Abstract
C1q nephropathy is a rare kidney disease that can present with nephrotic syndrome and typically has the histological phenotype of either minimal change disease (MCD) or focal segmental glomerulosclerosis (FSGS). Disagreement exists as to whether it is a distinct immune complex-mediated glomerulopathy or whether it resides in the spectrum of FSGS-MCD. Two African American patients with C1q nephropathy histologically presenting as the collapsing variant of FSGS (collapsing C1q nephropathy) and rapid loss of kidney function were genotyped for polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9). Both cases were homozygous for the MYH9 E1 risk haplotype; the variant strongly associated with idiopathic FSGS, collapsing FSGS in Human Immunodeficiency Virus-associated nephropathy and focal global glomerulosclerosis (historically attributed to hypertensive nephrosclerosis). Collapsing C1q nephropathy with rapid progression to ESRD appears to reside in the MYH9-associated disease spectrum.
Keywords: African American, C1q nephropathy, collapsing variant, focal segmental glomerulosclerosis, HIVAN, MYH9
Idiopathic C1q nephropathy with nephrotic syndrome is an uncommon form of steroid-resistant kidney disease that is most often reported in children and young adults.1,2 In one of two series,1 African Americans constituted the majority of patients. We report two patients with C1q nephropathy presenting with the histological phenotype of FSGS, collapsing variant, also known as collapsing glomerulopathy, who rapidly progressed to end-stage renal disease (ESRD). The potentially related disorder known as idiopathic FSGS, collapsing variant,also demonstrates African American ethnic predisposition, steroid-resistant nephrotic syndrome, and rapid progression to ESRD, and is seen in patients lacking Human Immunodeficiency Virus (HIV) infection.3 Since C1q nephropathy can occur as collapsing C1q nephropathy, we hypothesized that diverse forms of FSGS, collapsing variant (e.g., idiopathic, HIV-associated nephropathy [HIVAN] and C1q-associated) might share a common risk factor(s).
Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) underlie 40-45% of cases of ESRD in African Americans; 70% of cases with non-diabetic forms of ESRD (idiopathic FSGS, HIVAN) and focal global glomerulosclerosis historically labeled hypertensive nephrosclerosis), and approximately 16% of type 2 diabetes-associated ESRD.4-8 The prevalence of MYH9 E1 risk haplotype homozygosity is 36% in African Americans and 1% in European Americans; with approximately 60% of African Americans and 4% of European Americans inheriting one copy of the risk haplotype.4-8 Herein, two African American patients with collapsing C1q nephropathy were genotyped for single nucleotide polymorphisms (SNPs) that make up the MYH9 E1 risk haplotype.
Case Reports
Case 1
A 42 year old HIV negative African American man with no family history of kidney disease presented with the nephrotic syndrome (18 gm/day proteinuria), hypertension, and serum creatinine concentration 1.9 mg/dL (168 μmol/L) four years after donating a kidney to a friend. Prior to donation he was normotensive with serum creatinine 1.2 mg/dL (106.1 μmol/L) and urine protein-creatinine ratio 0.02 gm/gm. A laparoscopic kidney biopsy containing 78 glomeruli revealed focal segmental glomerular scarring; many glomeruli showed collapse of individual tuft lobules retaining their individuality with hyperplasia/hypertrophy of the overlying podocytes that displayed intraepithelial cell vacuoles containing protein reabsorption droplets, a pattern characteristic of collapsing glomerulopathy.9 A lymphocytic interstitial infiltrate was present as were occasional cystically dilated tubules. Immunofluorescence microscopy revealed granular predominantly mesangial IgA (1+), IgM (3+), C1q (4+), and kappa light chain (2+) deposits. There was no staining for IgG or lambda light chain. The only glomerulus available for electron microscopic examination displayed ischemic collapse (as opposed to collapsing glomerulopathy), the glomerular basement membrane was thickened and corrugated and not surprisingly there were no immune complex-type electron dense deposits; there were no endothelial tubuloreticular inclusions. He required kidney transplant within 2 years, despite treatment with oral prednisone 60 mg daily for 5 months.
Case 2
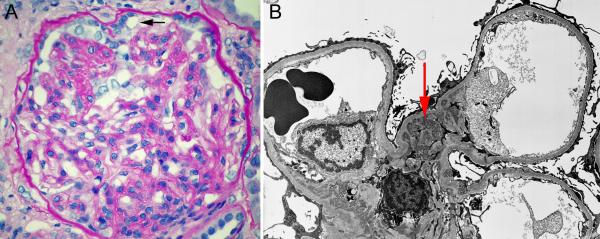
A 42 year old HIV negative gravida 2 para 2 African American female had a kidney biopsy after presenting with nephrotic syndrome (11 gm/day proteinuria) and serum creatinine concentration 2.4 mg/dL (212.2 μmol/L). Past history was significant for childhood obesity and “mild proteinuria” detected at age 21 that increased transiently during both pregnancies. A kidney biopsy contained 10 glomeruli; 5 were obsolescent and the remainder revealed segmental tuft sclerosis with at least one showing the pattern of collapsing glomerulopathy described above. Immunofluorescence microscopy revealed segmental mesangial deposits: IgG (2+), IgM (0.5+), and C1q (4+). electron microscopic examination confirmed the presence of massive mesangial immune complex-type electron-dense deposits without endothelial tubuloreticular inclusions (Figure 1). Kidney function deteriorated despite treatment with oral prednisone 120 mg every other day, followed by tacrolimus. She started hemodialysis less than one year after biopsy.
Figure 1.
Kidney biopsy findings for case 2. A. Photomicrograph of biopsy demonstrating the pattern of focal segmental glomerulosclerosis, collapsing variant. Note that collapsed lobules retain their individuality and are associated with hypertrophied/hyperplastic podocytes with intracytoplasmic vacuoles (arrow) some of which contain protein reabsortion droplets. (PAS stain. Original magnification 400X).
B. Electron micrograph of biopsy. Note the prominent mesangial immune complex-type electron-dense deposits (arrow). (Uranyl acetate– Lead citrate. Original magnification 3300X).
Three MYH9 E1 risk haplotype SNPs (rs4821480, rs4821481, rs3752462) were genotyped on the Sequenom Mass Array (www.sequenom.com). The fourth SNP (rs2032487) was imputed10 with a confidence score of 99.8%. Both cases were homozygous for risk alleles in all 4 MYH9 E1 SNPs (the identity of the nucleotide at each position was G, C, C, and T, respectively; referred to hereafter as the GCCT haplotype).
Discussion
We report two cases of collapsing C1q nephropathy with rapid progression to ESRD in African Americans homozygous for the GCCT MYH9 E1 risk haplotype. MYH9 gene expression is observed in the podocytes, peritubular capillaries’ endothelium and tubular epithelium.11 Although the pathogenesis remains unclear, MYH9-associated kidney disease may result from abnormal actin filament movement in podocytes with progressive cytoskeletal injury resulting in FSGS, FSGS collapsing variant, or focal global glomerulosclerosis. Alternatively, platelet activation may damage the glomerular capillaries, as platelets abnormalities are present in the MYH9-associated hematologic disorders May Hegglin anomaly, Fechtner, Epstein and Sebastian Syndromes.12 Strong associations exist between the MYH9 gene and multiple related kidney diseases, particularly in African Americans, including focal global glomerulosclerosis historically labeled as hypertensive ESRD, idiopathic FSGS, HIVAN, and approximately 16% of clinically diagnosed cases of type 2 diabetes-associated nephropathy.4-7 The Kopp et al. report 4 initially demonstrated the role of MYH9 in the histologically-related diseases idiopathic FSGS (characterized by podocyte depletion) and HIV-associated collapsing glomerulopathy (characterized by podocyte proliferation). However, MYH9 is strongly associated with FSGS and CKD in European-derived populations, as well.4,13
Jennette and Hipp 1 and Iskandar et al. 2 reported that C1q nephropathy is an immune complex-mediated glomerulopathy, whereas Markowitz et al. posit that the immunofluorescence microscopy and electron microscopic examination features of C1q nephropathy are the result of nonspecific deposition and that therefore C1q nephropathy falls along the spectrum of MCD-FSGS.14 The absence of immune complex-type electron-dense deposits in Case 1 was likely due to the fact that the only glomerulus available for electron microscopic examination showed ischemic collapse. These present cases suggest that collapsing C1q nephropathy is an MYH9-associated kidney disease. Notably, endothelial tubuloreticular inclusions and HIV infection were absent in both cases and neither patient had serologic or clinical manifestations of systemic lupus erythematosus. The similar histopathology of C1q collapsing nephropathy and HIV-associated collapsing glomerulopathy alerted us to the potential role of a common inciting factor. The series of Markowitz et al. included 6 cases of collapsing C1q nephropathy, none of which had the rapidly progressive course of collapsing glomerulopathy.14 In that respect, their cases are atypical. Although we could only evaluate two cases of this uncommon disorder, MYH9 appears to be a common genetic susceptibility factor underlying collapsing forms of both C1q nephropathy and HIVAN. Although we believe that C1q nephropathy is an immune complex-mediated disease, we are aware that others believe otherwise.14 In the present setting, we do not feel that immune complexes activating complement through the classical pathway, hence the presence of C1q per se, played a role in the association reported. Rather, it is the particular histologic phenotype of these two cases that links them to the genotype of interest.
We demonstrate homozygosity for common risk variants in the MYH9 gene in two patients with collapsing C1q nephropathy. This finding appears to extend the spectrum of MYH9-associated kidney diseases in African Americans to include collapsing C1q nephropathy, as well as suggest that collapsing C1q nephropathy resides in the FSGS-global glomerulosclerosis spectrum of kidney diseases.
Acknowledgements
Support: This work was supported by National Institutes of Health grants RO1 DK 070941 and RO1 DK084149 (both to Dr Freedman) and RO1 DK53591 (Dr Bowden).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Jennette JC, Hipp CG. C1q nephropathy: a distinct pathologic entity usually causing nephrotic syndrome. Am J Kidney Dis. 1985;6:103–110. doi: 10.1016/s0272-6386(85)80150-5. [DOI] [PubMed] [Google Scholar]
- (2).Iskandar SS, Browning MC, Lorentz WB. C1q nephropathy: a pediatric clinicopathologic study. Am J Kidney Dis. 1991;18:459–465. doi: 10.1016/s0272-6386(12)80114-4. [DOI] [PubMed] [Google Scholar]
- (3).Valeri A, Barisoni L, Appel GB, Seigle R, D’Agati V. Idiopathic collapsing focal segmental glomerulosclerosis: a clinicopathologic study. Kidney Int. 1996;50:1734–1746. doi: 10.1038/ki.1996.493. [DOI] [PubMed] [Google Scholar]
- (4).Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75:736–745. doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Freedman BI, Hicks PJ, Bostrom MA, et al. Non-muscle myosin heavy chain 9 gene MYH9 associations in African Americans with clinically diagnosed type 2 diabetes mellitus-associated ESRD. Nephrol Dial Transplant. 2009;24(11):3366–3371. doi: 10.1093/ndt/gfp316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lipkowitz MS, Iyengar S, Molineros J, Langefeld CD, Comeau ME, Klotman PE, Bowden DW, Freeman RG, Khitrov G, Zhang W, Kao WHL, Parekh RS, Choi M, Kopp JB, Winkler CA, Nelson G, Freedman BI, Bottinger EP, AASK Investigators Association analysis of the non-muscle myosin heavy chain 9 gene (MYH9) in hypertensive nephropathy: African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephol. 2009;20:56A. [Google Scholar]
- (9).Schwimmer JA, Markowitz GS, Valeri A, Appel GB. Collapsing glomerulopathy. Semin Nephrol. 2003;23:209–218. doi: 10.1053/snep.2003.50019. [DOI] [PubMed] [Google Scholar]
- (10).Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- (11).Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65–74. doi: 10.1681/ASN.V13165. [DOI] [PubMed] [Google Scholar]
- (12).Pecci A, Malara A, Badalucco S, et al. Megakaryocytes of patients with MYH9-related thrombocytopenia present an altered proplatelet formation. Thromb Haemost. 2009;102:90–96. doi: 10.1160/TH09-01-0068. [DOI] [PubMed] [Google Scholar]
- (13).Pattaro C, Aulchenko YS, Isaacs A, et al. Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney Int. 2009;76:297–306. doi: 10.1038/ki.2009.135. [DOI] [PubMed] [Google Scholar]
- (14).Markowitz GS, Schwimmer JA, Stokes MB, et al. C1q nephropathy: a variant of focal segmental glomerulosclerosis. Kidney Int. 2003;64:1232–1240. doi: 10.1046/j.1523-1755.2003.00218.x. [DOI] [PubMed] [Google Scholar]