Abstract
The GABA transporters GAT-1 and GAT-3 are abundant in the external and internal segments of the globus pallidus (GPe and GPi, respectively). We have shown that pharmacological blockade of either of these transporters results in decreased neuronal firing, and in elevated levels of extracellular GABA in normal monkeys. We now studied whether the electrophysiologic and biochemical effects of local intra-pallidal injections of GAT-1 and GAT-3 blockers, or the subcellular localization of these transporters, are altered in monkeys rendered parkinsonian by the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The subcellular localization of the transporters in GPe and GPi, studied with electron microscopy immunoperoxidase, was similar to that found in normal animals: i.e., GAT-3 immunoreactivity was mostly confined to glial processes, while GAT-1 labeling was expressed in unmyelinated axons and glial processes. A combined injection/recording device was used to record extracellular activity of single neurons in GPe and GPi, before, during and after administration of small volumes (1 μl) of either the GAT-1 inhibitor, SKF-89976A hydrochloride (720 ng), or the GAT-3 inhibitor, (S)-SNAP-5114 (500 ng). In GPe, the effects of GAT-1 or GAT-3 blockade were similar to those seen in normal monkeys. However, unlike the findings in the normal state, the firing of most neurons was not affected by blockade of either transporter in GPi. These results suggest that, after dopaminergic depletion, the functions of GABA transporters are altered in GPi; without major changes in their subcellular localization.
Keywords: Parkinsonism, globus pallidus external segment, globus pallidus internal segment, GABAergic transmission, single unit extracellular recording, electron microscope, subcellular distribution
INTRODUCTION
In parkinsonism, the activity of neurons in the external and internal pallidal segments (GPe and GPi, respectively) is abnormally patterned, with a pathologically high incidence of bursts, oscillatory activities, and aberrant synchronization among neurons (for a review see Galvan and Wichmann, 2008). It is likely that at least some of these abnormalities result from altered transmission at synapses utilizing the inhibitory transmitter γ-amino-butyric acid (GABA) in the pallidum (Galvan and Wichmann, 2007). GABA is released in GPe and GPi from terminals of the striato-pallidal projections, and local axon collaterals or connections between the two pallidal segments (Smith et al., 1998). Together, these GABAergic influences powerfully inhibit the activity of pallidal neurons (Kita, 2007, Nambu, 2007). According to the traditional model of the pathophysiology of parkinsonism, the striatal dopamine loss in Parkinson’s disease results in increased striatal GABAergic inhibition of GPe and decreased striatal GABAergic inhibition of GPi and the substantia nigra pars reticulata (SNr). In vivo microdialysis studies in parkinsonian animals have confirmed an elevated level of GABA in GPe (Galeffi et al., 2003, Robertson et al., 1991, Schroeder and Schneider, 2002), while there were no changes in GABA levels in the rat SNr (Galeffi et al., 2003, Ochi et al., 2004, but see Windels et al., 2005). Support for the notion that abnormalities of GABAergic transmission play a central role in the pathophysiology of parkinsonism also comes from studies of metabolic markers of GABAergic activity in the globus pallidus of dopamine-depleted animals. For instance, the level of glutamic acid decarboxylase (GAD) mRNA is increased in striatal neurons projecting to GPe (Laprade and Soghomonian, 1999, Soghomonian et al., 1992), and in GPi and SNr neurons (Soghomonian and Chesselet, 1992, Soghomonian et al., 1994). Furthermore, GABA-A and GABA-B receptor binding or mRNA expression are decreased in GPe, and increased in GPi and SNr in parkinsonian patients or animals (Calon et al., 1995, Calon et al., 2000, Calon et al., 2003, Chadha et al., 2000, Griffiths et al., 1990, Johnston and Duty, 2003, Katz et al., 2005, Robertson et al., 1990), perhaps as a compensatory mechanism in response to increased GABA release in GPe and reduced GABAergic inhibition of GPi and SNr neurons.
A key mechanism by which tissue concentrations of GABA, and thus GABAergic transmission, are regulated is the action of plasma-membrane bound GABA transporters (GATs, Dalby, 2003, Kanner, 2006, Richerson and Wu, 2003). These high-affinity transporters are thought to constrain the extent of diffusion of GABA from the release sites and, thus, the length of time which the transmitter spends in the synaptic cleft and in the extrasynaptic space. Of the four identified GAT genes (GAT-1, GAT-2, GAT-3 and B-GAT, Borden, 1996, Dalby, 2003), only GAT-1 and GAT-3 mRNA or protein expression have been described in the pallidum (Durkin et al., 1995, Ikegaki et al., 1994, Ng et al., 2000, Wang and Ong, 1999, Yasumi et al., 1997). We have previously shown that GAT-1 and GAT-3 are expressed in glia, and to a lesser extent in pre-terminal axons, and that both transporters modulate GABA levels and neuronal activity in the monkey GPe and GPi (Galvan et al., 2005).
GAT functions in other systems are regulated in response to changes in extracellular GABA concentrations (Bernstein and Quick, 1999, Chiu et al., 2002), likely as a compensatory phenomenon. In this study we sought to determine whether such compensatory changes in GAT functions are also triggered in response to the perturbations in pallidal GABAergic transmission in parkinsonian nonhuman primates. We therefore studied the ultrastructural localization of GAT-1 and GAT-3 in parkinsonian monkeys, and examined the effects of pharmacological blockade of GATs in GPe and GPi in these animals.
MATERIALS AND METHODS
Animals
Seven drug naïve rhesus monkeys (Macaca mulatta, 4-7 kg) were used for these studies. Four of these animals were used for the anatomical analysis and 3 for the electrophysiology studies. All animals were housed with ad libitum access to food and water. All experimental protocols were performed in accordance with the National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals” (1996) and the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals (amended 2002), and were approved by the Animal Care and Use Committee of Emory University. Before the start of the experiments, the monkeys were acclimated to the laboratory and trained to permit handling by the experimenter and sit in a primate chair.
MPTP administration
In order to induce dopamine depletion, 5 of the 7 animals received once weekly intramuscular injections of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP, 0.2-0.8 mg/kg, Sigma-Aldrich, St. Louis, MO) until moderate parkinsonism developed. The degree of parkinsonism was assessed weekly (see below). The total doses of MPTP and times needed to generate stable parkinsonian signs (as determined by the behavioral assessment) ranged from 12.3 to 36.5 mg/kg and 5 to 18 months, respectively.
Behavioral assessment
Before starting the MPTP treatment, the monkeys were habituated to a behavioral observation cage, and a baseline of motor behavior was established. During the MPTP treatment, the animals’ degree of parkinsonism was monitored once a week, using previously described methods (Wichmann et al., 2001). Briefly, the motor behavior was rated using a scale based on nine criteria (gross motor activity, balance, posture, arm bradykinesia, arm hypokinesia, leg bradykinesia, leg hypokinesia, arm tremor and leg tremor). Each criterion received a score between 0 and 3 (normal/absent to severe), for a maximum of 27 points. We also used a computer-assisted observation method and a cage activity monitoring system based on infrared beams. A monkey was considered as a stable parkinsonian animal if the score in the parkinsonian rating scale was at least 10, and if the counts in the activity monitoring remained 60% or below the baseline levels for at least six weeks after the last MPTP injection.
Immunohistochemical localization of GAT-1 and GAT-3
Two MPTP-treated and two untreated monkeys were deeply anesthetized with an overdose of pentobarbital (100 mg/kg, i.v.) and perfused transcardially with cold oxygenated Ringer’s solution, followed by 4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffered saline (PBS, 0.01 M, pH 7.4). The brains were removed from the skull and frontal sections (60 μm) were obtained with a vibratome and collected in cold PBS (0.01M, pH 7.4).
For the electron microscopic studies, sections were treated with 1% sodium borohydride, and then placed in a cryoprotectant solution, frozen at −80°C, thawed and washed in PBS. After blocking non-specific sites with 10% normal goat serum and 1% bovine serum albumin in PBS, the sections were incubated for two days at 4°C in the primary antibody solution (anti-GAT-1, 1:250 cat no. AB1570, or anti-GAT-3, 1:1000, cat no. AB1574; Millipore, Billerica, MA). The specificity of these antibodies was previously determined using immunoblot analysis with antigen preabsorption (Ikegaki et al., 1994).
The avidin-biotin complex (ABC) method (Hsu et al., 1981) was used to reveal the antibody binding sites, as follows. The sections were incubated in biotinylated goat anti-rabbit IgG (1:200; Vector labs, Burlingame, CA) for 2 hrs, and in the ABC solution (1:100; Vectastain Standard kit, Vector labs) for 90 min. All immuno-reagents were diluted in PBS containing 1% normal goat serum and 1% bovine serum albumin. Sections were rinsed in PBS and TRIS buffer (0.05 M, pH 7.6) before being placed in a solution containing 0.025% 3-3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich), 0.01M imidazole (Fisher Scientific) and 0.006% H2O2. The reaction was terminated by repeated washes in PBS.
The sections were transferred to PB for 10 min and postfixed in osmium tetroxide (1% in PB). This was followed by further washings in PB and dehydration in a graded series of ethanol and propylene oxide. Uranyl acetate (1%) was added to the 70% ethanol. The sections were embedded in resin (Durcupan ACM; Fluka, Ft. Washington, PA) on microscope slides and placed in the oven for 48 hours at 60°C. Blocks from GPe or GPi were cut out from the slides and glued on the top of resin blocks. Serial ultrathin sections were then cut on an ultramicrotome (Leica Ultracut T2). Sixty nanometer thick sections from the surface of the block were collected on Pioloform-coated copper grids, stained with lead citrate for 5 min to enhance tissue contrast, and examined on a Zeiss EM-10C electron microscope at 20,000-25,000X. Electron micrographs of fields containing immunoreactive elements were acquired using a digital camera (DualView 300W; Gatan, Inc., Pleasanton, CA) controlled by DigitalMicrograph software (version 3.10.1; Gatan).
For the light microscopy experiments, sections containing putamen, GPe and GPi were obtained from approximately the same antero-posterior level of the brain (14.70 to 12. 45 mm from the interaural line, according to Paxinos et al., 2000). To control for variability in GAT-1 or GAT-3 staining intensity caused by inherent differences between animals and immunohistochemical reactions, sections from all animals were incubated simultaneously. The sections were then processed as for the electron microscope analysis, except that the cryoprotectant solution and freezing steps were omitted and 0.1% Triton X-100 was added to all incubation solutions. After revealing the GAT-1 or GAT-3 antibodies, the sections were mounted on gelatin-coated slides, dehydrated in alcohol, immersed in toluene and a cover slip was applied with Cytoseal XYL (Richard-Allan Scientific, Kalamazoo, MI). The sections were examined with a Leica M420 microscope and low magnification images were acquired with a SPOT–RT camera (Diagnostic Instruments, Sterling Heights, MI) controlled by Adobe Photoshop software (version 9.0; Adobe System, Inc., San Jose, CA).
Surgical procedure and initial electrophysiological mapping
We gathered new electrophysiological data in three MPTP-treated animals, and compared the results with those previously obtained in normal animals (using identical recording methods, Galvan et al. 2005). At the beginning of the current studies, the monkeys were treated with MPTP. After they had developed stable parkinsonism, a standard stainless steel recording chamber (inner diameter 16 mm) was implanted under aseptic conditions and isoflurane anesthesia (1-3%). The chamber was stereotactically directed at the pallidum on the left side of the brain, with a 36° angle from the vertical in the coronal plane. The chamber was affixed to the skull with dental acrylic, along with metal head holders to permit head stabilization. Metal screws were used to anchor the acrylic to the bone. After surgery, the animals were allowed to recover for at least 1 week.
During all of the electrophysiological recording sessions, the animals were awake, seated in a primate chair with their heads restrained, but free to move their body and limbs. After perforating the dura with a 20-gauge guide tube, tungsten microelectrodes (Z = 0.5-1.0 MΩ at 1 kHz; FHC, Bowdoinham, ME) were lowered into the brain with a microdrive (MO-95B; Narishige, Tokyo, Japan), and neuronal electrical signals were recorded extracellularly, amplified (DAM-80 amplifier; World Precision Instruments, Sarasota, FL), filtered (400-10,000 Hz; Krohn-Hite, Brockton, MA), displayed on a digital oscilloscope (DL1540; Yokogawa, Tokyo, Japan), and made audible via an audio amplifier. Neurons in the GPe were identified by their characteristic high-frequency discharge, interspersed with pauses (DeLong, 1971, Elias et al., 2008), as well as by their relation to the neuronal activity of the striatum obtained dorsally in the same electrode trajectory (DeLong, 1973). Neurons in the GPi were identified by their high-frequency discharge, by their location, at least 2 mm ventral to the first GPe cell encountered in the same trajectory, and by the identification of ‘border’ cell activity between GPe and GPi (DeLong, 1971).
Intracerebral injections
Intracerebral injections were done using a device that combines an electrode with an injection tubing (Kliem and Wichmann, 2004). The device consists of a tungsten microelectrode and a segment of fused silica tubing (i.d. = 40 μm; o.d. = 103 μm; Polymicro Technologies, Phoenix, AZ), both placed inside a sleeve of polyimide tubing (o.d. = 0.5 mm; MicroLumen, Tampa, FL). The tip of the silica tubing was adjusted to project 1 mm from the polyimide sleeve, and the electrode tip extended 200 μm further. The injection tubing was connected to a 1 ml gas-tight syringe (CMA Microdialysis, Solna, Sweden), driven by a remotely controlled infusion pump (CMA/102) for pressure infusion of sub-microliter quantities of solutions. The recording-injection system was lowered into the brain using a microdrive.
After the electrode tip had been advanced into the target structure, neurons were isolated, and the spontaneous activity of the cell recorded for at least 90 s. The recording continued during the subsequent delivery of the drug under study (1 μl, at 0.3 μl/min, see below for drugs used), and for at least 300 s after the end of the infusion. In some cases more than one injection was done along the same penetration. In these cases, the injections were separated by at least 1 mm.
The neuronal signals were digitized (sampling rate 25 kHz) and stored onto computer disk using a data acquisition interface (Power1401; CED, Cambridge, UK) and commercial software (Spike2 v5, CED) for off-line analysis.
Drugs
We used SKF-89976A hydrochloride (720 ng, 2 mM, Tocris Bioscience, Ellisville, MO), a selective blocker of GAT-1 transporters (Borden, 1996, Borden et al., 1994), and the semi-selective GAT-3 inhibitor, (S)-SNAP-5114 (515 ng, 1 mM, Tocris), that has an 80-fold higher affinity for GAT-3 than for GAT-1 (Borden, 1996, Dalby, 2003, Dhar et al., 1994). The drugs were dissolved in artificial cerebrospinal fluid (aCSF, comprised of (in mM) 143 NaCl, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 1 Na2HPO4), and the pH adjusted to 7.2-7.4. Before being loaded into the injection systems, all solutions were filtered with a 0.2 μm pore size nylon membrane (Fisher Scientific, Hampton, NH). aCSF was used for control injections.
Perfusion and tissue processing
At the conclusion of the electrophysiology experiments, the animals were sacrificed and perfused as described above. Coronal sections of the pallidum were Nissl-stained to visualize electrode penetrations. In addition, immunostains for the neuronal marker microtubule associated protein 2 (mouse anti-MAP2, 1:1000, Millipore) were carried out to demonstrate areas of tissue damage. To assess the degree of dopaminergic denervation in the MPTP-treated monkeys, sections at the level of the striatum and the substantia nigra were stained with mouse anti-tyrosine hydroxylase (TH) antibodies (1:1000, Millipore). The immunohistochemistry procedures for MAP-2 and TH staining were performed using the ABC method (see above).
Analysis of immunohistochemical data
The intensity of peroxidase labeling was measured in the putamen, GPe and GPi using the ImageJ software (National Institutes of Health, Rasband, 1997-2009). Measurements of optical density at low magnification have been found to be linearly related to stereological counts of labeled fibers at higher magnifications (Scorcioni et al., 2008). Gray-scale light micrographs of GAT-1- or GAT-3- stained sections were generated as described above. In the putamen, GPe and GPi, the optical density was determined in four circular regions of the same size. To control for differences in background staining, the optical density in the insula was used as a reference. Thus, each value obtained in putamen, GPe and GPi was divided by the averaged value obtained in the insula. Comparisons among structures and treatments were carried out with non-parametric tests (Kruskal Wallis or Mann-Whitney tests). Statistical analyses were performed with SPSS (version 16.0, SPSS Inc, Chicago, Ill).
To assess the degree of dopaminergic denervation induced by MPTP treatments, we measured optical densities of TH immunostaining in the striatum of MPTP-treated monkeys and compared these against controls.
For the electron microscopic analysis of GATs localization, we examined a total of 1000 μm2 of immunostained tissue per structure (GPe or GPi) per monkey. The labeled elements were categorized based on ultrastructural features (Peters et al., 1991). Glial elements were further cataloged as follows: (1) ‘glia-terminal’ were glial processes that were immediately adjacent to an axon terminal, (2) ‘glia-unmyelinated axons’, referred to glial elements surrounded by bundles of unmyelinated axons, (3) ‘glia-dendrite’ were processes in close contact with dendrites and (4) ‘other’. If the glial element was in similar proximity to terminals and axons, it was catalogued as ‘glia-terminal’. Small (<0.5 μm) diameter elements cut in the transverse plane and surrounded by unmyelinated axons were categorized as unmyelinated axons.
The total number of immunoreactive elements was counted, and the relative proportion of each category of elements was calculated and expressed as percent of total labeled elements for each transporter in the areas examined, as we have previously reported (Charara et al., 2004, Galvan et al., 2004). Chi-square tests were used to compare the proportions of labeled elements between MPTP-treated and normal monkeys, and between GPe and GPi in each condition.
Analysis of electrophysiological data
The analysis was conducted only on those cells that were confirmed to be in the GPe or GPi based on depth readings during the recording sessions, and on the results of the histological analysis. Computer records of neuronal activity were used for off-line spike sorting with a waveform matching algorithm, followed by principal component analysis (Spike 2). Inter-spike interval distribution histograms were constructed to verify the recording quality for each spike train. All subsequent steps of the analysis were done in Matlab (Mathworks, Natick, MA).
Inter-spike intervals (ISIs) were used to calculate second-by-second readouts of firing rates or coefficients of variation (CV), which were subsequently smoothed using a sliding 21-point moving average.
A drug effect was considered significant if the firing rate or the CV remained continuously either above the 90th percentile, or below the 10th percentile of the pre-injection baseline activity, defined as the activity in the 60-s segment of data preceding the drug infusions, for at least 60 s, with an effect onset no longer than 200 s after the beginning of the drug injection. The 200-s latency parameter was chosen based on previous experiments (Galvan et al., 2005). Responses were classified into increases or decreases of firing. The ‘maximal effect period’ was defined as the 60-s period centered on the highest or lowest point of the firing rate or CV after the drug application. In cases in which no effect was observed (firing rate or CV did not change above or below the 90-10th percentile window after the injection within 200 s of the start of the injection), a 60s data segment centered on a randomly chosen time point within the 200 s period after the start of the injection was used as the ‘maximal effect period’. For each neuron, a ‘firing rate ratio’ and ‘CV ratio’ was calculated by dividing the firing rate or CV value of the maximal effect period by the baseline value, calculated across the 60 s period preceding the drug injections.
For statistical comparisons, the maximal change in discharge rate or CV after drug injections was expressed as the percentage of the baseline (control) firing rate, and compared with control (aCSF) experiments, using the Mann-Whitney U-test. For each type of drug injection (aCSF, SKF-89976A or SNAP-5114), the firing rate for the control and effect segments were compared using the Wilcoxon signed ranks test. The proportions of cells responding to each drug with increases, decreases or no changes in firing rate in GPe or GPi was compared between MPTP and normal monkeys using the chi-square test. A two-tailed Pearson test was used to assess the correlation between the firing rate and CV ratios.
RESULTS
All 7 MPTP-treated monkeys considered to be stably parkinsonian based on the behavioral assessment, with parkinsonian scores ranging from 17 to 24. All animals showed a >90% depletion of TH staining in the striatum, compared with normal monkeys. Supplemental Fig. A shows TH-stained sections at the level of the striatum/GP and SN in normal and MPTP-treated monkeys. As previously reported (i. e., Pifl et al., 1991, Villalba et al., 2009) we found a severe loss in the dorsal striatum, with relative preservation of TH labeling in the nucleus accumbens.
Light microscopic immunohistochemical analysis of GAT-1 and GAT-3 in GPe and GPi
Intense immunoreactivity for GAT-1 and GAT-3 was observed in both segments of the GP (Fig. 1), consistent with previous observations (Galvan et al., 2005, Ng et al., 2000, Wang and Ong, 1999). GAT-1 immunostaining was strong in putamen, GPe and GPi (Fig. 1A, B). The intensity of staining, assessed by measurements of optical density, was higher in GPe (optical densities, corrected by the values in cortex: 0.69 ± 0.05) than in the putamen (0.60 ± 0.04, different from GPe at p = 0.01, Mann-Whitney U-test), but lower than in GPi (0.77 ± 0.04; different from GPe at p = 0.021, Mann-Whitney U-test). GAT-3 labeling (Fig. 1C, D) was denser in both segments of the globus pallidus (GPe, 1.21 ± 0.07; GPi, 1.29 ± 0.08) than in the putamen (0.78 ± 0.07; different from GPe and GPi at p < 0.001, Mann-Whitney U). Based on optical density measurements, the intensity of GAT-1 or GAT-3 staining did not differ between normal (Fig. 1A, C) and MPTP-treated monkeys (Fig. 1B, D).
Fig. 1. GAT-1 and GAT-3 immunostaining in GPe and GPi of normal and MPTP-treated monkeys.
Scale bar in D: 2 mm, applies for the four panels.
Ultrastructural Localization of GAT-1 and GAT-3 in GPe and GPi
To determine if dopaminergic depletion induced changes in the ultrastructural localization of GAT-1 and GAT-3 transporters, we used electron microscopy immunoperoxidase to compare the relative percentage of labeled elements for either transporter subtypes between normal and MPTP-treated monkeys.
GAT-1 labeling
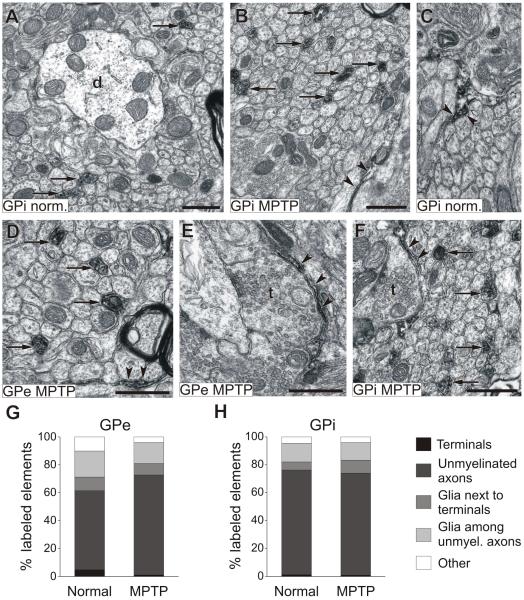
In both pallidal segments of normal monkeys, the majority of GAT-1-labeled elements were unmyelinated axonal segments (57% and 75% in GPe and GPi respectively, Fig. 2), followed by glial processes, which accounted for 27% of GAT-1-labeled elements in GPe and 18% in GPi, and GAT-1-positive axon terminals (less than 5% of labeled elements in GPe and GPi). GAT-1- immunopositive glial processes were mostly found among unmyelinated axons (Fig. 2C, D), and less frequently apposed to terminals (Figs. 2E, F).
Fig. 2. GAT-1 labeling in GPe and GPi of normal and MPTP-treated monkeys.
A to F show examples of GAT-1-labeled elements, arrows point to labeled unmyelinated axons, while arrowheads indicate glial processes. G-H display proportions of labeled elements in either pallidal segment in normal and MPTP-treated animals. Abbreviations: d, dendrites; t, terminals. All scale bars are 0.5 μm. The scale bar in F is valid for C.
The distribution of GAT-1 in the pallidum of MPTP-treated monkeys followed a similar pattern, i.e. the majority of labeling was found in unmyelinated axons (72% in GPe and 73% in GPi) and glial processes (GAT-1-positive glial elements combined accounted for 23% in GPe and 22% in GPi), with only scarce labeling in axon terminals (1% in GPe and GPi). There was no significant difference in the proportions of GAT-1-labeled elements between normal and MPTP-treated monkeys, or between GPe and GPi in each condition (Fig. 2G, H).
GAT-3 labeling
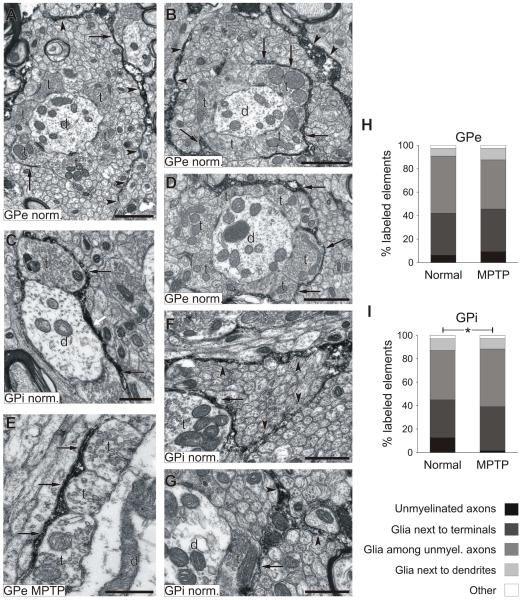
GAT-3-labeled elements were abundant in GPe and GPi. In both normal and MPTP-treated monkeys, GAT-3 immunoreactivity was confined almost exclusively to glial processes (representing more than 85% of labeled elements, Fig. 3), with the remainder found mostly in unmyelinated axons. A large proportion of GAT-3-immunopositive glial processes were found either surrounding terminals (for example, Fig. 3B, E) or within bundles of unmyelinated axons (Fig. 3A, B, F). GAT-3-labeled glial extensions frequently wrapped around axo-dendritic complexes consisting of numerous putative GABAergic boutons that formed symmetric synapses with pallidal dendrites (Fig. 3D), or followed tortuous routes through large bundles of unmyelinated axons (see for example Fig. 3F). In rare instances, GAT-3- positive glial processes were in close contact with dendritic processes (Fig. 3C).
Fig. 3. GAT-3 labeling in GPe and GPi of normal and MPTP-treated monkeys.
A to G show examples of GAT-3-labeled elements, arrows point to GAT-3-positive glial processes next to terminals, while arrowheads indicate GAT-3-immunoreactive glial elements among unmyelinated axons. The white arrow indicates a glial process closely apposed to a dendrite. H and I illustrate the relative percentage of different categories of labeled elements. *, Difference between normal and MPTP-treated monkeys, p<0.05. Abbreviations: d, dendrites; t, terminals. Scale bars: 1 μm in A, B and D; 0.5 μm in C, E, F and G.
In GPi, the distribution of GAT-3-labeled elements differed between normal and MPTP-treated monkeys (Pearson chi-square = 11.391, p = 0.023), due to a lower proportion of unmyelinated axons in the MPTP-treated animals. We did not find any other significant difference between normal and MPTP-treated groups, or between GPe and GPi (Fig. 3H, I).
Effects of GAT-1 and GAT-3 transporters blockade on the firing of GPe and GPi neurons in MPTP-treated monkeys
Pallidal neurons were recorded from 3 MPTP-treated stable parkinsonian monkeys. We report results obtained from a total of 32 GPe and 20 GPi neurons in which the stability and isolation of single units was maintained before, during and after injections in the vicinity of the recorded cells. The local injections of GABA transporters in GPe and GPi did not result in any behavioral effects.
The baseline firing properties of pallidal neurons in MPTP-treated and normal monkeys is shown in Table 1, contrasted to the information from neurons recorded in normal monkeys (obtained from Galvan et al, 2005). GPe or GPi neurons exposed to either aCSF, SKF-89976A or SNAP-5114 were not significantly different from one another in terms of firing rates or coefficients of variation (CV). In agreement with our earlier observations (Galvan et al., 2005), aCSF control injections in both GPe and GPi did not change firing rates or CV values.
Table 1.
Descriptors of basal firing in GPe and GPi neurons
| GPe | GPi | |||
|---|---|---|---|---|
| Normal (n=15) |
MPTP (n=32) |
Normal (n=12) |
MPTP (n=20) |
|
| Firing rate | 65.83 (29.88) | 63.38 (31.63) | 56.53 (30.6) | 62.05 (30.69) |
| CV | 1.37 (1.21) | 1.50 (0.65) | 0.83 (0.25) | 1.42 (0.64) |
Data are means (± SD)
Lack of average population differences in physiological activity induced by GAT blockade in GPe and GPi
As a first approach to analyze potential effects of GAT-1 and GAT-3 blockade on the electrophysiological activity of pallidal neurons, the maximal changes in discharge rate or CV values after the drug injections were expressed as a percentage of the respective baseline, and compared with control (aCSF) experiments, using the non-parametric Mann-Whitney U-test. This analysis revealed no significant difference between the activity of aCSF-treated GPe and GPi neurons and those exposed to GAT-1 or GAT-3 blockers. In some of the groups, the lack of average population differences was due to a lack of significant drug effect on individual neurons, while in others, it resulted from even distributions of significant increases or decreases in activity between individual cells. Thus, to take into consideration the direction of changes in discharge rate after drug injections, neurons were classified into those that responded with increases, decreases or no change in firing rates or CV values in response to drug applications (see Methods for criteria used to define effects).
Effects of GAT-1 or GAT-3 blockade in the GPe of MPTP-treated monkeys
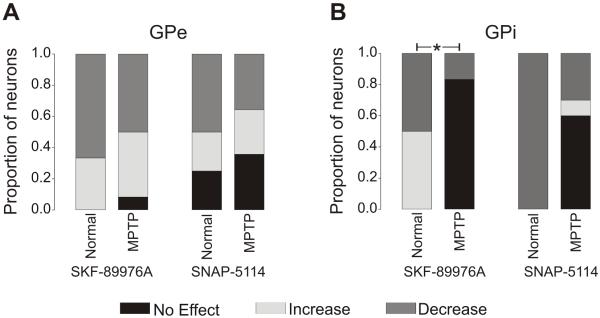
Most GPe cells showed a significant change in firing rate in response to GAT-1 or GAT-3 blockade. The GAT-1 inhibitor, SKF-89976A, reduced firing in 6/12 cells and increased it in 5 cells; while the GAT-3 blocker, SNAP-5114, reduced firing in 5/14 cells, and increased it in 4. An example of a GPe neuron that responded to SNAP-5114 application with a decreased firing is shown in Fig. 4. The proportions of responders are shown in Fig. 5A.
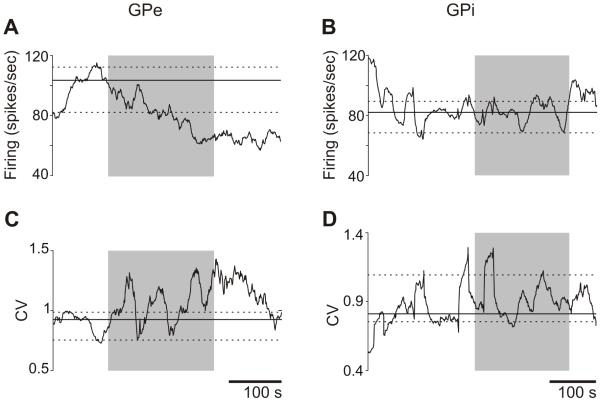
Fig. 4. Examples of changes in firing rates and coefficients of variation (CV), in a GPe and a GPi neuron after the local administration of a GAT-3 blocker.
The shaded area indicates the period of drug injection (SNAP-5114, 515 ng/1 μl). The solid black lines indicate the median firing rates or CV values at baseline (60 s before start of drug infusion), along with the corresponding 90th and 10th percentiles (dashed lines). The GPe cell responded to the drug infusion with a reduction in firing rate, and an increase in CV, while the GPi cell did not respond.
Fig. 5. Proportions of responses of pallidal cells to GATs blockade.
GPe (A) and GPi (B) cells showing no effect, increased or decreased firing rates after administration of the GAT-1 blocker SKF-89976A (744 ng/μl), or the GAT-3 blocker SNAP-5114 (515 ng/μl). Significant differences (using Chi-square tests) were found in the distribution of SKF-89976A-induced responses of GPi neurons between normal and MPTP-treated monkeys (* p=0.02). The data from normal monkeys are from Galvan et al. (2005).
These effects of SKF-89976A and SNAP-5114 on the firing rate of GPe cells were not significantly different from those observed in GPe of normal monkeys, as shown in Fig. 5A. We found that GPe neurons with low firing rates at baseline responded with larger changes after GAT-3 blockade than cells with high baseline firing rates (Pearson Correlation = −0.552, p = 0.041).
Effects of GAT-1 or GAT-3 blockade in the GPi of MPTP-treated monkeys
SKF-89976A and SNAP-5114 were less effective in altering the firing of GPi than GPe cells (see summary in Fig. 5B). Blockade of GAT-1 with SKF-89976A produced a decrease in firing in only 1/6 cells (and no increase), while treatment with the GAT-3 blocker SNAP-5114 lead to decreased firing in 3/10 cells and increased firing in 1/10 cells, as shown in the example in Fig. 4.
These results were strikingly different to those in normal monkeys, in which all of the tested GPi cells responded to the blockade of GAT-1 or GAT-3 transporters with changes in firing rate (Fig. 5B).
We found a significant difference in the proportions of responders to the GAT-1 inhibitor (SKF-89976A) between normal and MPTP-treated GPi neurons (p = 0.02), and a tendency towards a significant difference (p = 0.06) in GPi neurons treated with the GAT-3 blocker SNAP-5114.
Changes in coefficient of variations after SKF-89976A or SNAP-5114
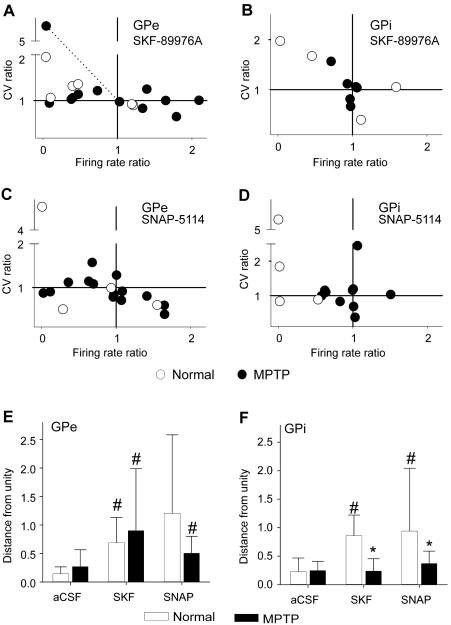
In addition to the rate changes evoked by GAT-1 or GAT-3 blockade, we analyzed the drug effects on the CV of inter-spike intervals (ISIs), to assess whether the variability of firing was influenced by GAT blockade, regardless of changes in firing rate. While changes in CV accompanied changes in firing rates in some cases (see the example in Fig. 4A), the plots in Fig. 6 show that changes in CV and firing rates were largely independent from one another and frequently displayed opposite polarities. However, the proportions of GPe or GPi neurons with increases, decreases or no changes in CV after SKF-8976A or SNAP-5114 was not significantly different between normal and MPTP-treated groups (not shown). As a measure of the overall magnitude of drug effects, regardless of its polarity, we calculated the distances to a point at which the firing rate and CV ratios were both at unity. These measurements are shown in Fig. 6 (bottom panels). In normal monkeys, infusions of GAT-1 and GAT-3 blockers in GPe and GPi had a significant effect compared to aCSF treatment (except for normal GPe cells treated with SNAP-5114, likely due to the large variability of responses in this small group of recorded cells). In the GPe of MPTP-treated monkeys, the effect of both GAT blockers was similar to normal animals; that is, the effects were different from control aCSF injections. However, in the GPi of MPTP treated monkeys neither of the two GAT blockers had significant effects when compared to control aCSF injections.
Fig. 6. MPTP-induced changes in the effects of GAT blockers on the activity of pallidal neurons.
Shown are ratios of changes in firing rates and coefficients of variation (CV) in response to GAT-1 (SKF-89976A) and GAT-3 (SNAP-5114) blockers. The two bottom panels show means ± SD of distances from each data point to the data point at which the CV ratio and firing rate ratio was 1. An example of how these distances were calculated is represented by the dotted line in the first panel. #, Different from the respective aCSF at p 0.05; and *, different from the normal state at p < 0.05.
DISCUSSION
We found that GAT-1 and GAT-3 in GPe and GPi display a similar pattern of distribution in normal and parkinsonian conditions. The blockade of GAT-1 and GAT-3 affects most GPe cells in either condition, while the effects of GAT-1 or GAT-3 blockade in GPi neurons were less pronounced in parkinsonian than in normal monkeys, suggesting that in parkinsonism GATs may be less active in GPi.
Immunohistochemical localization of GATs in GP
Our immunohistochemical data in this and our previous study (Galvan et al., 2005) indicate that GAT-1 and GAT-3 are heavily expressed in both segments of the primate pallidum. The robust immunostaining of GAT-3 in GPe and GPi compared with the weak expression of this transporter in the putamen is consistent with the massive arborization of striatopallidal axons in either pallidal segment (Kita, 2007, Nambu, 2007), compared with the more modest intrastriatal GABAergic innervation (Wilson, 2007) GAT-1 is primarily found in neuronal elements (axonal segments and a few terminals), and in glial processes in both segments of the pallidum, while GAT-3 is expressed almost exclusively in glia. Except for a reduction in the relative percentage of GAT-3-positive unmyelinated axons in the GPi, the distributions of GAT-1 and GAT-3 labeled elements was generally similar between normal and MPTP-treated monkeys
Various neurochemical markers of GABAergic transmission (i.e., GAD mRNA, GABA receptor binding, and GABA levels) are altered in parkinsonism (see Introduction), supporting the notion that GABAergic transmission is increased in GPe and decreased in GPi (as reviewed in Galvan and Wichmann, 2007). As the expression and function of GATs are modulated by the extracellular GABA concentration in other brain regions and in cell expression systems (Bernstein and Quick, 1999, Hu and Quick, 2008, Whitworth and Quick, 2001), we hypothesized that GAT expression or function would be increased in the GPe and decreased in the GPi of parkinsonian animals compared to controls. However, we found no significant changes in the intensity of pallidal immunostaining for GAT-1 or GAT-3 at the light microscopic level, nor did we find any major alterations in the overall pattern of GAT-1 and GAT-3 localization at the ultrastructural level between normal and MPTP-treated monkeys.
It has been proposed that neurotransmitter transporters can enforce or reduce synaptic independence by curtailing the overflow of transmitter from sites of release to extrasynaptic receptors, or promoting cooperation between nearby release sites (Overstreet and Westbrook, 2003, Sonders et al., 2005). The finding that GAT expression in GABAergic terminals is very low in the primate globus pallidus suggests that the uptake of GABA occurs at sites that are distant from its synaptic release, thereby allowing diffusion of GABA from the synaptic release sites to extrasynaptic GABA-A and GABA-B receptors (Charara et al., 2005). However, because the extent of extrasynaptic diffusion of neurotransmitter relies on complex structural, biophysical and neurochemical factors (Sykova and Nicholson, 2008) that have not been addressed in this study, our findings do not allow a precise assessment of the degree of non-synaptic GABAergic neuronal communication in the monkey GP. On the other hand, the elaborated wrapping of axo-dendritic complexes by GAT-3-immunoreactive glial processes suggests a GAT-3-mediated regulation of GABA diffusion between functional axo-dendritic complexes, while allowing GABA to spread across nearby synapses within individual complexes.
It is important to keep in mind that changes in the ultrastructural localization of GATs in parkinsonian animals may be more pronounced than our data suggest, but cannot be fully assessed because of the limited spatial resolution of the peroxidase method used in our study. The use of pre- and post-embedding immunogold methods to localize GATs could reveal additional changes in their pattern of subcellular distribution (Galvan et al., 2006). Another limitation of the chosen experimental approach is that it does not allow us to study dynamic changes in the trafficking of transporters between cytoplasm and cell surface. However, the importance of this factor has been demonstrated in previous studies of rat hippocampal cultures in which elevated extracellular GABA levels significantly slowed the internalization of GAT-1, thereby increasing its surface expression during active GABAergic activity (Bernstein and Quick, 1999).
Effects of GAT-1 and GAT-3 blockade in GPe
Blockade of GAT-1 and GAT-3 decreased the firing rate of a large proportion of GPe neurons in MPTP-treated monkeys, most likely as a consequence of increased GABA in the extracellular space, and enhanced activation of postsynaptic GABA receptors. Another way by which increased GABA spillover may decrease pallidal activity is by activation of presynaptic GABA-B receptors on glutamatergic terminals, as has recently been demonstrated in vitro in the rat GP (Jin and Smith, 2009).
In other pallidal cells, GAT-1 and GAT-3 blockade increased the firing rate. Conceivably, this may result from activation of presynaptic GABA-B autoreceptors which, in turn, could reduce GABA release and increase the firing rate of the recorded pallidal neurons. However, this possibility is unlikely because of the scarcity of GABA-B autoreceptors in the monkey GP (Charara et al., 2005), and because an increase in pallidal GABA levels that is high enough to activate presynaptic GABA-B receptors should also activate postsynaptic GABA-A receptors, and thereby inhibit pallidal neurons. A more plausible explanation for the increase in firing after GAT blockade is that the increased GABA levels induced by GAT blockade may have affected neighboring GP neurons more strongly than the recorded neurons themselves, thereby reducing ‘lateral’ inhibition of the recorded neurons (Sadek et al., 2007, Sato et al., 2000, Shink and Smith, 1995).
In summary, blockade of GAT-1 and GAT-3 resulted in heterogeneous effects on the firing of GPe neurons, suggesting that the mechanisms triggered by GABA uptake blockade are not only a mere increase in postsynaptic GABA-A mediated inhibition, induced by enhanced GABA concentration. The similarity of responses of GPe neurons to GAT-1 or GAT-3 blockade in parkinsonian animals compared with our previous results in normal monkeys suggests that the MPTP treatment did not substantially alter the functions of these transporters in GPe. Along with our anatomical data, these findings suggest that the uptake of GABA is not strongly affected in GPe under parkinsonian conditions.
Effects of GAT-1 and GAT-3 blockade in GPi
The activity of GPi neurons remained largely unaffected by SKF-89976A or SNAP-5114 treatments in MPTP-treated monkeys. Even the combined measurement of changes in the CV and firing rates was not different from aCSF injections, confirming that the two blockers had weak or no significant effect on GPi cells under these conditions. These results are different from those gathered previously in normal animals in which both blockers (used at the same concentrations as used in this study) frequently affected the activity of GPi cells.
Several possibilities can explain the differences between normal and MPTP-treated animals. One possibility would be that GABA receptors are down-regulated in GPi of parkinsonian monkeys. However, this is unlikely because an upregulation of GABA-A and GABA-B receptor expression has been demonstrated in the GPi of dopamine-depleted primates and other animals (see Introduction). Moreover, we have found that GABA-A and GABA-B receptor agonists strongly inhibit the firing of GPi neurons in MPTP-treated monkeys (Galvan et al., 2009). Another possibility is that dopamine depletion induced changes in the pharmacological properties of GAT-1 and GAT-3 in the GPi of MPTP-treated monkeys, resulting in a reduced sensitivity, or in an overall reduction of effectiveness of GABA reuptake. Finally, the minor changes found in the distribution of GAT-3 in the GPi of MPTP-treated monkeys could also play a role in these functional differences.
The most parsimonious way to reconcile our physiological and anatomical data in normal and dopamine-depleted animals is that GATs have a similar expression level and ultrastructural distribution in both segments of the GP after dopaminergic depletion, but that these transporters are less effective in GPi. This could be due to increased internalization, or altered pharmacological properties of the transporters or the receptors. If this is the case, this may represent a mechanism to compensate for diminished release of GABA from striatal and GPe terminals in parkinsonism. However, given the fact that the monkeys presented clear parkinsonian signs, this compensatory mechanism was, at best, partially effective in preventing behavioral deficits.
In summary, despite the lack of obvious changes in the distribution and expression of GATs in the pallidum of parkinsonian animals, the function of these transporters is reduced in the GPi of parkinsonian animals compared with the normal state.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yuxian Ma, Jean-Francois Pare and Susan Jenkins for excellent technical support. This work was supported by NIH grants R01-NS042937 (Y.S.) and RR-00165 (Yerkes Center base grant).
ABBREVIATIONS
- aCSF
Artificial cerebro-spinal fluid
- CV
Coefficient of variation
- GABA
γ-amino-butyric acid
- GAD
Glutamic acid decarboxylase
- GAT
GABA transporter
- GAT-1
GABA transporter type 1
- GAT-3
GABA transporter type 3
- GP
Globus pallidus
- GPe
External segment of globus pallidus
- GPi
Internal segment of globus pallidus
- MAP-2
Microtubule associated protein -2
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- TH
Tyrosine hydroxylase
- SN
Substantia nigra
- SNc
Substantia nigra pars compacta
- SNr
Substantia nigra pars reticulata
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bernstein EM, Quick MW. Regulation of gamma-aminobutyric acid (GABA) transporters by extracellular GABA. J. Biol. Chem. 1999;274:889–895. doi: 10.1074/jbc.274.2.889. [DOI] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem. Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur. J. Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Calon F, Goulet M, Blanchet PJ, Martel JC, Piercey MF, Bédard PJ, Di Paolo T. Levodopa or D2 agonist induced dyskinesia in MPTP monkeys: correlation with changes in dopamine and GABAA receptors in the striatopallidal complex. Brain Res. 1995;680:43–52. doi: 10.1016/0006-8993(95)00229-j. [DOI] [PubMed] [Google Scholar]
- Calon F, Morissette M, Goulet M, Grondin R, Blanchet PJ, Bedard PJ, Di Paolo T. 125I-CGP 64213 binding to GABA(B) receptors in the brain of monkeys: effect of MPTP and dopaminomimetic treatments. Exp. Neurol. 2000;163:191–199. doi: 10.1006/exnr.2000.7366. [DOI] [PubMed] [Google Scholar]
- Calon F, Morissette M, Rajput AH, Hornykiewicz O, Bedard PJ, Di Paolo T. Changes of GABA receptors and dopamine turnover in the postmortem brains of parkinsonians with levodopa-induced motor complications. Mov. Disord. 2003;18:241–253. doi: 10.1002/mds.10343. [DOI] [PubMed] [Google Scholar]
- Chadha A, Howell O, Atack JR, Sur C, Duty S. Changes in [3H]zolpidem and [3H]Ro 15-1788 binding in rat globus pallidus and substantia nigra pars reticulata following a nigrostriatal tract lesion. Brain Res. 2000;862:280–283. doi: 10.1016/s0006-8993(00)02081-3. [DOI] [PubMed] [Google Scholar]
- Charara A, Galvan A, Kuwajima M, Hall RA, Smith Y. An electron microscope immunocytochemical study of GABA(B) R2 receptors in the monkey basal ganglia: a comparative analysis with GABA(B) R1 receptor distribution. J. Comp. Neurol. 2004;476:65–79. doi: 10.1002/cne.20210. [DOI] [PubMed] [Google Scholar]
- Charara A, Pare JF, Levey AI, Smith Y. Synaptic and Extrasynaptic GABA-A and GABA-B receptors in the Globus Pallidus: an Electron Microscopic immunogold analysis in monkeys. Neuroscience. 2005;131:917–933. doi: 10.1016/j.neuroscience.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Chiu C-S, Jensen K, Sokolova I, Wang D, Li M, Deshpande P, Davidson N, Mody I, Quick MW, Quake SR, Lester HA. Number, Density, and Surface/Cytoplasmic Distribution of GABA Transporters at Presynaptic Structures of Knock-In Mice Carrying GABA Transporter Subtype 1-Green Fluorescent Protein Fusions. J. Neurosci. 2002;22:10251–10266. doi: 10.1523/JNEUROSCI.22-23-10251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby NO. Inhibition of [gamma]-aminobutyric acid uptake: anatomy, physiology and effects against epileptic seizures. Eur. J. Pharmacol. 2003;479:127–137. doi: 10.1016/j.ejphar.2003.08.063. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J. Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Putamen: activity of single units during slow and rapid arm movements. Science. 1973;179:1240–1242. doi: 10.1126/science.179.4079.1240. [DOI] [PubMed] [Google Scholar]
- Dhar TG, Borden LA, Tyagarajan S, Smith KE, Branchek TA, Weinshank RL, Gluchowski C. Design, synthesis and evaluation of substituted triarylnipecotic acid derivatives as GABA uptake inhibitors: identification of a ligand with moderate affinity and selectivity for the cloned human GABA transporter GAT-3. J. Med. Chem. 1994;37:2334–2342. doi: 10.1021/jm00041a012. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Brain Res. Mol.Brain Res. 1995;33:7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- Elias S, Ritov Y, Bergman H. Balance of increases and decreases in firing rate of the spontaneous activity of basal ganglia high-frequency discharge neurons. J. Neurophysiol. 2008;100:3086–3104. doi: 10.1152/jn.90714.2008. [DOI] [PubMed] [Google Scholar]
- Galeffi F, Bianchi L, Bolam JP, Della Corte L. The effect of 6-hydroxydopamine lesions on the release of amino acids in the direct and indirect pathways of the basal ganglia: a dual microdialysis probe analysis. Eur. J. Neurosci. 2003;18:856–868. doi: 10.1046/j.1460-9568.2003.02795.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Charara A, Pare JF, Levey AI, Smith Y. Differential subcellular and subsynaptic distribution of GABA(A) and GABA(B) receptors in the monkey subthalamic nucleus. Neuroscience. 2004;127:709–721. doi: 10.1016/j.neuroscience.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience. 2006;143:351–375. doi: 10.1016/j.neuroscience.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Nanda B, Hu X, Smith Y, Wichmann T. Changes in the subcellular localization and functions of GABA-B receptors in the Globus Pallidus of MPTP-treated monkeys. In: Groenewegen H, Voorn P, Berendse HW, Mulder AB, Cools AR, editors. The Basal Ganglia IX. Springer; 2009. [Google Scholar]
- Galvan A, Villalba RM, West SM, Maidment NT, Ackerson LC, Smith Y, Wichmann T. GABAergic Modulation of the Activity of Globus Pallidus Neurons in Primates: In Vivo Analysis of the Functions of GABA Receptors and GABA Transporters. J. Neurophysiol. 2005;94:990–1000. doi: 10.1152/jn.00068.2005. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. GABAergic circuits in the basal ganglia and movement disorders. Prog. Brain Res. 2007;160:287–312. doi: 10.1016/S0079-6123(06)60017-4. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin. Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths PD, Sambrook MA, Perry R, Crossman AR. Changes in benzodiazepine and acetylcholine receptors in the globus pallidus in Parkinson’s disease. J. Neurol. Sci. 1990;100:131–136. doi: 10.1016/0022-510x(90)90023-g. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hu J, Quick MW. Substrate-mediated regulation of gamma-aminobutyric acid transporter 1 in rat brain. Neuropharmacology. 2008;54:309–318. doi: 10.1016/j.neuropharm.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaki N, Saito N, Hashima M, Tanaka C. Production of specific antibodies against GABA transporter subtypes (GAT1, GAT2, GAT3) and their application to immunocytochemistry. Brain Res. Mol. Brain Res. 1994;26:47–54. doi: 10.1016/0169-328x(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Jin X, Smith Y. GABA transporters modulate glutamatergic transmission in the rat globus pallidus. Soc. Neurosci. Abstr. 2009;35:134–118. [Google Scholar]
- Johnston T, Duty S. Changes in GABA(B) receptor mRNA expression in the rodent basal ganglia and thalamus following lesion of the nigrostriatal pathway. Neuroscience. 2003;120:1027–1035. doi: 10.1016/s0306-4522(03)00418-4. [DOI] [PubMed] [Google Scholar]
- Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J. Membr. Biol. 2006;213:89–100. doi: 10.1007/s00232-006-0877-5. [DOI] [PubMed] [Google Scholar]
- Katz J, Nielsen KM, Soghomonian JJ. Comparative effects of acute or chronic administration of levodopa to 6-hydroxydopamine-lesioned rats on the expression of glutamic acid decarboxylase in the neostriatum and GABAA receptors subunits in the substantia nigra, pars reticulata. Neuroscience. 2005;132:833–842. doi: 10.1016/j.neuroscience.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Kita H. Globus pallidus external segment. Prog. Brain Res. 2007;160:111–133. doi: 10.1016/S0079-6123(06)60007-1. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Wichmann T. A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J. Neurosci. Methods. 2004;138:45–49. doi: 10.1016/j.jneumeth.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Laprade N, Soghomonian JJ. Gene expression of the GAD67 and GAD65 isoforms of glutamate decarboxylase is differentially altered in subpopulations of striatal neurons in adult rats lesioned with 6-OHDA as neonates. Synapse. 1999;33:36–48. doi: 10.1002/(SICI)1098-2396(199907)33:1<36::AID-SYN4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Nambu A. Globus pallidus internal segment. Prog. Brain Res. 2007;160:135–150. doi: 10.1016/S0079-6123(06)60008-3. [DOI] [PubMed] [Google Scholar]
- Ng CH, Wang XS, Ong WY. A light and electron microscopic study of the GABA transporter GAT-3 in the monkey basal ganglia and brainstem. J. Neurocytol. 2000;29:595–603. doi: 10.1023/a:1011076219493. [DOI] [PubMed] [Google Scholar]
- Ochi M, Shiozaki S, Kase H. Adenosine A(2A) receptor-mediated modulation of GABA and glutamate release in the output regions of the basal ganglia in a rodent model of Parkinson’s disease. Neuroscience. 2004;127:223–231. doi: 10.1016/j.neuroscience.2004.04.050. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. Synapse Density Regulates Independence at Unitary Inhibitory Synapses. J. Neurosci. 2003;23:2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 2000. [Google Scholar]
- Peters A, Palay S, Webster HD. The Fine Structure of the Nervous System. Oxford University Press; New York: 1991. [Google Scholar]
- Pifl C, Schingnitz G, Hornykiewicz O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience. 1991;44:591–605. doi: 10.1016/0306-4522(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Rasband W. ImageJ. U.S. National Insitutes of Health; Bethesda, MD: 1997-2009. [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J. Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Robertson RG, Clarke CA, Boyce S, Sambrook MA, Crossman AR. The role of striatopallidal neurones utilizing gamma-aminobutyric acid in the pathophysiology of MPTP-induced parkinsonism in the primate: evidence from [3H]flunitrazepam autoradiography. Brain Res. 1990;531:95–104. doi: 10.1016/0006-8993(90)90762-z. [DOI] [PubMed] [Google Scholar]
- Robertson RG, Graham WC, Sambrook MA, Crossman AR. Further investigations into the pathophysiology of MPTP-induced parkinsonism in the primate: an intracerebral microdialysis study of gamma-aminobutyric acid in the lateral segment of the globus pallidus. Brain Res. 1991;563:278–280. doi: 10.1016/0006-8993(91)91545-c. [DOI] [PubMed] [Google Scholar]
- Sadek AR, Magill PJ, Bolam JP. A Single-Cell Analysis of Intrinsic Connectivity in the Rat Globus Pallidus. J. Neurosci. 2007;27:6352–6362. doi: 10.1523/JNEUROSCI.0953-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Lavallee P, Levesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J. Comp. Neurol. 2000;417:17–31. [PubMed] [Google Scholar]
- Schroeder JA, Schneider JS. GABA-opioid interactions in the globus pallidus: [D-Ala2]-Met-enkephalinamide attenuates potassium-evoked GABA release after nigrostriatal lesion. J. Neurochem. 2002;82:666–673. doi: 10.1046/j.1471-4159.2002.01010.x. [DOI] [PubMed] [Google Scholar]
- Scorcioni R, Wright SN, Patrick Card J, Ascoli GA, Barrionuevo G. Point Analysis in Java applied to histological images of the perforant pathway: a user’s account. Neuroinformatics. 2008;6:63–67. doi: 10.1007/s12021-008-9011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Smith Y. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J. Comp. Neurol. 1995;358:119–141. doi: 10.1002/cne.903580108. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Chesselet MF. Effects of nigrostriatal lesions on the levels of messenger RNAs encoding two isoforms of glutamate decarboxylase in the globus pallidus and entopeduncular nucleus of the rat. Synapse. 1992;11:124–133. doi: 10.1002/syn.890110205. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Gonzales C, Chesselet MF. Messenger RNAs encoding glutamate-decarboxylases are differentially affected by nigrostriatal lesions in subpopulations of striatal neurons. Brain Res. 1992;576:68–79. doi: 10.1016/0006-8993(92)90610-l. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Pedneault S, Audet G, Parent A. Increased glutamate decarboxylase mRNA levels in the striatum and pallidum of MPTP-treated primates. J. Neurosci. 1994;14:6256–6265. doi: 10.1523/JNEUROSCI.14-10-06256.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonders MS, Quick M, Javitch JA. How did the neurotransmitter cross the bilayer? A closer view. Curr. Opin. Neurobiol. 2005;15:296–304. doi: 10.1016/j.conb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Sykova E, Nicholson C. Diffusion in Brain Extracellular Space. Physiol. Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp. Neurol. 2009;215:220–227. doi: 10.1016/j.expneurol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Ong WY. A light and electron microscopic study of GAT-1 in the monkey basal ganglia. J. Neurocytol. 1999;28:1053–1061. doi: 10.1023/a:1007056608820. [DOI] [PubMed] [Google Scholar]
- Whitworth TL, Quick MW. Substrate-induced regulation of gamma-aminobutyric acid transporter trafficking requires tyrosine phosphorylation. J. Biol. Chem. 2001;276:42932–42937. doi: 10.1074/jbc.M107638200. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp. Neurol. 2001;167:410–424. doi: 10.1006/exnr.2000.7572. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog. Brain Res. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]
- Windels F, Carcenac C, Poupard A, Savasta M. Pallidal origin of GABA release within the substantia nigra pars reticulata during high-frequency stimulation of the subthalamic nucleus. J. Neurosci. 2005;25:5079–5086. doi: 10.1523/JNEUROSCI.0360-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumi M, Sato K, Shimada S, Nishimura M, Tohyama M. Regional distribution of GABA transporter 1 (GAT1) mRNA in the rat brain: comparison with glutamic acid decarboxylase67 (GAD67) mRNA localization. Brain Res. Mol. Brain Res. 1997;44:205–218. doi: 10.1016/s0169-328x(96)00200-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.