased interest in tau-based drug discovery approaches. It is thus timely to review these recent tau-focused efforts, with a particular emphasis here on the identification of compounds that affect tau assembly into oligomers and/or fibrils.
TAU IN NEURODEGENERATIVE DISEASE
Fibrils comprised of hyper-phosphorylated tau (Avila, 2006b; Avila, 2006a; Lee et al., 1991) accumulate within neuron cell bodies and dendrites in AD (Avila, 2006b; Brandt et al., 2005), forming paired-helical filaments (PHFs) that coalesce into NFTs (Hyman, 1997; Kosik et al., 1986; Lee et al., 1991). The presence of intraneuronal tau aggregates is not restricted to AD and FTDP-17, but also occurs in a group of neurodegenerative diseases referred to as “tauopathies” that include progressive supranuclear palsy, corticobasal degeneration and Pick’s disease (Ballatore et al., 2007; Forman et al., 2004; Lee et al., 2001). Tau is normally a highly soluble protein whose primary intracellular role appears to be the stabilization of microtubules (MTs) (Cleveland et al., 1977b; Cleveland et al., 1977a). There are six isoforms of tau which are generated through alternative splicing of exons 2, 3 and 10, and the inclusion/exclusion of exon 10 results in tau species that contain either three (3-R) or four (4-R) carboxyl-terminal MT-binding repeat motifs (Andreadis et al., 1992; Goedert et al., 1989). The 4-R forms of tau appear to bind to MTs with greater affinity than the 3-R versions (Goedert and Jakes, 1990; Panda et al., 2003), and in normal cells the amount of 4-R and 3-R tau species are approximately equal. Interestingly, many of the tau mutations identified in FTDP-17 occur within intronic regions that affect exon 10 splicing, leading to an increase of the 4-R:3-R tau ratio (Hutton et al., 1998; Hong et al., 1998b). This increase of 4-R tau could lead to neuropathology through a change of MT dynamics that result from over-stabilization as FTDP-17 patients with exon 10 mutations were reported to show only 4-R tau within brain deposits (Spillantini et al., 1997).
FTDP-17 mutations within the tau coding region are clustered in the vicinity of the MT-binding domains and typically result in an enhancement of tau assembly into fibrils (Barghorn et al., 2000; Gamblin et al., 2000; Nacharaju et al., 1999) and a decrease in MT binding (Dayanandan et al., 1999; Hasegawa et al., 1998; Hong et al., 1998a). The intronic and exonic tau FTDP-17 mutations thus teach that tau-mediated neurodegeneration likely results from altered stabilization of MTs and/or from the formation of toxic tau oligomers or fibrils. Interestingly, no tau mutations have been identified in AD, even though tau aggregates in AD are similar to those seen in FTDP-17 and the other tauopathies. It is likely that other modifications of tau in AD, particularly hyper-phosphorylation, cause changes to the protein that mimic the effects of FTDP-17 mutations. For example, tau phosphorylation plays an important physiological role in regulating MT dynamics and several studies have demonstrated that tau hyper-phosphorylation reduces its ability to bind to MTs (Baudier and Cole, 1987; Bramblett et al., 1993; Drechsel et al., 1992; Alonso et al., 1997) and alters MT function in cell-based models (Merrick et al., 1997; Wagner et al., 1996). Moreover, increasing tau phosphorylation at certain residues also enhances its ability to aggregate (Alonso et al., 1996; Necula and Kuret, 2004).
TAU-DIRECTED THERAPEUTIC STRATEGIES
Since tau-associated neuropathology likely results from reduced MT stabilization and/or the formation of toxic oligomers/fibrils, exploratory therapeutic strategies have been directed to these tau loss-of-function (LOF) and gain-of-function (GOF) mechanisms. Evidence for tau LOF has been demonstrated in transgenic mice expressing the smallest wild type human tau isoform (Ishihara et al., 1999; Zhang et al., 2005)‥ These animals have reduced MT density and motor impairments which can be overcome by treatment with the MT-stabilizing agent, paclitaxel (Zhang et al., 2005). More recently, a peptide-based MT stabilizer has also been shown to improve cognitive performance in a tau transgenic mouse model (Matsuoka et al., 2008). While these studies provide important proof-of-principle, a challenge to this approach of compensating for tau LOF will be identifying drugs that have adequate central nervous system (CNS) exposure to stabilize affected axons without causing the peripheral side-effects, including myelo-suppression and neuropathy, that plague existing MT-directed oncology therapeutics.
The propensity of hyper-phosphorylated tau to aggregate and bind with lower affinity to MTs suggests that reducing tau phosphorylation in AD might provide therapeutic benefit. Several kinases are capable of phosphorylating tau in vitro, and a variety of data suggest that glycogen synthase kinase-3 (GSK-3), cell-cycle dependent kinase-5 (CDK5), extracellular signal-related kinase 2 (ERK2) and microtubule affinity-regulating kinase (MARK) may be the most relevant kinases in vivo (Mazanetz and Fischer, 2007; Churcher, 2006). While not the focus of this review, a number of drug discovery programs have been initiated to identify selective tau kinase inhibitors for the treatment of AD (Mazanetz and Fischer, 2007; Barten and Albright, 2008). Because these kinases are involved in many cellular processes, it remains to be seen whether inhibitors of tau phosphorylation can be identified that are both safe and efficacious.
Alternative approaches to reducing pathologic tau are also being pursued, with these efforts largely being undertaken in academic centers. For example, strategies to increase the activity of cellular pathways that can degrade misfolded or aggregated tau are being investigated. Literature reports indicate that inhibition of the molecular chaperone, heat shock protein 90 (HSP90), can reduce the levels of highly phosphorylated tau (Dickey et al., 2007; Dickey et al., 2006) and improve behavioral endpoints in transgenic mice that express human tau with one of the FTDP-17 mutations (Luo et al., 2007). Moreover, there is emerging evidence that an up-regulation of cellular autophagy can result in a clearance of misfolded protein aggregates, including those comprised of tau (Williams et al., 2006; Hamano et al., 2008). Finally, as discussed in greater detail below, several laboratories have identified compounds that can prevent the formation of tau oligomers/fibrils which are believed to be responsible for eliciting LOF and/or GOF neurotoxicity.
INHIBITORS OF TAU FIBRILLIZATION
The fibrillar tau deposits that are seen in AD and other tauopathies are readily stained by dyes such as thioflavine S (ThS) that recognize a cross-β-fibril structure that is common to all amyloids (Berriman et al., 2003; Margittai and Langen, 2004). Fibril formation by tau and other amyloids appears to proceed through a common mechanism whereby oligomeric nucleating cores are formed during an initial lag-phase, followed by a period of rapid fibril growth (Morris et al., 2008). As discussed, the precipitating events in AD that causes tau to assemble and fibrillize are not completely understood, but hyper-phosphorylation is a consistent distinguishing feature of pathological versus normal tau. Normal tau, which is largely unstructured in solution, can be made to fibrillize in vitro in the presence negatively charged co-factors such as heparin, fatty acid and other lipids, or RNA (Hasegawa et al., 1997; Chirita et al., 2003; Wilson and Binder, 1997). These anionic molecules have been proposed to facilitate tau-tau interaction by inducing conformational changes that result from electrostatic interactions with positively charged basic residues in the protein (Perez et al., 1996; Goedert et al., 1996), and thus tau hyper-phosphorylation might achieve a similar effect. However, it should be noted that others have proposed that anionic polymers such as heparin and lipids act to facilitate tau nucleation by presenting negatively-charged surfaces (Congdon et al., 2008) and it is not clear that tau hyper-phosphorylation mimics this mechanism of action.
A number of laboratories have taken advantage of the ability of full-length tau or shorter tau fragments to form fibrils under defined conditions, and have conducted experiments to identify compounds that inhibit this fibrillization process. It should be noted that the inhibition of protein-protein interactions of the type that occur during fibril assembly is considered quite challenging with small molecules due to the large protein surface areas involved. For example, there has been only modest success in the identification of non-peptide inhibitors of Aβ peptide fibrillization. The challenge of small molecule fibrillization inhibitors becomes even greater when searching for compounds that have the requisite properties of true drug candidates; i.e., that meet the standard physical-chemical properties of drugs (Lipinski, 2000) and which show good brain penetration, oral absorption and metabolic stability. Nonetheless, there is reason for cautious optimism regarding the inhibition of tau fibril assembly with small molecules. First, it is known that a change of a single amino acid residue within tau can prevent its ability to form fibrils (Li and Lee, 2006), and thus it is conceivable that a small molecule that interacts at or near this site could affect tau assembly. Moreover, a number of structurally distinct compounds have been identified that inhibit tau fibril formation, albeit many with features which are not consistent with drug-like candidates.
One of the first compounds identified that blocked tau-tau interaction was the phenothiazine, methylene blue (Wischik et al., 1996). This compound (Figure 1) was shown to partially disrupt the structure of isolated PHFs and subsequent studies showed that methylene blue could affect tau multimerization (Wischik et al., 1996), although the assay that was utilized did not assess actual fibril formation. Interestingly, this dye molecule has now progressed into clinical testing in AD patients, and Phase II data presented at the 2008 International Conference on AD appear intriguing. In general, planar dye-like compounds have not been thought of as likely drug candidates, in part because they frequently show non-specific interactions with multiple protein targets. In addition, colored compounds can complicate the conduct of clinical trials if they can be detected upon excretion since patients on drug can be distinguished from those receiving placebo.
Figure 1. Inhibitors of tau aggregation.
1Wischik et al., 1996; 2Taniguchi et al., 2005; 3Necula et al., 2005; 4Pickhardt et al., 2005; 5Khlistunova et al., 2006; 6Pickhardt et al., 2007; 7Bulic et al., 2007; 8Crowe et al., 2007.
Another compound, a cyanine dye termed N744 (Figure 1), was found to inhibit arachidonic acid-induced fibrillization of full-length 4-R tau, and sub-stoichiometric amounts of this compound caused disaggreagation of pre-formed tau filaments (Chirita et al., 2003). An analysis of the time-course and dose-dependency of N744 suggested that it inhibits tau filament extension but not its nucleation (Necula et al., 2005). Interestingly, this compound and certain other dye-like molecules form aggregates at higher concentrations that enhance tau fibrillization (Congdon et al., 2007). This feature complicates the potential use of such compounds in vivo since excessive dosing could result in drug levels that worsen tau pathology. Whereas aggregation of N774 reduced its ability to inhibit tau fibrillization, in other cases it is the formation of compound aggregates that results in the inhibition of amyloid fibril formation (Feng et al., 2008).
In a small screen of 42 compounds, Taniguchi and colleagues (Taniguchi et al., 2005) identified molecules from several chemical classes that inhibited heparin-facilitated fibrillization of full-length tau as judged by electron microscopy (EM) and ThS fluorescence. These compounds included phenothiazines, polyphenols and porphyrins (Figure 1). In the same year, another research team (Pickhardt et al., 2005) conducted a large-scale screen of ~200,000 compounds in an assay in which heparin-promoted fibrillization of a tau fragment comprised only of three MT-binding repeats (termed K19) was monitored by ThS fluorescence. A number of anthraquinones, including the anti-cancer drugs daunorubicin and adriamycin, were identified as inhibitors of tau fibril formation (Figure 1). Further analyses revealed that these compounds also blocked fibrillization of a peptide comprised of all four MT-binding repeats (K18), as well as of full-length 3-R tau, although there were minor differences in compound potency with the different tau species. Generally, the anthraquinones could also induce disassembly of pre-formed tau fibrils, although in all cases the molecules were much more effective in disaggregating the shorter tau fragments than full-length tau. Finally, one of the anthraquinones (emodin; Figure 1) was demonstrated by the Mandelkow laboratory to reduce tau aggregates in N2a neuroblastoma cells that over-express K18 containing an FTLD-17 mutation. They also demonstrated that certain N-phenylamines initially identified in their screening effort could inhibit the formation of tau aggregates and reduce pre-existing tau inclusions in the N2a cell model (Khlistunova et al., 2006).
The Mandelkow team has subsequently identified two additional classes of tau aggregation inhibitors. Using a pharmacophore model (Larbig et al., 2007) that was derived from the active compounds identified in their prior high-throughput screening (HTS) effort, a phenylthiazolyl-hydrazide (PTH) compound was predicted to be active and was subsequently shown to prevent tau fibrillization (Pickhardt et al., 2007). Further medicinal chemistry efforts led to the synthesis of additional active analogs (Figure 1), and these were found to cause disassembly of preexisting K18 fibrils. Moreover, several of the PTH compounds prevented tau aggregation in the N2a cell model. Although the PTH compounds appeared to be free of overt cellular toxicity, the researchers did find that one of the more potent members of this series showed irreversible binding to immobilized K18. It is unclear if covalent interaction with tau is a general property of this class of inhibitor. An additional rhodanine series of tau fibril inhibitors (Figure 1) was also identified (Bulic et al., 2007). Synthesis of a number of analogs resulted in the identification of key structural elements within this class that were important for activity. As with the PTH series, the rhodanines also caused disaggregation of pre-formed K19 filaments, and several compounds from this chemotype were shown to reduce tau aggregates in N2a cells.
ADDITIONAL TAU ASSEMBLY INHIBITORS
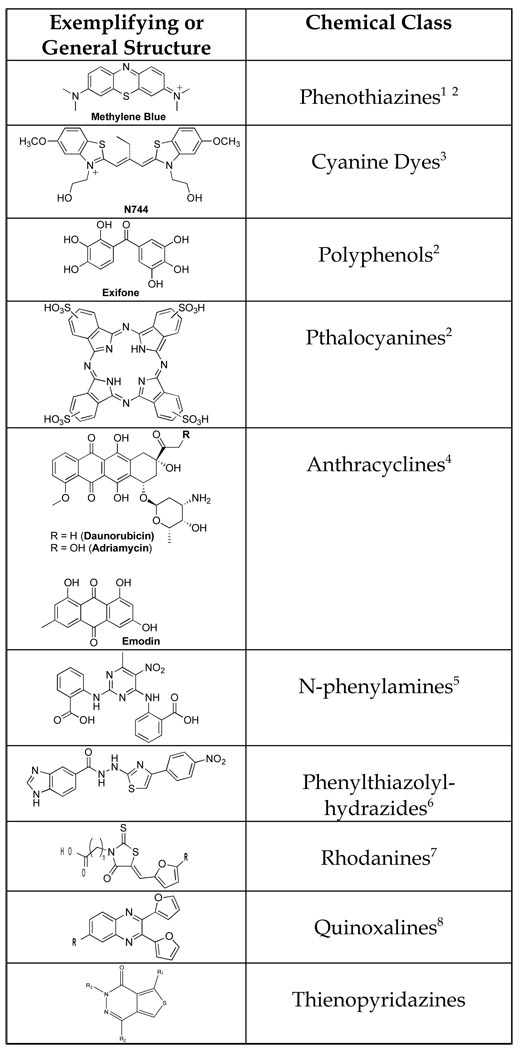
Our laboratory conducted HTS of ~51,000 compounds in an assay of heparin-induced K18 fibrillization (Crowe et al., 2007). Tau fibril formation was monitored via thioflavine T (ThT) binding and fluorescence, and a non-fibrillizing K18 mutant obtained by substituting lysine 311 with aspartic acid was utilized as a negative control. Compounds that showed ≥40% inhibition of K18 fibrillization underwent secondary analyses (Fig. 2) to confirm fibril inhibition through an evaluation of the amount of material that sedimented upon centrifugation as well as EM examination of this insoluble material. Eleven compounds were identified that were judged to be effective inhibitors of tau fibril formation in both the primary screen and secondary assays, with these molecules falling into eight structural classes (Crowe et al., 2007). Among these active entities were examples from previously described compound classes, including anthraquinones, sulfonated dyes, porphyrins, and phenothiazines, as well as new scaffolds including quinoxalines and pyrimidotriazines. Interestingly, several of the active compounds required the presence of dithiothreitol (DTT) in the reaction mixture for activity (Crowe et al., 2007). One possible explanation for this DTT-dependence is that the compounds formed peroxides in the presence of DTT that inhibit tau fibrillization. Among the compounds that were active inhibitors of K18 fibril formation in the absence or presence of DTT were members of a novel quinoxaline series (Figure 1). These molecules were of particular interest due to their uniqueness and compliance with standard physical-chemical parameters that define drug-like molecules (Lipinski, 2000). A bioinformatic analysis of the screening library revealed ~200 additional entries that contained the quinoxaline scaffold. However, all of these analogs showed little activity when tested at 10 µM concentration in the K18 fibrillization assay. Thus, activity seemed to depend on the presence of the 2,3-di(furan-2yl) moieties that were found on the two active quinoxalines, but additional medicinal chemistry efforts will be required to achieve a further understanding of the structure-activity relationships (SAR) within this chemical series.
Figure 2. Characterization of a tau fibrillization inhibitor.
A unique quinoxaline (113F08) identified from HTS of ~51,000 compounds (Crowe et al., 2007) underwent A) a full-dose response analysis in a K18 tau fibrillization reaction as monitored by ThT fluorescence. B) K18 fibrillization reaction mixtures that were incubated with increasing concentrations of 113F08, as indicated, were subjected to centrifugation and the supernatant and pellet fractions were subjected to SDS-PAGE followed by densitometry to ascertain the amount of K18 in each fraction. The black and white bar graph for each concentration represents the percentage of K18 that was in the pellet and supernatant fractions, respectively. Much more soluble K18 was found in the samples containing 2.5 and 20 µM compound, and the pellet fraction from these samples had significantly fewer K18 fibrils as judged by EM than the samples containing lower concentrations of 113F08.
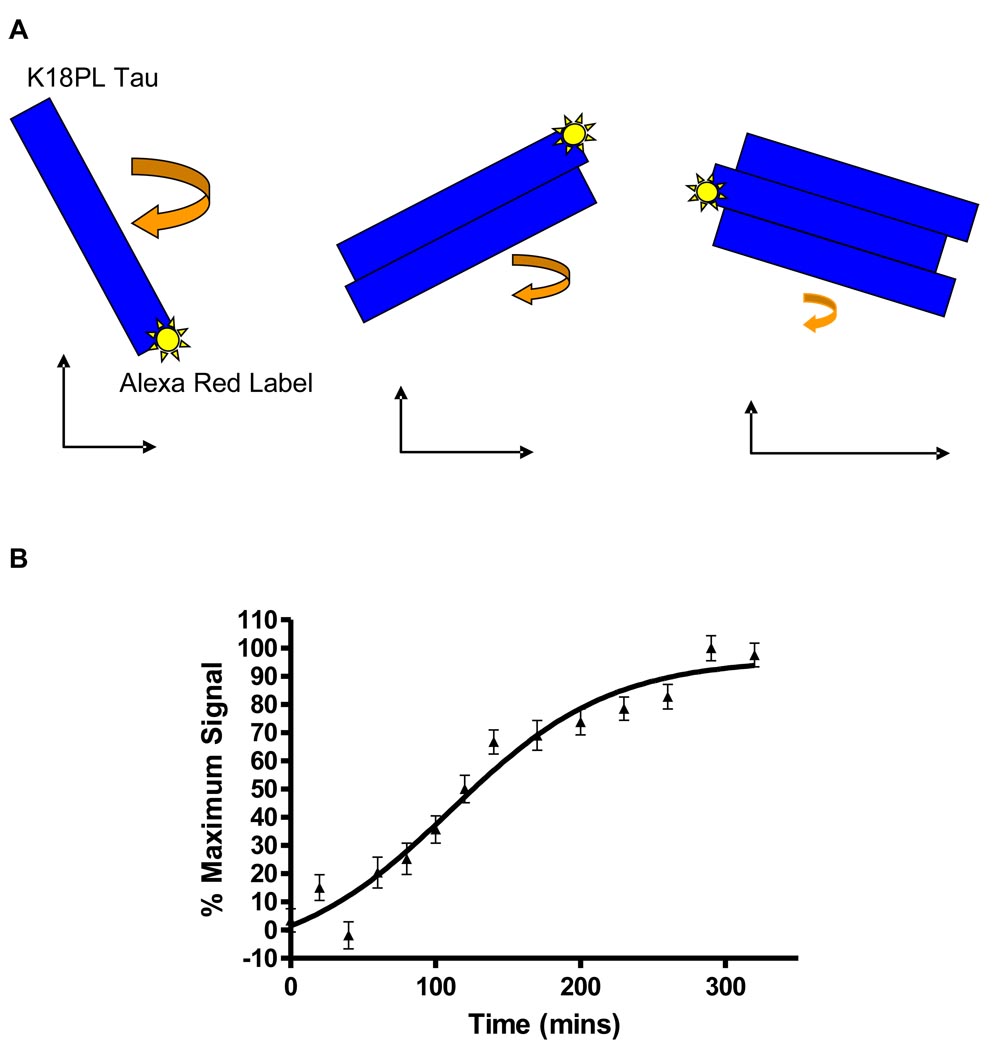
In an effort to explore a significantly greater number of chemical scaffolds, we have recently completed quantitative HTS of nearly 300,000 compounds in collaboration with the Chemical Genomics Center at the National Institutes of Health (Crowe et al., 2009). All compounds were analyzed in the absence of DTT at six concentrations in a 1536-well screening format in which heparin-facilitated fibrillization of K18 with the P301L mutation (K18PL) was assessed by ThT fluorescence. In addition, the screen also incorporated a unique fluorescence polarization (FP) endpoint that allowed for the measurement of smaller tau multimers. The FP assay depends on the incorporation of Alexa Red-labeled K18PL into newly formed tau multimers, with the rotational freedom of the Alex Red probe slowing as tau multimers grow in size, thereby resulting in an increased FP signal (depicted in Figure 3). We observed a time-dependent increase of FP in the K18PL fibrillization assay that plateaued after 6–7 hours (Fig. 3), matching the overall kinetics of fibril formation as assessed by ThT fluorescence.
Figure 3. Schematic representation of a FP-based tau assembly assay and the kinetics of tau fibrillization.
A FP assay was utilized to monitor tau assembly in which Alexa Red-labeled K18PL was mixed at a 1:62.5 molar ratio with unlabeled K18PL. A) Upon addition of heparin and initiation of tau assembly, Alexa Red-labeled K18PL is incorporated into tau multimers, thereby decreasing the rotational freedom of the fluorescent label with a resulting increase in FP signal. B) FP signal as a function of time in a K18PL fibrillization reaction.
A total of 285 compounds were identified from the screening campaign that showed full dose-dependent inhibition of both the FP and ThT signals, and many of these active molecules belonged to previously described classes of tau fibril inhibitors. Essentially all of the active compounds had IC50 values of 1–10 µM in the FP and ThT assays, and as 15 µM K18PL was employed in the reactions, it would appear that nearly equimolar amounts of inhibitory compound were required to prevent tau oligomerization/fibrillization. Of particular note was a new aminothienopyridazine class of inhibitors that had drug-like physical-chemical features (see generic structure shown in Figure 1). Several analogs of this series were synthesized and tested, and certain structure-activity relationships were defined (Crowe et al., 2009). This series is being further explored as the features of certain of these compounds suggest that they may have good brain penetration and oral bioavailability.
SUMMARY OF TAU FIBRILLIZATION INHIBITORS
A number of inhibitors of tau fibril formation from multiple chemical classes have been identified, with most of these discovered over the last five years. To date, there has not been a peer-reviewed publication in which a tau fibrillization inhibitor has been tested in one of the several established tau transgenic mouse models (Lee et al., 2005). Thus, although an inhibitor of tau assembly has conceptual appeal, it remains to be demonstrated whether any of the existing candidate compounds reduce tau deposition in vivo. Moreover, it is also unknown if a compound that diminishes somatodendritic tau inclusions will result in meaningful improvements of memory or learning. The generation of such proof-of-principle data will require that compounds have appropriate chemical and biological properties, including good pharmacokinetic behavior, adequate CNS exposure and a lack of toxicity. Unfortunately, many of the existing tau fibrillization inhibitors have chemical or biological features that are likely to preclude them from testing in tau transgenic models, and thus it will be critical in the near-term to identify compounds with good blood-brain barrier (BBB) penetration, suitable half-lives (e.g., >2–3 hrs in mice) and reasonable safety so as to allow for multi-month assessment in mouse tauopathy models.
Another consideration in the evaluation of tau assembly inhibitors is the concentration of compound that will be required to interrupt multimerization in vivo. Many of the published tau fibrillization inhibitors require concentrations that are approximately equimolar to the amount of tau used in the assays. This is not surprising, as it is likely that these compounds block tau assembly by interacting with tau monomers, small oligomers or protofibrils. It is estimated that intraneuronal tau concentrations are low µM and that under normal circumstances >99% is bound to MTs (Congdon et al., 2008). Although the amount of free tau is almost certainly increased in tauopathies (and animal models thereof) due to reduced MT binding, it is not unreasonable to assume that free tau concentrations are sub-µM in affected neurons. It should thus be possible to achieve efficacious CNS drug levels with compounds that readily penetrate the BBB.
Assuming that tau fibrillization inhibitors emerge that are suitable for efficacy testing in transgenic mice, it will be important to understand how these molecules interrupt tau assembly. It is presently unclear whether mature tau fibrils or smaller tau multimers confer toxicity, as certain data support a role for the latter species (Brunden et al., 2008). If diffusible tau multimers (e.g., dimmers or oligomers) are harmful, a compound that blocks fibril formation but allows the formation of multimers may actually exacerbate tau pathology. Similarly, a compound that disrupts existing fibrils may prove to be detrimental if pathologic tau multimers are released. On the other hand, it is possible that the primary cause of tau-mediated toxicity is LOF due to its sequestration into fibrils. In this case, fibril disruption might be desirable and compounds that increase both oligomer and monomer levels could be acceptable. Given the uncertainty of the mechanism of tau toxicity, it would seem that compounds that inhibit initial tau-tau interactions so that no multimeric species are formed would be the most desired. Unfortunately, there has been very little characterization of the tau species that are formed in the presence of the existing repertoire of fibrillization inhibitors, and such information will likely be critical to the full interpretation of in vivo efficacy studies. We are thus in the early stages of tau drug discovery, particularly as it relates to the development of tau assembly inhibitors. However, the pace of this research has increased considerably over the last several years, and in view of concerns about the clinical failure of several Aβ-directed therapies, it seems likely that increasing attention will be focused on therapeutic approaches that target tau-mediated neurodegeneration. It is therefore hoped that one or more prototype compounds resulting from these efforts will soon undergo in vivo efficacy and safety testing.
ACKNOWLEDGEMENTS
This work was supported by grants from the NIH (P01 AG09215, P30 AG10124, P01 AG11542, P01 AG14382, P01 AG14449, P01 AG17586, PO1 AG19724, P01 NS-044233, UO1 AG24904) and the Marian S. Ware Alzheimer Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alonso AD, GrundkeIqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tan and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso AD, GrundkeIqbal I, Iqbal K. Alzheimer's disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nature Medicine. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 3.Andreadis A, Brown WM, Kosik KS. Structure and Novel Exons of the Human-Tau Gene. Biochemistry. 1992;31:10626–10633. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- 4.Avila J. Tau and tauopathies: tau phosphorylation and tau assembly. Febs Journal. 2006a;273:23. [Google Scholar]
- 5.Avila J. Tau phosphorylation and aggregation in Alzheimer's disease pathology. Febs Letters. 2006b;580:2922–2927. doi: 10.1016/j.febslet.2006.02.067. [DOI] [PubMed] [Google Scholar]
- 6.Ball M, Braak H, Coleman P, Dickson D, Duyckaerts C, Gambetti P, Hansen L, Hyman B, Jellinger K, Markesbery W, Perl D, Powers J, Price J, Trojanowski JQ, Wisniewski H, Phelps C, Khachaturian Z. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiology of Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 7.Ballatore C, Lee VMY, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nature Reviews Neuroscience. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 8.Barghorn S, Zheng-Fischhofer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000;39:11714–11721. doi: 10.1021/bi000850r. [DOI] [PubMed] [Google Scholar]
- 9.Barten DM, Albright CF. Therapeutic strategies for Alzheimer's disease. Molecular Neurobiology. 2008;37:171–186. doi: 10.1007/s12035-008-8031-2. [DOI] [PubMed] [Google Scholar]
- 10.Baudier J, Cole RD. Phosphorylation of Tau-Proteins to A State Like That in Alzheimers Brain Is Catalyzed by A Calcium Calmodulin-Dependent Kinase and Modulated by Phospholipids. Journal of Biological Chemistry. 1987;262:17577–17583. [PubMed] [Google Scholar]
- 11.Berriman J, Serpell LC, Oberg KA, Fink AL, Goedert M, Crowther RA. Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9034–9038. doi: 10.1073/pnas.1530287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VMY. Abnormal Tau-Phosphorylation at Ser(396) in Alzheimers-Disease Recapitulates Development and Contributes to Reduced Microtubule-Binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- 13.Brandt R, Hundelt M, Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2005;1739:331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Brunden K, Trojanowski JQ, Lee VMY. Evidence that non-fibrillar tau causes pathology linked to neurodegeneration and behavioral impairments. J Alzheimers Dis. 2008;14:393–399. doi: 10.3233/jad-2008-14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulic B, Pickhardt M, Khlistunova I, Biernat J, Mandelkow EM, Mandelkow E, Waldmann H. Rhodanine-based tau aggregation inhibitors in cell models of tauopathy. Angewandte Chemie-International Edition. 2007;46:9215–9219. doi: 10.1002/anie.200704051. [DOI] [PubMed] [Google Scholar]
- 16.Chirita CN, Necula M, Kuret J. Anionic micelles and vesicles induce tau fibrillization in vitro. Journal of Biological Chemistry. 2003;278:25644–25650. doi: 10.1074/jbc.M301663200. [DOI] [PubMed] [Google Scholar]
- 17.Churcher I. Tau therapeutic strategies for the treatment of Alzheimer's disease. Current Topics in Medicinal Chemistry. 2006;6:579–595. doi: 10.2174/156802606776743057. [DOI] [PubMed] [Google Scholar]
- 18.Cleveland DW, Connolly JA, Kalnins VI, Spiegelman BM, Kirschner MW. Physical-Properties and Cellular Localization of Tau, A Microtubule Associated Protein Which Induces Assembly of Purified Tubulin. Journal of Cell Biology. 1977a;75:A283. [Google Scholar]
- 19.Cleveland DW, Hwo SY, Kirschner MW. Purification of Tau, A Microtubule-Associated Protein That Induces Assembly of Microtubules from Purified Tubulin. Journal of Molecular Biology. 1977b;116:207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- 20.Congdon EE, Kim S, Bonchak J, Songrug T, Matzavinos A, Kuret J. Nucleation-dependent tau filament formation - The importance of dimerization and an estimation of elementary rate constants. Journal of Biological Chemistry. 2008;283:13806–13816. doi: 10.1074/jbc.M800247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Congdon EE, Necula M, Blackstone RD, Kuret J. Potency of a tar fibrillization inhibitor is influenced by its aggregation state. Archives of Biochemistry and Biophysics. 2007;465:127–135. doi: 10.1016/j.abb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowe A, Huang W, Ballatore C, Johnson R, Hogan A, Huang R, Wichtermann J, McCoy J, Huryn D, Auld D, Smith AI, Inglese J, Trojanowski J, Austin C, Brunden K, Lee V. The identification of aminothienopyridazine inhibitors of tau assembly by quantitative high-throughput screening. Biochemistry. 2009;48:7732–7745. doi: 10.1021/bi9006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowe A, Ballatore C, Hyde E, Trojanowski JQ, Lee VMY. High throughput screening for small molecule inhibitors of heparin-induced tau fibril formation. Biochemical and Biophysical Research Communications. 2007;358:1–6. doi: 10.1016/j.bbrc.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayanandan R, Van Slegtenhorst M, Mack TGA, Ko L, Yen SH, Leroy K, Brion JP, Anderton BH, Hutton M, Lovestone S. Mutations in tau reduce its microtubule binding properties in intact cells and affect its phosphorylation. Febs Letters. 1999;446:228–232. doi: 10.1016/s0014-5793(99)00222-7. [DOI] [PubMed] [Google Scholar]
- 25.Dickey CA, Dunmore J, Lu BW, Wang JW, Lee WC, Kamal A, Burrows F, Eckman C, Hutton M, Petrucelli L. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. Faseb Journal. 2006;20 doi: 10.1096/fj.05-5343fje. 753-+. [DOI] [PubMed] [Google Scholar]
- 26.Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Patterson C, Dickson DW, Nahman NS, Hutton M, Burrows F, Petrucelli L. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. Journal of Clinical Investigation. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the Dynamic Instability of Tubulin Assembly by the Microtubule-Associated Protein Tau. Molecular Biology of the Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng BY, Toyama BH, Wille H, Colby DW, Collins SR, May BCH, Prusiner SB, Weissman J, Shoichet BK. Small-molecule aggregates inhibit amyloid polymerization. Nature Chemical Biology. 2008;4:197–199. doi: 10.1038/nchembio.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman MS, Trojanowski JQ, Lee VMY. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nature Medicine. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 30.Gamblin TC, Berry RW, Binder LI. Modeling Tau polymerization in vitro: A review and synthesis. Biochemistry. 2003;42:15009–15017. doi: 10.1021/bi035722s. [DOI] [PubMed] [Google Scholar]
- 31.Gamblin TC, King ME, Dawson H, Vitek MP, Kuret J, Berry RW, Binder LI. In vitro polymerization of tau protein monitored by laser light scattering: Method and application to the study of FTDP-17 mutants. Biochemistry. 2000;39:6136–6144. doi: 10.1021/bi000201f. [DOI] [PubMed] [Google Scholar]
- 32.George-Hyslop PH, Petit A. Molecular biology and genetics of Alzheimer's disease. C R Biol. 2005;328:119–130. doi: 10.1016/j.crvi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Goedert M. Tau gene mutations and their effects. Movement Disorders. 2005;20:S45–S52. doi: 10.1002/mds.20539. [DOI] [PubMed] [Google Scholar]
- 34.Goedert M, Jakes R. Expression of Separate Isoforms of Human Tau-Protein - Correlation with the Tau-Pattern in Brain and Effects on Tubulin Polymerization. Embo Journal. 1990;9:4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2005;1739:240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 37.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple Isoforms of Human Microtubule-Associated Protein-Tau - Sequences and Localization in Neurofibrillary Tangles of Alzheimers-Disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 38.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, DeTure M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. European Journal of Neuroscience. 2008;27:1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- 39.Hardy J, Selkoe DJ. Medicine - The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa M, Crowther RA, Jakes B, Goedert M. Alzheimer-like changes in microtubule-associated protein tau induced by sulfated glycosaminoglycans - Inhibition of microtubule binding, stimulation of phosphorylation, and filament assembly depend on the degree of sulfation. Journal of Biological Chemistry. 1997;272:33118–33124. doi: 10.1074/jbc.272.52.33118. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa M, Smith MJ, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. Febs Letters. 1998;437:207–210. doi: 10.1016/s0014-5793(98)01217-4. [DOI] [PubMed] [Google Scholar]
- 42.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VMY. Mutation-specific functional impairments in distinct Tau isoforms of hereditary FTDP-17. Science. 1998b;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 43.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VMY. Mutation-specific functional impairments in distinct Tau isoforms of hereditary FTDP-17. Science. 1998a;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 44.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5 '-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 45.Hyman BT. The neuropathological diagnosis of Alzheimer's disease: Clinical-pathological studies. Neurobiology of Aging. 1997;24:S27–S32. doi: 10.1016/s0197-4580(97)00066-3. [DOI] [PubMed] [Google Scholar]
- 46.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VMY. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 47.Khlistunova I, Biernat J, Wang YP, Pickhardt M, von Bergen M, Gazova Z, Mandelkow E, Mandelkow M. Inducible expression of tau repeat domain in cell models of tauopathy - Aggregation is toxic to cells but can be reversed by inhibitor drugs. Journal of Biological Chemistry. 2006;281:1205–1214. doi: 10.1074/jbc.M507753200. [DOI] [PubMed] [Google Scholar]
- 48.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-Associated Protein Tau (Tau) Is A Major Antigenic Component of Paired Helical Filaments in Alzheimer-Disease. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larbig G, Pickhardt M, Lloyd DG, Schmidt B, Mandelkow E. Screening for inhibitors of tau protein aggregation into Alzheimer paired helical filaments: A ligand based approach results in successful scaffold hopping. Current Alzheimer Research. 2007;4:315–323. doi: 10.2174/156720507781077250. [DOI] [PubMed] [Google Scholar]
- 50.Lee VMY, Balin BJ, Otvos L, Trojanowski JQ. A68 - A Major Subunit of Paired Helical Filaments and Derivatized Forms of Normal-Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 51.Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annual Review of Neuroscience. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 52.Lee VMY, Kenyon TK, Trojanowski JQ. Transgenic animal models of tauopathies. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2005;1739:251–259. doi: 10.1016/j.bbadis.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Li WK, Lee VMY. Characterization of two VQIXXK motifs for tau fibrillization in vitro. Biochemistry. 2006;45:15692–15701. doi: 10.1021/bi061422+. [DOI] [PubMed] [Google Scholar]
- 54.Lipinski C. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 55.Luo WJ, Dou F, Rodina A, Chip S, Kim J, Zhao Q, Moulick K, Aguirre J, Wu N, Greengard P, Chiosis G. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9511–9516. doi: 10.1073/pnas.0701055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Margittai M, Langen R. Template-assisted filament growth by parallel stacking of tau. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10278–10283. doi: 10.1073/pnas.0401911101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF, Feng L, Lecanu L, Walker BR, Planel E, Arancio O, Gozes I, Aisen PS. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer's disease. Journal of Pharmacology and Experimental Therapeutics. 2008;325:146–153. doi: 10.1124/jpet.107.130526. [DOI] [PubMed] [Google Scholar]
- 58.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nature Reviews Drug Discovery. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 59.Merrick SE, Trojanowski JQ, Lee VMY. Selective destruction of stable microtubules and axons by inhibitors of protein serine/threonine phosphatases in cultured human neurons (NT2N cells) Journal of Neuroscience. 1997;17:5726–5737. doi: 10.1523/JNEUROSCI.17-15-05726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris AM, Watzky MA, Agar JN, Finke RG. Fitting neurological protein aggregation kinetic data via a 2-step, Minimal/"Ockham's Razor" model: The Finke-Watzky mechanism of nucleation followed by autocatalytic surface growt. Biochemistry. 2008;47:2413–2427. doi: 10.1021/bi701899y. [DOI] [PubMed] [Google Scholar]
- 61.Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, Yen SH. Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. Journal of Neuropathology and Experimental Neurology. 1999;58:545. doi: 10.1016/s0014-5793(99)00294-x. [DOI] [PubMed] [Google Scholar]
- 62.Necula M, Chirita CN, Kuret J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: Implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry. 2005;44:10227–10237. doi: 10.1021/bi050387o. [DOI] [PubMed] [Google Scholar]
- 63.Necula M, Kuret J. Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. Journal of Biological Chemistry. 2004;279:49694–49703. doi: 10.1074/jbc.M405527200. [DOI] [PubMed] [Google Scholar]
- 64.Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Differential regulation of microtubule dynamics by three- and four-repeat tau: Implications for the onset of neurodegenerative disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9548–9553. doi: 10.1073/pnas.1633508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perez M, Valpuesta JM, Medina M, deGarcini EM, Avila J. Polymerization of tau into filaments in the presence of heparin: The minimal sequence required for tau-tau interaction. Journal of Neurochemistry. 1996;67:1183–1190. doi: 10.1046/j.1471-4159.1996.67031183.x. [DOI] [PubMed] [Google Scholar]
- 66.Pickhardt M, Gazova Z, von Bergen M, Khlistunova I, Wang YP, Hascher A, Mandelkow EM, Biernat J, Mandelkow E. Anthraquinones inhibit tau aggregation and dissolve Alzheimer's paired helical filaments in vitro and in cells. Journal of Biological Chemistry. 2005;280:3628–3635. doi: 10.1074/jbc.M410984200. [DOI] [PubMed] [Google Scholar]
- 67.Pickhardt M, Larbig G, Khlistunova I, Coksezen A, Meyer B, Mandelkow EM, Schmidt B, Mandelkow E. Phenylthiazolyl-hydrazide and its derivatives are potent inhibitors of tau aggregation and toxicity in vitro and in cells. Biochemistry. 2007;46:10016–10023. doi: 10.1021/bi700878g. [DOI] [PubMed] [Google Scholar]
- 68.Selkoe DJ, Wolfe MS. Presenilin: Running with scissors in the membrane. Cell. 2007;131:215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B. Familial multiple system tauopathy with presenile dementia: A disease with abundant neuronal and glial tau filaments. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4113–4118. doi: 10.1073/pnas.94.8.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. Journal of Biological Chemistry. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 71.Wagner U, Utton M, Gallo JM, Miller CCJ. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. Journal of Cell Science. 1996;109:1537–1543. doi: 10.1242/jcs.109.6.1537. [DOI] [PubMed] [Google Scholar]
- 72.Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, Ravikumar B, Rubinsztein DC. Aggregate-prone proteins are cleared from the cytosol by autophagy: Therapeutic implications. Current Topics in Developmental Biology. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. Vol 76. [DOI] [PubMed] [Google Scholar]
- 73.Wilson DM, Binder LI. Free fatty acids stimulate the polymerization of tau and amyloid beta peptides - In vitro evidence for a common effector of pathogenesis in Alzheimer's disease. American Journal of Pathology. 1997;150:2181–2195. [PMC free article] [PubMed] [Google Scholar]
- 74.Wischik CM, Edwards PC, Lai RYK, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, Toleikis PM, Lee VMY, Trojanowski JQ. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]