Summary
Advances in multiple myeloma support the notion that the associated bone disease, characterized by increased osteoclastogenesis and suppressed osteoblastogenesis, is both a consequence and necessity of tumour progression. Osteoblastogenesis is suppressed by secreted inhibitors and dysregulation of cell-surface “coupling” factors on osteogenic cells. Osteoclastogenesis is increased as a consequence of osteoblast deactivation and of production of osteoclast-activating factors. Osteoclasts express soluble and cell-surface factors that stimulate myeloma growth, while osteoblasts produce bone-building factors that restrain growth of myeloma cells that are dependent on the microenvironment; detailed molecular mechanisms are discussed. Experimental and clinical findings indicate that pharmacological and experimental osteoblast-activating agents that effectively promote bone formation also reduce growth of myeloma cells within bone, seemingly by simultaneously stimulating osteoblastogenesis and restraining osteoclastogenesis. Unravelling mechanisms of myeloma bone disease expands horizons for developing novel interventions and also facilitates better understanding of the association between induction of osteolysis and disease progression.
Keywords: myeloma, osteoblasts, osteoclasts, Wnt, proteasome
Introduction
During the recent decade, an explosion of studies investigated the role of the bone marrow (BM) microenvironment in the pathogenesis of multiple myeloma (MM). These studies revealed that, while cells of hematopoietic lineage seem to support MM progression (Yaccoby et al, 2004; Kukreja et al, 2006; Chauhan et al, 2009; Zheng et al, 2009; Nakayama et al, 2004), certain cells of mesenchymal origin may restrain myeloma cell growth (Yaccoby et al, 2006; Li et al, 2008). Exceptional are neovascular endothelial cells, which are increased in number in myelomatous BM (Vacca et al, 1994), but their role in disease progression is not well delineated. Due to its unique growth pattern and the defined form of benign disease, MM is an excellent model for studying tumour–microenvironment interactions.
MM is unique among most hematologic malignancies because of its induction of bone disease. In addition, while in many other bone-associated malignancies (e.g. prostate and breast cancer) tumor growth is associated with increased numbers of osteoclasts and osteoclasts, osteolytic bone resorption in MM is caused by stimulation of osteoclastogenesis and suppression of osteoblastogenesis in areas adjacent to tumour foci (Bataille et al, 1991; Barille-Nion & Bataille, 2003; Roodman, 2009; Sezer, 2009). This phenomenon supports the notion that bone disease is both a consequence and necessity of MM progression and metastasis, which is frequently described as a “vicious cycle” between tumour cells and their surrounding cellular environment.
Factual analysis of focal and bone lesions in patients with active MM is achieved by combining computed tomography (CT) with novel, sensitive imaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) (Walker et al, 2007; Bartel et al, 2009). These imaging techniques and histomorphometric analyses revealed that changes in bone microstructures are typical characteristics of focal lesions and also are evident in patients with benign disease and in those with early-stage MM (Drake M, 2009). Interestingly, novel drugs that are exceptionally effective at clinically controlling MM, such as the immunomodulatory agents (IMiDs) thalidomide and lenalidomide and the proteasome inhibitor bortezomib, seem to affect osteoclast and osteoblast activity in myelomatous bones (Zangari et al, 2005; Terpos et al, 2007; Sezer, 2009). Moreover, molecular classification of patients’ plasma cells, based on global gene expression profiling, identified a unique group of patients characterized by low level of bone disease and revealed that these patients enjoyed favourable event-free survival (Zhan et al, 2006). These clinical findings suggest that continued dissemination of myeloma cells is facilitated by myeloma cell interactions with bone cells within focal lesions.
This review focuses on the effects of bone cells—bone-destroying osteoclasts and bone-building osteoblasts—on MM progression. While the reciprocal interactions between myeloma-induced osteoclastogenesis and osteoclast-induced myeloma growth have been extensively studied, emerging data are revealing the molecular mechanisms of myeloma-induced suppression of osteoblastogenesis and of osteoblasts’ regulation of myeloma cell growth.
Activation of osteoclastogenesis in myeloma
The molecular mechanisms by which myeloma cells stimulate osteoclast activity are multifactorial and involve osteoclast-differentiation and -survival factors that are produced by microenvironmental cells and myeloma cells (Figure 1). Several osteoclastogenic factors that are highly produced in myelomatous bone have been shown in vitro and/or in vivo to be directly involved in MM-induced osteoclast activity: receptor activator of NF-κB ligand (RANKL) (Pearse et al, 2001), inflammatory protein-1 alpha (MIP1α) (Choi et al, 2000; Han et al, 2001), interleukin (IL)-3 (Lee et al, 2004), and stroma-derived factor (SDF)-1 (Zannettino et al, 2005). Other factors, including thrombospondin-1 (TSP-1) (Kukreja et al, 2009), IL-17 (Dhodapkar et al, 2008), and inflammatory cytokines such as IL-6 and tumor necrosis factor alpha (TNFα) (for review see Ehrlich & Roodman, 2005), that are associated with bone remodeling have increased levels in BM and/or blood of patients with myeloma, but their roles in this process require further validation.
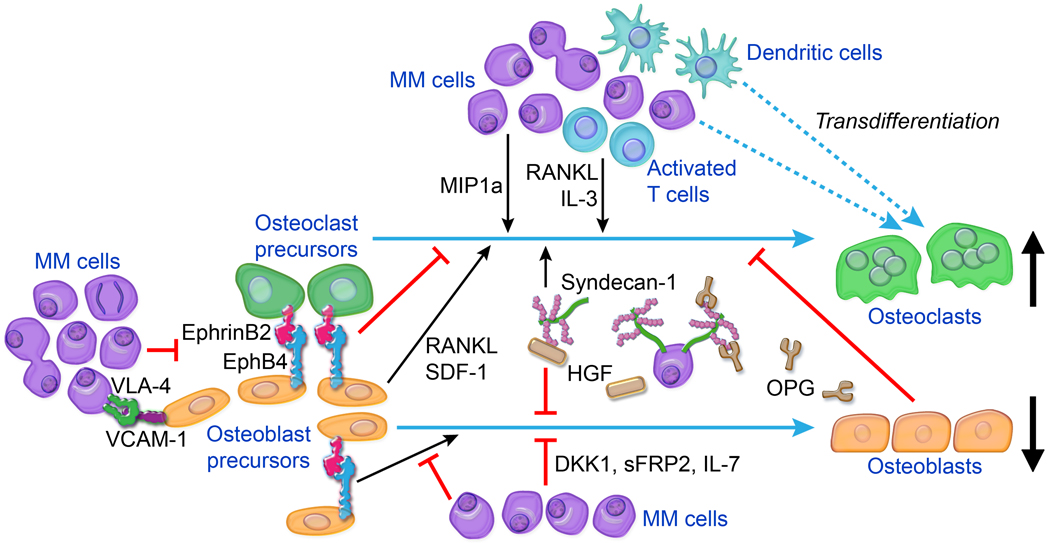
Figure 1.
Main cellular and molecular factors associated with MM-induced bone disease. Osteolytic lesions are induced by uncoupling of bone metabolism, resulting in increased bone resorption by osteoclasts and reduced bone formation by osteoblasts. Interactions between MM cells and various BM cells (e.g., osteoblast and osteoblast precursors, activated T lymphocytes, dendritic cells) result in increased production of osteoclast-activating factors (e.g., RANKL, IL-3, MIP1α) and/or osteoblast-inactivating factors (e.g., DKK1, sFRP2, IL-7). OPG levels in lytic lesions are low as a consequence of reduced osteoblast numbers and of OPG internalization and degradation by MM cells in a mechanism mediated by binding to syndecan-1 on MM cell surfaces. Syndecan-1 molecules shed from MM cells also bind and localize factors such as HGF that act on bone cells to promote osteolysis. Osteoblastogenesis is impaired by cell–cell contact mediated by VCAM-1 on MM cells and VLA-4 on osteoblast precursors and also by dysregulation of cell-surface coupling factors such as ephrinB2 and EphB4 on osteoblast precursors. Osteoclasts may form by MM cells fusing together or by dendritic cells fusing with osteoclast precursors, contributing to increased numbers of osteoclasts. Other cellular elements of BM, such as megakaryocytes, macrophages, and Th17 T lymphocytes, may also be cultivated by MM to promote bone disease (see text for details).
Roles of osteoblasts
Myeloma cells’ cultivation of BM stromal cells or mesenchymal stem cells (MSCs) is a central mechanism associated with increased osteoclastogenesis in MM. Pearse et al (Pearse et al, 2001), followed by others (Giuliani et al, 2001; Sezer et al, 2003), demonstrated that myeloma cells induce stromal cells to upregulate osteoclastogenic factor RANKL and to downregulate RANKL-decoy receptor osteoprotegerin (OPG). Further detailed studies revealed that the ratio of RANKL-to-OPG expressed by osteogenic cells reflects osteoblast differentiation: as osteoblasts mature, their production of RANKL is reduced and OPG is increased (Glass et al, 2005; Spencer et al, 2006;Qiang et al, 2008b). Recently, Qiang et al (Qiang et al, 2008a) demonstrated that myeloma cell production of Wnt antagonist dickkopf 1 (DKK1) abrogates the ability of the canonical Wnt ligand, Wnt3a, to stabilize β-catenin and commit immature cells to osteoblastogenesis. As a result, osteoprogenitor cells increased expression of RANKL and reduced expression of OPG, ultimately increasing RANKL/OPG ratios. Immature osteogenic cells and other mesenchymal elements in BM are also significant sources of IL-6 (Gunn et al, 2006) and SDF-1 (Kortesidis et al, 2005), which may be indirectly involved in inducing local bone resorption in osteolytic lesions (Sandhu et al, 1999; Zannettino et al, 2005). These studies suggest that suppression of osteoblastogenesis contributes to increased osteoclast activity in MM lytic lesions.
Roles of hematopoietic cells
Hematopoietic cells of various lineages are expanded in or recruited to BM focal lesions and contribute to osteoclastogenesis in MM. Myeloma cells attract osteoclast precursors and macrophages (Yaccoby et al, 2004; Zheng et al, 2009), which produce high levels of IL-8, a chemokine that directly promotes osteoclast formation (Bendre et al, 2003). RANKL and IL-3 are produced by activated T lymphocytes in BM of patients with MM (Giuliani et al, 2002; Giuliani et al, 2006). IL-3 is also expressed by myeloma cells, which enhances the effects of RANKL and MIP1α on stimulating osteoclast formation (Lee et al, 2004).
Recent studies suggest that myeloma cells are infiltrated by dendritic cells (DCs) and that DCs contribute to increased osteoclastogenesis and myeloma cell growth (Kukreja et al, 2006; Kukreja et al, 2009; Chauhan et al, 2009). DCs produce RANKL (Wong et al, 1999), and plasmacytoid DC interactions with myeloma cells results in upregulation of other osteoclastogenic factors, such as IL-3, SDF-1, CD40L, and TNFα (Chauhan et al, 2009). Interestingly, upon engagement with myeloma cells, DCs produce increased levels of TSP-1, which mediates DC transdifferentiation into bone-resorbing osteoclasts (Kukreja et al, 2009). DCs also induce expansion of IL-17-producing T lymphocytes (Dhodapkar et al, 2008), which may promote osteoclast formation via their release of IL-17 (Sato et al, 2006). Expanded megakaryocytes are also detected in the vascular niche of BM of patients with MM, probably due to high production of cytokines such as IL-6, IL-3, and IL-11. Megakaryocytes produce various bone-associated factors, including RANKL, OPG, and M-CSF, and megakaryocyte RANKL/OPG ratios increase as a result of their interactions with myeloma cells (Yaccoby et al, 2005).
Taken together, these studies indicate that hematopoietic cells play critical roles in increasing the gradient of osteoclastogenic factors in focal lesions. The precise involvement of specific hematopoietic elements, particularly certain immune cells, in myeloma bone disease requires further investigation because induction of severe bone resorption occurs in immunodeficient mice after engraftment of MM.
Roles of myeloma cells
In the early 1970s, Mundy and colleagues proposed that myeloma cells produce osteoclast-activating factors (Mundy et al, 1974). Osteoclastogenic factors potentially produced by myeloma cells, particularly in response to interactions with BM microenvironmental cells, include MIP1α, IL-3, IL-7, IL-8, and RANKL. Expression of RANKL by myeloma cells is controversial (Farrugia et al, 2003; Giuliani et al, 2005a; Heider et al, 2003), and the roles of IL-7 and IL-8 in forming osteoclasts within the myelomatous bone await validation; however, MIP1α has been extensively studied by Roodman and colleagues. They demonstrated high levels of this chemokine in BM plasma of patients with MM, and they showed MIP1α promotes osteoclastogenesis directly and indirectly by increasing production of RANKL and IL-6 (Choi et al, 2000;Han et al, 2001). This group also showed that IL-3 is similarly involved in myeloma-induced osteoclastogenesis (Lee et al, 2004). Syndecan-1 (CD138), a heparan sulfate proteoglycan highly produced by myeloma cells, along with heparanase, an enzyme involved in syndecan-1 shedding from surfaces of myeloma cells, regulates osteoclastogenesis by sequestering OPG (Standal et al, 2002) and by concentrating factors (e.g., HGF, IL-6, IL-8, SDF-1) that promote osteoclastogenesis, angiogenesis, and myeloma growth (Sanderson et al, 2004).
In addition to osteoclastogenic factors, growth factors produced by myeloma cells may increase the life span of monocytes and osteoclast precursors, enhancing their potential to form osteoclasts. Also noteworthy, studies suggest that myeloma cells actively participate in formation of osteoclasts by fusing into osteoclasts (Andersen et al, 2007) or by forming themselves into multinucleated cells capable of bone resorption (Silvestris et al, 2009).
In summary, levels of multiple key osteoclastogenic factors are increased in the BM milieu of patients with MM. Myeloma cells secrete some of the factors, but the main mechanisms that result in increased levels of these factors and subsequent osteolysis result from myeloma-cell–induced suppression of differentiation and recruitment of osteoblasts and from expansion and cultivation of various hematopoietic and immune elements (Figure 1).
Inactivation of osteoblastogenesis in myeloma
In 1991, Bataille et al (Bataille et al, 1991) originally reported that increased osteoblast activity coupled with increased bone resorption rate synchronically occur early in conversion from the benign precursor condition (monoclonal gammopathy of undetermined significance, MGUS) to overt MM and that patients who maintained high osteoblast activity did not develop osteolytic disease. MSCs isolated from BM of patients with MM are genetically and phenotypically abnormal and have impaired osteogenic potential (Corre et al, 2007; Garayoa et al, 2009; Wallace et al, 2001). In addition, myeloma cells actively suppress osteoblastogenesis through direct cell–cell contact and production of soluble factors. Co-culturing myeloma cells with osteoblasts resulted in downregulation of the osteoblast marker, osteocalcin (Barille et al, 1995), and direct contact between myeloma cells and MSCs, mediated by cell-surface molecules very late antigen 4 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1), resulted in MSCs downregulating expression of the critical osteoblast transcription factor Runt-related transcription factor 2 (RUNX2). These reports are in accord with histopathological analyses indicating that osteolytic lesions in MM often occur adjacent to the tumour area (Roodman, 2004).
It is now evident also that osteoblast differentiation is inhibited by factors secreted by myeloma cells (e.g., Wnt-signaling inhibitors DKK1 [Tian et al, 2003] and secreted frizzled-related protein-2 [Oshima et al, 2005], IL-7 [Giuliani et al, 2005b], and hepatocyte growth factor (HGF) [Standal et al, 2007]) and by microenvironmental cells within myelomatous bone (e.g., IL-3 [Lee et al, 2004; Ehrlich et al, 2005]). Importantly, expression or circulating levels of the osteoblast-inactivating factors often varied among patients, indicating multiple mechanisms by which myeloma cells suppress osteoblastogenesis.
Recently, Pennisi et al demonstrated that cell-surface “coupling” factors ephrinB2 and EphB4, which mediate communication between osteoblasts and osteoclasts by bidirectional signaling (Zhao et al, 2006), are underexpressed in MSCs from patients with myeloma and that myeloma cells induce downregulation of these genes in healthy MSCs (Pennisi et al, 2009c). Osteoclast precursors mainly express ephrinB2, whereas osteoblasts and their precursors, MSCs, express ephrinB2 and EphB4. Forward signaling in MSCs promotes their osteogenic differentiation, and reverse signaling in osteoclast precursors inhibits their differentiation into multinucleated bone-resorbing osteoclasts (Zhao et al, 2006; Pennisi et al, 2009c). Activating ephrinB2–EphB4 bidirectional signaling by using chimeric proteins ephrinB2-Fc and EphB4-Fc in the SCID-hu model for MM resulted in increased osteoblast activity and bone mass of the myelomatous bone (Pennisi et al, 2009c). Inhibition of osteoclastogenesis, angiogenesis, and myeloma growth also were associated with treating mice with clustered EphB4-Fc (which stimulates reverse signaling) but not with clustered ephrinB2-Fc (which stimulates forward signaling). These studies indicate that cell-surface factors for coupling bone remodeling, such as ephrinB2 and particularly EphB4, are potential therapeutic targets for MM bone disease (Figure 1).
Role of bone disease in myeloma progression: In vitro studies
Involvement of osteoclasts
The absolute numbers of osteoclasts in healthy bone and in MM focal lesions are low compared to other cellular compartments; nevertheless, the impact of osteoclasts on myeloma cell survival and growth is significant. Osteoclasts influence myeloma cells directly, by physical cell–cell contact and production of growth factors, and indirectly, by effects on the extracellular compartment (e.g., increased levels of bone-resorption products) and other cellular elements (e.g., angiogenesis stimulation) (Tanaka et al, 2007). For instance, we showed that co-culturing osteoclasts and primary myeloma plasma cells in a 1:1,000 ratio was sufficient to effectively support survival of myeloma cells and protect them from drug-induced apoptosis (Yaccoby et al, 2004; Yaccoby, 2005); cell contact seems to be essential for these effects to occur (Abe et al, 2004; Yaccoby et al, 2004), but it is not yet clear whether osteoclasts significantly affect survival of distant myeloma cells in lytic lesions. In patients with MM, some osteoclasts may be formed by fused myeloma cells (Andersen et al, 2007; Silvestris et al, 2009) or transdifferentiation of DCs (Kukreja et al, 2009), but little is known about the properties, structure, and functional activities of osteoclasts from myeloma patients.
An intriguing observation indicated that after in vitro interactions with osteoclasts (Yaccoby, 2005) or stromal cells (Dezorella et al, 2009), myeloma cells acquire an immature phenotype. This suggests that, through an adhesion mechanism, certain microenvironmental elements can revert or induce de-differentiation of the recognizable myeloma plasma cells into an immature phenotype that protects these cells from spontaneous and drug-induced apoptosis. This notion is in line with in vivo studies that demonstrated high aggressiveness of murine myeloma cells due to alteration of bone turnover by external factors (Libouban et al, 2003; Libouban et al, 2004). Recently, imaging techniques such as MRI and PET-microCT revealed that plasma cells can persist in focal lesions of patients who achieve clinical complete remission (defined by detection of monoclonal protein and random BM examination [Walker et al, 2007; Bartel et al, 2009]), suggesting the presence of immature, resilient, apoptosis-resistant malignant plasma cells in clinical myeloma.
As a result of the influences of osteoclasts, myeloma cells decrease JNK activation (Colla et al, 2007) and activate survival signaling pathways that include p44/p42 MAPK, STAT3, and PI3K/Akt pathways (Hecht et al, 2008). Soluble growth factors and cytokines in the MM microenvironment, such as IL-6, osteopontin (Abe et al, 2004; Yaccoby et al, 2004), B cell activating factor of the TNF family (BAFF), and a proliferation-inducing ligand (APRIL) (Yaccoby et al, 2008), have been implicated in osteoclast-induced myeloma cell survival. Global gene expression profiling in osteoclasts cultured alone or co-cultured with primary myeloma cells identified few genes not expressed by myeloma cells but commonly upregulated in osteoclasts after co-culture with myeloma cells (Ge et al, 2006); notable among these was fibroblast activation protein (FAP), a type-II integral-membrane glycoprotein that belongs to the serine protease family known as DASH (dipeptidyl peptidase-IV activity and/or structure homologs). Further studies revealed that it is also upregulated in MSCs after co-culture with myeloma cells and that inhibiting FAP (with siRNA or specific inhibitor) reduced the stimulatory effects of osteoclasts and MSCs on myeloma cell growth in co-cultures (Pennisi et al, 2009a) and in vivo in SCID-hu mice (Pennisi et al, 2009a). The study using FAP inhibitor suggests that this serine protease is involved in regulating adhesion molecules in osteoclasts, some of which are implicated in tumourigenesis and osteoclastogenesis (Pennisi et al, 2009a). Together, the studies of interactions between myeloma cells and osteoclasts shed light on critical cell-surface and soluble factors associated with myeloma cell survival in bone, which are potentially important for innovative targeted therapies (Figure 2).
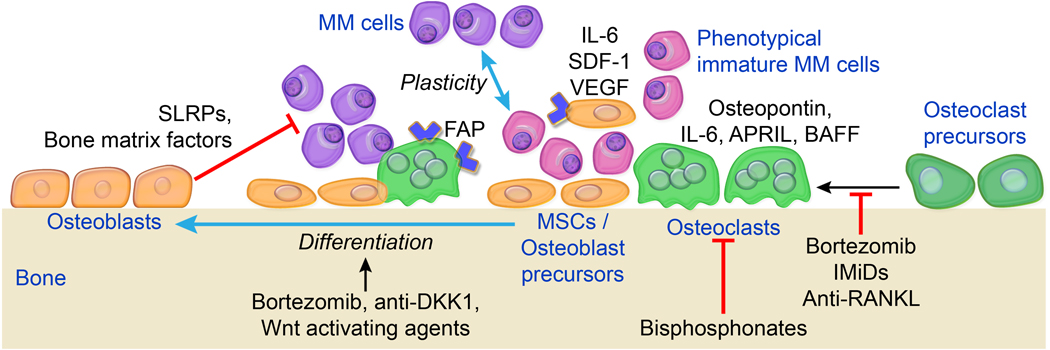
Figure 2.
Roles of bone cells in myeloma cell survival and growth. In focal/lytic lesions, osteoclasts and mesenchymal cells (e.g., MSCs) are cultivated by myeloma cells to express tumour-promoting factors (e.g., FAP) and produce MM growth factors (e.g., osteopontin, IL-6), which induce survival signalling and promote dedifferentiation of myeloma cells into an immature, apoptosis-resistant phenotype. In contrast, bone-building osteoblasts may inhibit myeloma cell growth by producing bone-building products, including some SLRPs. Agents that suppress osteoclast activity and/or stimulate osteoblastogenesis help re-establish balanced bone remodeling and contribute to controlling MM (see text for details); these agents include bortezomib, anti-DKK1, Wnt-activators, bisphosphonates, IMiDs, and anti-RANKL.
Involvement of osteoblasts
Studies of the roles of osteoblasts on myeloma cell growth have been inconclusive, partially due to heterogeneous characteristics of MM and to the use of ill-defined populations of osteoblasts (e.g., using differentiating rather than terminally differentiated osteoblasts). In addition to their production of bone-building products, mature osteoblasts differ from MSCs and immature osteoblasts by their expression of cytokines, osteoclastogenic factors, and “coupling factors.” It is now recognized that bone-building osteoblasts produce high levels of OPG and reduced levels of RANKL (Glass et al, 2005; Spencer et al, 2006; Qiang et al, 2008b). Expression of myeloma growth factor IL-6 is reduced as MSCs differentiate into osteoblasts (Gunn et al, 2006). It is proposed, therefore, that inhibition of osteogenic differentiation in MM creates favourable conditions for propagating myeloma cells and inducing osteolytic lesions (Gunn et al, 2006; Corre et al, 2007; Stewart & Shaughnessy, Jr., 2006).
Although BM stromal cells and MSCs often protect myeloma cells from spontaneous and drug-induced apoptosis (Mitsiades et al, 2006), recent findings indicate that mature osteoblasts inhibit survival of myeloma cells taken from a large subset of patients, and they interfere with the stimulatory effects of osteoclasts on myeloma cell survival and growth (Yaccoby et al, 2006). Li et al (Li et al, 2008) demonstrated that decorin, a small leucine-rich proteoglycan that is highly produced by bone-building osteoblasts, inhibited survival of myeloma cells and attenuated the stimulatory effects of osteoclasts on myeloma cells. They also demonstrated that blocking activity or expression of decorin reduced osteoblasts’ inhibitory effects on myeloma cell growth and survival, but overexpression of decorin in MSCs lessened the ability of these cells to support myeloma cell survival (Li et al, 2008). Mechanistically, decorin may directly induce tumour cell apoptosis by activating caspase 3 and upregulating p21WAF, and it may indirectly inhibit tumour growth by degrading critical cell-surface growth-factor receptors such as MET and epidermal growth factor receptor (EGFR) (for review, see Goldoni & Iozzo, 2008) and by suppressing myeloma-induced osteoclastogenesis and angiogenesis (Li et al, 2008). It is likely that bone-building osteoblasts produce other factors that negate myeloma cell growth. Collectively, these findings suggest that increasing numbers of terminally differentiated osteoblasts may help control MM by producing bone-building factors that directly and indirectly restrain myeloma growth by inhibiting osteoclastogenesis and angiogenesis and by altering the net balance between osteoclastogenic and antiosteoclastogenic factors in myelomatous bone (Figure 2).
Role of bone disease in myeloma progression: Clinical and in vivo experimental studies
Osteoclast inhibitors
Until recently, in vivo animal studies to unravel the roles of bone disease in myeloma tumour growth focused on the use of osteoclast inhibitors. Currently, MM bone disease is treated mainly by controlling MM tumour burden and inhibiting osteoclast activity with bisphosphonates. Osteoclasts have heterogeneous enzymatic repertoires and bisphosphonate sensitivities (Andersen et al, 2004;Everts et al, 2006). In fact, the report that trabecular osteoclasts are more responsive to bisphosphonates than are cortical osteoclasts (Chappard et al, 1991) may explain why bisphosphonates are better for reducing fractures of the appendicular skeleton, which is mainly composed of trabecular bone, than fractures of long bones, which are made of compact Haversian bone (Kanis & McCloskey, 2000). Experimental studies using animal models, such as the 5T or SCID-hu mouse models, have shown that inhibition of osteoclast activity by bisphosphonates or inhibitors of RANKL (e.g., RANK-Fc or OPG) effectively prevented MM-induced osteolysis (Yaccoby et al, 2002; Pearse et al, 2001; Croucher et al, 2003; Vanderkerken et al, 2003; Croucher et al, 2001). In many of these experiments, inhibition of osteoclast activity reduced growth of medullary but not extramedullary myeloma. These findings, however, are inconsistent with clinical observations: bisphosphonates, mainly pamidronate and zoledronate, reduce skeletal complications in patients with MM, but the beneficial effects on myeloma progression are inconclusive (Berenson et al, 1996; Roodman, 2008). In a phase II clinical trial with patients with relapsed or plateau-phase MM, some patients who were treated with RANKL-neutralizing antibody denosumab experienced MM disease stabilization (Vij et al, 2009).
The discrepancies in anti-myeloma efficacy of osteoclast inhibitors seen in experimental and clinical studies could be due to several factors. Clinically, bisphosphonates do not completely block bone resorption, and it often progresses (Roodman, 2008). This point could be significant in light of recent clinical observations suggesting that certain bisphosphonates may induce formation of viable, detached giant osteoclasts in BM of patients with osteoporosis (Weinstein et al, 2009). In experimental systems, however, significant inhibition of bone disease serves as an indicator of drug efficacy that allows investigation of the association between inhibition of bone disease and tumour progression. Conversely, many experimental animal studies initiated treatment when tumour burden was low. These studies also utilized young animals or bones capable of compensating for bone resorption by increasing osteoblast activity; thus, the experimental conditions may not reflect the bone biology of elderly MM patients. For instance, Croucher et al (Croucher et al, 2001) showed that, in the 5T mouse model, treatment with OPG prevented development of osteolytic bone disease and preserved bone mineral density and bone volume to levels similar to or higher than nonmyelomatous mice, suggesting restoration of normal osteoblastogenesis or perhaps a concomitant increase in osteoblast activity. Overall, these studies suggest that, by effectively inhibiting osteoclast activity and retaining normal levels of osteoblast activity, osteoclast inhibitors may create an inhospitable BM microenvironment and restrain growth of medullary myeloma cells, which otherwise induce lytic lesions that facilitate their growth within the bone.
Wnt activation
Recent in vivo studies dealt with the effects of using osteoblast-activating agents to inhibit MM bone disease and tumour progression. Studies demonstrating the critical role of Wnt signaling in bone hemostasis (Clevers, 2006), along with the discovery that myeloma cells produce Wnt inhibitors, prompted the examination of agents that promote Wnt signaling in bone indirectly (e.g., neutralizing activity of MM-produced Wnt antagonist DKK1 [Yaccoby et al, 2007]) or directly (e.g., Wnt3a [Qiang et al, 2008c], lithium chloride [Edwards et al, 2008]). The effects of DKK1-neutralizing antibody on MM bone disease and tumour growth were investigated in SCID-rab mice that were each engrafted with myeloma cells from one of 11 patients (Yaccoby et al, 2007). In this animal model, rabbit bone is implanted in a SCID mouse, and primary human myeloma cells are engrafted in the implanted bone; typical MM disease manifestations occur, including induction of severe osteolytic bone disease. In this animal study, as in the clinical setting, anti-DKK1 heterogeneously affected bone disease and tumour burden, but the overall effects were significantly increased osteoblast numbers and bone mass and reduced osteoclast numbers and myeloma burden in myelomatous bones (Yaccoby et al, 2007). A humanized DKK1-neutralizing antibody, BHQ880, which is currently being evaluated in clinical trials, has shown similar effects in SCID-hu mice engrafted with IL6-dependent myeloma cell line INA6 (Fulciniti et al, 2009). In the 5T2MM murine model of MM, BHQ880 promoted osteoblastogenesis but had no effect on osteoclastogenesis or tumour growth (Heath et al, 2009), suggesting that inducing a BM microenvironment that is inhospitable for myeloma cells by such intervention requires simultaneous stimulatory effects on osteoblasts and inhibitory effects on osteoclasts.
In an attempt to directly increase canonical Wnt signaling, Qiang et al (Qiang et al, 2008c) demonstrated that the canonical Wnt ligand, Wnt3a, similarly reduced bone disease and growth of myeloma cell line H929 and primary myeloma cells that were engrafted in the human bone in SCID-hu mice, but Wnt3a had no effect when H929 myeloma cells were grown subcutaneously in SCID mice. In a different approach to directly stimulate Wnt signaling, Edwards et al (Edwards et al, 2008) used lithium chloride, a GSK-3β inhibitor, in the 5TGM1 myeloma mouse model. Treatment with lithium chloride resulted in stabilization of β-catenin, prevention of MM-induced bone disease, and reduction in tumour burden in bone; however, lithium chloride treatment stimulated tumour growth when 5TGM1 cells were inoculated subcutaneously (Edwards et al, 2008). Because growth of most patients’ MM cells is restricted to bone and requires a specialized BM microenvironment, the animal studies investigating the consequences of increased Wnt signaling on medullary myeloma (Qiang et al, 2008c) are clinically relevant and suggest that increased Wnt signaling may restrain myeloma growth by increasing bone formation and preventing bone resorption, which result from stimulation of osteoblastogenesis and suppression of osteoclastogenesis.
Proteasome inhibitors
Incorporating proteasome inhibitor bortezomib into clinical treatment of myeloma uncovered its unique effects on bone remodeling (Zangari et al, 2005; Zangari et al, 2007; Zangari et al, 2008). Proteasome inhibitors stimulate osteoblastogenesis and bone formation (Garrett et al, 2003; Mukherjee et al, 2008), which supports the notion that the ubiquitin–proteasome pathway is a promising therapeutic target for inhibiting myeloma cell growth and bone disease. In successive clinical observations, Zangari et al (Zangari et al, 2007; Zangari et al, 2008) demonstrated that the anti-myeloma response of bortezomib is associated with increases in both bone mass and circulating levels of bone alkaline phosphatase (Zangari et al, 2008) and that increased levels of alkaline phosphatase predict treatment response (Zangari et al, 2007). Clinical observations by others support the perception that bortezomib is a bone-anabolic agent in MM (Terpos et al, 2006; Heider et al, 2006). In addition to their clinical value, these observations demonstrate that osteoblast activation is feasible in patients with MM, despite reports indicating that patients’ MSCs are genetically, phenotypically, and functionally abnormal (Corre et al, 2007; Garayoa et al, 2009; Wallace et al, 2001;Todoerti et al, 2009).
The clinical association between increased osteoblast activity and response to bortezomib therapy is supported by experimental data. Edwards et al (Edwards et al, 2009) reported that, in the 5TGM1 model, the effect of bortezomib on tumour reduction was higher when myeloma cells were grown within the BM than in extra-osseous sites (Edwards et al, 2009). In the SCID-rab model for primary myeloma, the anti-myeloma effects of bortezomib, but not melphalan, were associated with increased bone mass in responding hosts (Pennisi et al, 2009b), suggesting that bortezomib’s effects on skeletal homeostasis are not a consequence of reduced tumour burden but, rather, of direct effects on bone cells (Pennisi et al, 2009b).
Indeed, accumulating data shed light on mechanisms by which proteasome inhibitors directly affect the fate of bone cells. Inhibition of the ubiquitin–proteasome pathway induces osteoblast differentiation by increasing expression of bone morphogenetic protein 2 (Garrett et al, 2003) and preventing proteolytic degradation of RUNX2 (Bellido et al, 2003; Giuliani et al, 2007). Bortezomib also promotes bone formation by inhibiting DKK1 expression in osteogenic cells (Oyajobi et al, 2007) and by stabilizing β-catenin in osteogenic cells in a mechanism that is independent of canonical Wnt ligands (Qiang et al, 2009). Proteasome inhibitors also reduce NF-κB activity in osteoclast precursors, resulting in inhibition of osteoclast formation (Zavrski et al, 2005; von Metzler et al, 2007). Bortezomib reduces circulating levels of DKK1 and RANKL in patients with relapsed MM (Terpos et al, 2006; Kaiser et al, 2008), further suggesting that proteasome inhibitors increase bone mass by stimulating osteoblastogenesis and inhibiting osteoclastogenesis. Despite these convincing data, the long-term effects of proteasome inhibition on bone remodeling, particularly with regard to the fate of osteoprogenitor cells, should be carefully investigated because the stimulatory effects of bortezomib on circulating levels of osteoblastic markers, at least in patients with MM, seem to be transient (Zangari et al, 2005).
Conclusions
Understanding the roles of bone disease in myeloma tumour growth is challenging due to the heterogeneous characteristics of the disease. Nevertheless, clinical observations reveal a high correlation between numbers of focal and/or lytic lesions and disease stage, as assessed by standard clinical parameters and molecular classification of myeloma cells (Walker et al, 2007; Bartel et al, 2009; Zhan et al, 2006). Because osteoclasts and bone-building osteoblasts appear to induce contrasting effects on myeloma cell survival and proliferation, induction of osteolytic bone destruction in MM is likely to promote disease progression. In animal studies, pretreating with bisphosphonates to inhibit osteoclast activity (Yaccoby et al, 2002) or with intermittent parathyroid hormone to increase bone mass (Pennisi A et al, 2007) significantly slowed engraftment of MM, suggesting that, in some cases, bone disease drives development of MM. Partially inhibiting osteoclast activity without maintaining normal or increased levels of osteoblast activity may not be sufficient to create an inhospitable BM environment for myeloma cells. Therefore, development of interventions that simultaneously stimulate osteoblastogenesis and restrain osteoclastogenesis may also control myeloma progression while effectively promoting bone formation.
Acknowledgments
The current research in the author’s laboratory related to the work reported in this publication is supported by grants from the National Cancer Institute (CA-093897) and from the Multiple Myeloma Research Foundation (Senior and Translational Research Award). The author recognizes the efforts of Xin Li, Angela Pennisi, Wen Ling, Sharmin Khan, Jianmei Chen, and Yuping Yang. The author also wishes to thank the faculty, staff, and patients of the Myeloma Institute for Research and Therapy for their support, as well as the Office of Grants and Scientific Publications at the University of Arkansas for Medical Sciences for editorial assistance during the preparation of this manuscript.
Footnotes
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- Andersen TL, Boissy P, Sondergaard TE, Kupisiewicz K, Plesner T, Rasmussen T, Haaber J, Kolvraa S, Delaisse JM. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: a new type of cancer-host partnership? Journal of Pathology. 2007;211:10–17. doi: 10.1002/path.2078. [DOI] [PubMed] [Google Scholar]
- Andersen TL, del Carmen OM, Kirkegaard T, Lenhard T, Foged NT, Delaisse JM. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 2004;35:1107–1119. doi: 10.1016/j.bone.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Barille S, Collette M, Bataille R, Amiot M. Myeloma cells upregulate interleukin-6 secretion in osteoblastic cells through cell-to-cell contact but downregulate osteocalcin. Blood. 1995;86:3151–3159. [PubMed] [Google Scholar]
- Barille-Nion S, Bataille R. New insights in myeloma-induced osteolysis. Leukaemia & Lymphoma. 2003;44:1463–1467. doi: 10.3109/10428190309178765. [DOI] [PubMed] [Google Scholar]
- Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr, van Ree F, Anaissie E, Alpe T, Angtuaco E, Walker R, Epstein J, Crowley J, Barlogie B. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–2076. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille R, Chappard D, Marcelli C, Dessauw P, Baldet P, Sany J, Alexandre C. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. Journal of Clinical Investigation. 1991;88:62–66. doi: 10.1172/JCI115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. Journal of Biological Chemistry. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- Bendre MS, Montague DC, Peery T, Akel NS, Gaddyz D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs MJ, Blacklock HA, Bell R, Simeone J, Reitsma DJ, Heffernan M, Seaman J, Knight RD Myeloma Aredia Study Group. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. New England Journal of Medicine. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- Chappard D, Petitjean M, Alexandre C, Vico L, Minaire P, Riffat G. Cortical osteoclasts are less sensitive to etidronate than trabecular osteoclasts. Journal of Bone Mineral Research. 1991;6:673–680. doi: 10.1002/jbmr.5650060704. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, Bianchi G, Podar K, Tai YT, Mitsiades C, Raje N, Jaye DL, Kumar SK, Richardson P, Munshi N, Anderson KC. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–323. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Cruz JC, Craig F, Chung H, Devlin RD, Roodman GD, Alsina M. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Colla S, Zhan F, Xiong W, Wu X, Xu H, Stephens O, Yaccoby S, Epstein J, Barlogie B, Shaughnessy JD., Jr The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood. 2007;109:4470–4477. doi: 10.1182/blood-2006-11-056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, Danho C, Laharrague P, Klein B, Reme T, Bourin P. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher PI, De Hendrik R, Perry MJ, Hijzen A, Shipman CM, Lippitt J, Green J, Van Marck E, Van Camp B, Vanderkerken K. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. Journal of Bone Mineral Research. 2003;18:482–492. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, Brabbs AC, van Beek EJ, Holen I, Skerry TM, Dunstan CR, Russell GR, Van Camp B, Vanderkerken K. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–3540. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- Dezorella N, Pevsner-Fischer M, Deutsch V, Kay S, Baron S, Stern R, Tavor S, Nagler A, Naparstek E, Zipori D, Katz BZ. Mesenchymal stromal cells revert multiple myeloma cells to less differentiated phenotype by the combined activities of adhesive interactions and interleukin-6. Experimental Cell Research. 2009;315:1904–1913. doi: 10.1016/j.yexcr.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, Jagannath S, Dhodapkar MV. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112:2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M, Ng A, Kumar S, Charatcharoenwitthaya N, Achenbach S, Holets M, McCready L, Rajkumar S, Kyle R. Increases in serum levels of dickkopf 1 are associated with alterations in skeletal microstructure in monoclonal gammopathy of undetermined significance. Journal of Bone Mineral Research. 2009;24 Suppl 1 [Google Scholar]
- Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CM, Lwin ST, Fowler JA, Oyajobi BO, Zhuang J, Bates AL, Mundy GR. Myeloma cells exhibit an increase in proteasome activity and an enhanced response to proteasome inhibition in the bone marrow microenvironment in vivo. American Journal of Hematology. 2009;84:268–272. doi: 10.1002/ajh.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S, Rizzoli V, Roodman GD, Giuliani N. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106:1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget's disease and multiple myeloma. Immunology Review. 2005;208:252–266. doi: 10.1111/j.0105-2896.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- Everts V, Korper W, Hoeben KA, Jansen ID, Bromme D, Cleutjens KB, Heeneman S, Peters C, Reinheckel T, Saftig P, Beertsen W. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: differences between calvaria and long bone. Journal of Bone Mineral Research. 2006;21:1399–1408. doi: 10.1359/jbmr.060614. [DOI] [PubMed] [Google Scholar]
- Farrugia AN, Atkins GJ, To LB, Pan B, Horvath N, Kostakis P, Findlay DM, Bardy P, Zannettino AC. Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Research. 2003;63:5438–5445. [PubMed] [Google Scholar]
- Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garayoa M, Garcia JL, Santamaria C, Garcia-Gomez A, Blanco JF, Pandiella A, Hernandez JM, Sanchez-Guijo FM, Del Canizo MC, Gutierrez NC, San Miguel JF. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia. 2009;23:1515–1527. doi: 10.1038/leu.2009.65. [DOI] [PubMed] [Google Scholar]
- Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, Harris SE, Gallwitz W, Kim KB, Hu S, Crews CM, Mundy GR. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. Journal of Clinical Investigation. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Zhan F, Barlogie B, Epstein J, Shaughnessy J, Yaccoby Y. Fibroblast activation protein (FAP) is upregulated in myelomatous bone and supports myeloma cell survival. British Journal of Haematology. 2006;133:83–92. doi: 10.1111/j.1365-2141.2006.05976.x. [DOI] [PubMed] [Google Scholar]
- Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98:3527–3533. doi: 10.1182/blood.v98.13.3527. [DOI] [PubMed] [Google Scholar]
- Giuliani N, Colla S, Morandi F, Barille-Nion S, Rizzoli V. Lack of receptor activator of nuclear factor-kB ligand (RANKL) expression and functional production by human multiple myeloma cells. Haematologica. 2005a;90:275–278. [PubMed] [Google Scholar]
- Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005b;106:2472–2483. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S, Bonomini S, Hojden M, Sammarelli G, Barille S, Bataille R, Rizzoli V. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood. 2002;100:4615–4621. doi: 10.1182/blood-2002-04-1121. [DOI] [PubMed] [Google Scholar]
- Giuliani N, Morandi F, Tagliaferri S, Colla S, Bonomini S, Sammarelli G, Rizzoli V. Interleukin-3 (IL-3) is overexpressed by T lymphocytes in multiple myeloma patients. Blood. 2006;107:841–842. doi: 10.1182/blood-2005-07-2719. [DOI] [PubMed] [Google Scholar]
- Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, Mancini C, Martella E, Ferrari L, Tabilio A, Rizzoli V. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Develomental Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. International Journal of Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97:3349–3353. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD, Jr, Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. Journal of Bone Mineral Research. 2009;24:425–436. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- Hecht M, von MI, Sack K, Kaiser M, Sezer O. Interactions of myeloma cells with osteoclasts promote tumour expansion and bone degradation through activation of a complex signalling network and upregulation of cathepsin K, matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPA) Experimental Cell Research. 2008;314:1082–1093. doi: 10.1016/j.yexcr.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Heider U, Kaiser M, Muller C, Jakob C, Zavrski I, Schulz CO, Fleissner C, Hecht M, Sezer O. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. European Journal of Haematology. 2006;77:233–238. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- Heider U, Langelotz C, Jakob C, Zavrski I, Fleissner C, Eucker J, Possinger K, Hofbauer LC, Sezer O. Expression of receptor activator of nuclear factor kappaB ligand on bone marrow plasma cells correlates with osteolytic bone disease in patients with multiple myeloma. Clinical Cancer Research. 2003;9:1436–1440. [PubMed] [Google Scholar]
- Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, Stover D, Sezer O, Heider U. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. European Journal of Haematology. 2008;80:490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- Kanis JA, McCloskey EV. Bisphosphonates in multiple myeloma. Cancer. 2000;88:3022–3032. doi: 10.1002/1097-0142(20000615)88:12+<3022::aid-cncr19>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, Jagannath S, Dhodapkar MV. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. Journal of Experimental .Medicine. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja A, Radfar S, Sun BH, Insogna K, Dhodapkar MV. Dominant role of CD47-thrombospondin-1 interactions in myeloma induced fusion of human dendritic cells: implications for bone disease. Blood. 2009;16:3413–3421. doi: 10.1182/blood-2009-03-211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, Choi SJ. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103:2308–2315. doi: 10.1182/blood-2003-06-1992. [DOI] [PubMed] [Google Scholar]
- Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112:159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libouban H, Moreau MF, Basle MF, Bataille R, Chappard D. Increased bone remodeling due to ovariectomy dramatically increases tumoral growth in the 5T2 multiple myeloma mouse model. Bone. 2003;33:283–292. doi: 10.1016/s8756-3282(03)00196-0. [DOI] [PubMed] [Google Scholar]
- Libouban H, Moreau MF, Basle MF, Bataille R, Chappard D. Selection of a highly aggressive myeloma cell line by an altered bone microenvironment in the C57BL/KaLwRij mouse. Biochemical and Biophysical Research Communications. 2004;316:859–866. doi: 10.1016/j.bbrc.2004.02.131. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: Interplay of growth factors, their receptors and stromal interactions. European Journal of Cancer. 2006;42:1564–1573. doi: 10.1016/j.ejca.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. Journal of Clinical Investigation. 2008;118:491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR, Raisz LG, Cooper RA, Schechter GP, Salmon SE. Evidence for the secretion of an osteoclast stimulating factor in myeloma. New England Journal of Medicine. 1974;291:1041–1046. doi: 10.1056/NEJM197411142912001. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Yao L, Tosato G. Mast cell-derived angiopoietin-1 plays a critical role in the growth of plasma cell tumors. Journal of Clinical Investigation. 2004;114:1317–1325. doi: 10.1172/JCI22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- Oyajobi BO, Garrett IR, Gupta A, Flores A, Esparza J, Munoz S, Zhao M, Mundy GR. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: implications for myeloma bone disease. British Journal of Haematology. 2007;139:434–438. doi: 10.1111/j.1365-2141.2007.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proceedings of the National Academy of Sciences USA. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi A, Li X, Zangari M, Yaccoby S. Intermittent PTH treatment inhibits development and progression of primary myeloma by promoting bone formation in the SCID-rab model; 29th Annual Meeting of the American Society for Bone and Mineral Research; 2007. Abstract 1172. [Google Scholar]
- Pennisi A, Li X, Ling W, Khan S, Gaddy D, Suva LJ, Barlogie B, Shaughnessy JD, Aziz N, Yaccoby S. Inhibitor of DASH proteases affects expression of adhesion molecules in osteoclasts and reduces myeloma growth and bone disease. British Journal of Haematology. 2009a;145:775–787. doi: 10.1111/j.1365-2141.2009.07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. American Journal of Hematology. 2009b;84:6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD, Jr, Barlogie B, Yaccoby S. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009c;114:1803–1812. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD., Jr Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008a;42:669–680. doi: 10.1016/j.bone.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, Barlogie B, Shaughnessy JD., Jr Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008b;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B, Shaughnessy JD., Jr Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang YW, Shaughnessy JD, Jr, Yaccoby S. Wnt3a signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood. 2008c;112:374–382. doi: 10.1182/blood-2007-10-120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman GD. Mechanisms of bone metastasis. New England Journal of Medicine. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Skeletal imaging and management of bone disease. Hematology (American Society of Hematology Education Program) 2008:313–319. doi: 10.1182/asheducation-2008.1.313. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y, Suva LJ, Kelly T. Heparan sulfate proteoglycans and heparanase--partners in osteolytic tumor growth and metastasis. Matrix Biology. 2004;23:341–352. doi: 10.1016/j.matbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Sandhu JS, Gorczynski RM, Waddell J, Nguyen H, Squires J, Waddell J, Boynton EL, Hozumi N. Effect of interleukin-6 secreted by engineered human stromal cells on osteoclasts in human bone. Bone. 1999;24:217–227. doi: 10.1016/s8756-3282(98)00172-0. [DOI] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. Journal of Experimental Medicine. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezer O. Myeloma bone disease: recent advances in biology, diagnosis, and treatment. Oncologist. 2009;14:276–283. doi: 10.1634/theoncologist.2009-0003. [DOI] [PubMed] [Google Scholar]
- Sezer O, Heider U, Zavrski I, Kuhne CA, Hofbauer LC. RANK ligand and osteoprotegerin in myeloma bone disease. Blood. 2003;101:2094–2098. doi: 10.1182/blood-2002-09-2684. [DOI] [PubMed] [Google Scholar]
- Silvestris F, Ciavarella S, De MM, Tucci M, Dammacco F. Boneresorbing cells in multiple myeloma: osteoclasts, myeloma cell polykaryons, or both? Oncologist. 2009;14:264–275. doi: 10.1634/theoncologist.2008-0087. [DOI] [PubMed] [Google Scholar]
- Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. Journal of Cell Science. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- Standal T, Abildgaard N, Fagerli UM, Stordal B, Hjertner O, Borset M, Sundan A. HGF inhibits BMP-induced osteoblastogenesis: possible implications for the bone disease of multiple myeloma. Blood. 2007;109:3024–3030. doi: 10.1182/blood-2006-07-034884. [DOI] [PubMed] [Google Scholar]
- Standal T, Seidel C, Hjertner O, Plesner T, Sanderson RD, Waage A, Borset M, Sundan A. Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood. 2002;100:3002–3007. doi: 10.1182/blood-2002-04-1190. [DOI] [PubMed] [Google Scholar]
- Stewart JP, Shaughnessy JD., Jr Role of osteoblast suppression in multiple myeloma. Journal of Cellular Biochemistry. 2006;98:1–13. doi: 10.1002/jcb.20774. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Abe M, Hiasa M, Oda A, Amou H, Nakano A, Takeuchi K, Kitazoe K, Kido S, Inoue D, Moriyama K, Hashimoto T, Ozaki S, Matsumoto T. Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: a role for vascular endothelial cell growth factor and osteopontin. Clinical Cancer Research. 2007;13:816–823. doi: 10.1158/1078-0432.CCR-06-2258. [DOI] [PubMed] [Google Scholar]
- Terpos E, Dimopoulos MA, Sezer O. The effect of novel anti-myeloma agents on bone metabolism of patients with multiple myeloma. Leukemia. 2007;21:1875–1884. doi: 10.1038/sj.leu.2404843. [DOI] [PubMed] [Google Scholar]
- Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A, Pouli A, Katodritou E, Verrou E, Vervessou EC, Dimopoulos MA, Croucher PI. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. British Journal of Haematology. 2006;135:688–692. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. New England Journal of Medicine. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Todoerti K, Lisignoli G, Storti P, Agnelli L, Novara F, Manferdini C, Codeluppi K, Colla S, Crugnola M, Abeltino M, Bolzoni M, Sgobba V, Facchini A, Lambertenghi-Deliliers G, Zuffardi O, Rizzoli V, Neri A, Giuliani N. Distinct transcriptional profiles characterize bone microenvironment mesenchymal cells rather than osteoblasts in relationship with multiple myeloma bone disease. Experimental Hematology. 2009 Dec 4; doi: 10.1016/j.exphem.2009.11.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, Dammacco F. Bone marrow angiogenesis and progression in multiple myeloma. British Journal of Haematology. 1994;87:503–508. doi: 10.1111/j.1365-2141.1994.tb08304.x. [DOI] [PubMed] [Google Scholar]
- Vanderkerken K, De Leenheer E, Shipman C, Asosingh K, Willems A, Van Camp B, Croucher P. Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Research. 2003;63:287–289. [PubMed] [Google Scholar]
- Vij R, Horvath N, Spencer A, Taylor K, Vadhan-Raj S, Vescio R, Smith J, Qian Y, Yeh H, Jun S. An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. American Journal of Hematology. 2009;84:650–656. doi: 10.1002/ajh.21509. [DOI] [PubMed] [Google Scholar]
- von Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, Kaiser M, Jakob C, Sterz J, Kleeberg L, Heider U, Sezer O. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21:2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD, Jr, Epstein J, van HR, Erdem E, Hoering A, Crowley J, Ferris E, Hollmig K, van RF, Zangari M, Pineda-Roman M, Mohiuddin A, Yaccoby S, Sawyer J, Angtuaco EJ. Magnetic Resonance Imaging in Multiple Myeloma: Diagnostic and Clinical Implications. Journal of Clinical Oncology. 2007;25:1121–1128. doi: 10.1200/JCO.2006.08.5803. [DOI] [PubMed] [Google Scholar]
- Wallace SR, Oken MM, Lunetta KL, Panoskaltsis-Mortari A, Masellis AM. Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer. 2001;91:1219–1230. doi: 10.1002/1097-0142(20010401)91:7<1219::aid-cncr1122>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. New England Journal of Medicine. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BR, Josien R, Choi Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. Journal of Leukocyte Biology. 1999;65:715–724. doi: 10.1002/jlb.65.6.715. [DOI] [PubMed] [Google Scholar]
- Yaccoby S. The phenotypic plasticity of myeloma plasma cells as expressed by dedifferentiation into an immature, resilient, and apoptosis-resistant phenotype. Clinical Cancer Research. 2005;11:7599–7606. doi: 10.1158/1078-0432.CCR-05-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaccoby S, Pearse RN, Johnson CL, Barlogie B, Choi Y, Epstein J. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. British Journal of Haematology. 2002;116:278–290. doi: 10.1046/j.1365-2141.2002.03257.x. [DOI] [PubMed] [Google Scholar]
- Yaccoby S, Pennisi A, Li X, Dillon SR, Zhan F, Barlogie B, Shaughnessy JD., Jr Atacicept (TACI-Ig) inhibits growth of TACI(high) primary myeloma cells in SCID-hu mice and in coculture with osteoclasts. Leukemia. 2008;22:406–413. doi: 10.1038/sj.leu.2405048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, Epstein J. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Research. 2004;64:2016–2023. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- Yaccoby S, Wezeman MJ, Zangari M, Walker R, Cottler-Fox M, Gaddy D, Ling W, Saha R, Barlogie B, Tricot G, Epstein J. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- Yaccoby S, Saha R, Kozlowska E, Ge Y, Ling W, Cottler-Fox M, Barlogie B, Tricot G. Myeloma cells cultivate megakaryocytes to promote lytic bone disease. Blood (ASH Annual Meeting Abstracts) 2005;106:2495. [Google Scholar]
- Zangari M, Esseltine D, Cavallo F, Neuwirth R, Elice F, Burns MJ, Yaccoby S, Richardson P, Sonneveld P, Tricot G. Predictive value of alkaline phosphatase for response and time to progression in bortezomib-treated multiple myeloma patients. American Journal of Hematology. 2007;82:831–833. doi: 10.1002/ajh.20961. [DOI] [PubMed] [Google Scholar]
- Zangari M, Esseltine D, Lee CK, Barlogie B, Elice F, Burns MJ, Kang SH, Yaccoby S, Najarian K, Richardson P, Sonneveld P, Tricot G. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. British Journal of Haematology. 2005;131:71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- Zangari M, Pappas L, Zhan F, kumar NS, Cavallo F, Suva LJ, Tricot G, Esseltine DL, Yaccoby S. Parathyroid hormones (PTH) serum variations are associated with bortezomib response in multiple myeloma patients. Blood (ASH Annual Meeting Abstracts) 2008;112:2783. [Google Scholar]
- Zannettino AC, Farrugia AN, Kortesidis A, Manavis J, To LB, Martin SK, Diamond P, Tamamura H, Lapidot T, Fujii N, Gronthos S. Elevated serum levels of stromal-derived factor-1alpha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Research. 2005;65:1700–1709. doi: 10.1158/0008-5472.CAN-04-1687. [DOI] [PubMed] [Google Scholar]
- Zavrski I, Krebbel H, Wildemann B, Heider U, Kaiser M, Possinger K, Sezer O. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochemical and Biophysical Research Communications. 2005;333:200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B, Anaissie E, Hollmig K, Pineda-Roman M, Tricot G, van RF, Walker R, Zangari M, Crowley J, Barlogie B, Shaughnessy JD., Jr The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metabolism. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]