Abstract
Temporal lobe epilepsy (TLE) is a condition characterized by an imbalance between excitation and inhibition in the temporal lobe. Hallmarks of this change are axon sprouting and accompanying synaptic reorganization in the temporal lobe. Synthetic and endogenous cannabinoids have variable therapeutic potential in treating intractable temporal lobe epilepsy, in part because cannabinoid ligands can bind multiple receptor types. This study utilized in vitro electrophysiological methods to examine the effect of transient receptor potential vanilloid type 1 (TRPV1) activation in dentate gyrus granule cells in a murine model of TLE. Capsaicin, a selective TRPV1 agonist had no measurable effect on overall synaptic input to granule cells in control animals, but significantly enhanced spontaneous and miniature EPSC frequency in mice with TLE. Exogenous application of anandamide, an endogenous cannabinoid that acts at both TRPV1 and cannabinoid type 1 receptors (CB1R), also enhanced glutamate release in the presence of a CB1R antagonist. Anandamide reduced the EPSC frequency when TRPV1 were blocked with capsazepine. Western blot analysis of TRPV1 receptor indicated protein expression was significantly greater in the dentate gyrus of mice with TLE compared with control mice. This study indicates that a prominent cannabinoid agonist can increase excitatory circuit activity in the synaptically reorganized dentate gyrus of mice with TLE by activating TRPV1 receptors, and suggests caution in designing anticonvulsant therapy based on modulating the endocannabinoid system.
Keywords: anandamide, endocannabinoid, mossy fiber sprouting, vanilloid, VR1, seizure
Introduction
Temporal lobe epilepsy (TLE) is a common neurological condition affecting approximately 1% of human population. It is associated with characteristic changes in neuronal circuitry, which render the brain more susceptible to seizure generation (Ben-Ari et al., 1981; Babb et al., 1991; Dudek and Spitz, 1997). Circuit modification includes hippocampal cell loss and axon sprouting, which have been associated with the development of TLE in animal models and humans (Ben-Ari, 1985; Tauck and Nadler, 1985; Sutula et al., 1989; Shibley and Smith, 2002; Smith and Dudek, 2001). Mossy fibers of the granule cells sprout into the inner molecular layer of the dentate gyrus where they form excitatory synapses with the other granule cells, resulting in a recurrent excitatory circuit associated with TLE (Sutula et al., 1989; Franck et al., 1995; Wuarin and Dudek, 1996; Winokur et al., 2004).
Recent evidence suggests that cannabinoids and endocannabinoids play a protective role under excitatory conditions in the brain and in modulating circuits activated during seizures (Wallace et al., 2002; Marsicano et al., 2003; Wallace et al., 2003). Release of the endocannabinoids, arachidonoylethanolamide (anandamide; AEA) and 2-arachydonoylglycerol (2-AG) is increased during seizures and the synthetic cannabinoid receptor agonist WIN 55,212-2 can suppress seizure development in animal models of TLE (Marsicano et al., 2003; Wallace et al., 2003). Thus, agonist binding to cannabinoid type receptors (CB1R) tends to suppress seizure activity in experimental epilepsy.
In addition to binding CB1R centrally, several eicosanoid endocannabinoid agonists, including AEA, also functionally activate TRPV1. TRPV1 receptors are non-specific cation channels and are believed to be molecular integrators of various stimuli, including capsaicin, pH, and high temperature, being well characterized in peripheral pain pathways. TRPV1 is also expressed in the brain, including the hippocampus and the dentate gyrus (Mezey et al., 2000; Roberts et al., 2004; Toth et al., 2005; Cristino et al., 2006), but little is known about the physiological functions of central TRPV1 receptors. Increasing evidence suggests activation of both TRPV1 and CB1R by AEA centrally (Al-Hayani et al., 2001; Derbenev et al., 2006; Kauer and Gibson, 2009; Kofalvi et al., 2007; Starowicz, Cristino and DiMarzo, 2008;), implying a dual role for endocannabinoid agonists in brain areas containing both receptors. It is necessary to understand such dual roles for endocannbinoids to design effective therapies based on the endocannabinoid system.
In preliminary studies, we observed a transient increase in EPSC frequency in many granule cells treated with AEA in mice with TLE (Bhaskaran and Smith, 2007). Based on the findings that AEA activates TRPV1 as well as cannabinoid receptors, a mixed AEA response due to TRPV1 and CB1R activation is observed in other brain systems where both receptors are located (Derbenev et al., 2006), and blockade of endocannabinoid activity actually exacerbates seizures (Wallace et al., 2003; Baakman et al., 2009), we hypothesized that TRPV1 activation and expression would be observed in the new recurrent excitatory circuitry formed between granule cells in association with TLE development in pilocarpine-treated mice.
Methods
Animals
Adult male CD1 mice weighing 25–30g were housed individually on a 12-hour day and night cycle. Food and water were available ad libitum. The animals were housed at least 7 days prior to beginning treatment. All procedures were approved by the Tulane University and University of Kentucky Animal Care and Use Committees.
Pilocarpine-injection
Mice were administered an intraperitoneal injection (i.p.) of methylscopolamine in sterile saline (1 mg/kg) 15–30 min prior to injection of pilocarpine to reduce the peripheral cholinergic effects of the pilocarpine. Experimental animals were then injected i.p. with a single dose of pilocarpine hydrochloride (280–290 mg/kg) in sterile saline vehicle (0.9% NaCl; 0.1 ml total volume) as described previously (Shibley and Smith, 2002). Control mice were age-matched with treated mice and were administered a comparable volume of vehicle or were not injected after the initial methylscopolamine treatment.
Seizure behavior was observed for at least 2 hours starting immediately after the pilocarpine injection. The category and the number of generalized convulsive seizures in each ½ hour period were tallied. A modified version of the seizure scale described by Racine (1972) was used to identify seizure severity, with primary attention given to convulsive seizures (i.e., categories 3 to 5) as they were correlated with the eventual development of spontaneous seizures, mossy fiber sprouting, and increased recurrent excitation in the dentate gyrus (Shibley and Smith, 2002; Winokur et al., 2004). Convulsive seizures in mice most often included unilateral limb myoclonus, loss of postural control and repetitive jumping. Seizures were typically of 30–90s duration and were separated by variable duration periods of relative inactivity. The periods between convulsive seizures were marked by continuous low-level seizure-like activity (i.e., categories 1–2) that typically included continuous head bobbing, chewing, wet dog shakes, and stiff tail. A mouse that experienced a minimum of 3 generalized convulsive seizure events within the 2 hours following pilocarpine injection was considered to have undergone status epilepticus (SE). In addition to standard rodent chow and water, mice were supplied with water-moistened chow and a 5% sucrose solution in water in a petri dish inside the cage for 4 days after SE induction to help replenish fluids.
Slice preparation
Mice were anesthetized to effect by halothane inhalation and then decapitated. The brains were rapidly removed and immersed in oxygenated (95% O2/5% CO2) ice-cold (0–4ºC) artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 3 KCl, 26 NaHCO3, 11 glucose, 2 CaCl2, and 1.3 MgCl2, pH=7.3–7.4, with an osmolality of 290 – 305 mOsm/kg. Brains were then blocked and glued to a sectioning stage and sliced in the horizontal plane such that transverse slices (300–400 μm thickness) of the ventral ~2/3 of the hippocampal formation were cut in cold oxygenated ACSF using a vibrating microtome (Vibratome Series 1000; Technical Products Intl, St Louis, MO, USA). The hippocampus was then separated from the surrounding tissue, being sure to completely remove any entorhinal cortex from the slice. Slices were then transferred to a storage chamber, where they were perfused with warm (32–35ºC) and oxygenated ACSF.
Whole-cell patch-clamp recordings
After an equilibration period of 1–2h, granule cells in the dentate gyrus were targeted for recording under a 40x water-immersion objective (NA=0.8) with infrared-differential interference contrast (IR-DIC) optics (Olympus, BX51WI) using a CCD video camera. Whole-cell voltage-clamp recordings from granule cells of the dentate gyrus were obtained using pipettes with open tip resistance of 2–5 MΩ using a Multiclamp 700A or Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA). Signals were low-pass filtered at 2–5kHz, digitized at 88kHz (Neurocorder, Cygnus Technology, Delaware Water Gap, PA, USA), and recorded onto videotape as well as to a PC-style computer (Digidata 1440A or 1320A, Axon Instruments). Data were captured using pCLAMP programs (Axon Instruments) and analyzed using pCLAMP or Mini-Analysis (Synaptosoft, Decatur, GA, USA). Recording pipettes were pulled from borosilicate glass (1.65 mm outer diameter and 0.45 mm wall thickness, Garner Glass Co., Claremont, CA, USA) with a P-87 puller (Sutter Instruments) and were filled with (in mM): 130 K+-gluconate, 10 HEPES, 1 NaCl, 1 MgCl2, 1 CaCl2, 3 KOH, 5 EGTA, 2 Mg-ATP. Seal resistance was typically 1–4 GΩ and series resistance, measured from brief voltage steps (10 mV, 5 ms) applied through the recording pipette was typically <30 MΩ, uncompensated. Spontaneous excitatory postsynaptic currents (sEPSCs) were examined at a holding potential of −70 mV. Synaptic events were characterized by a typically fast rising phase and exponential decay phase, and only currents with amplitudes greater than twice peak-to-peak noise level were included for analysis. Recordings in which a >20% change in series resistance was measured during drug application were excluded from the analysis. Input conductance was estimated by measuring the current at the end of brief (20–400 ms) voltage pulses of 5–10 mV. Resting membrane potential was determined by periodically monitoring the voltage at which no current was measured (i.e. briefly removing voltage-clamp control of the neuron by switching to I = 0) during the recording.
Drug application
Whole cell recordings were usually done in the absence of added Mg2+ in the ACSF in order to remove the Mg2+-dependent blockade of the NMDA receptors near resting membrane potential and expose network excitability (Mayer et al., 1984; Smith and Dudek, 2001,2002; Winokur et al., 2004). The GABAA receptor antagonist, bicuculline methiodide (30 μM; Sigma) was added to ACSF to reduce synaptic inhibition. Tetrodotoxin (TTX; 1 μM) (Sigma or Alomone labs, Jerusalem, Israel) was applied to study action-potential independent synaptic events (i.e., ‘miniature’ EPSCs). The selective TRPV1 agonist (E)-N-[(4-Hydroxy-3-methoxyphenyl) methyl]-8-methyl-6-nonenamide (capsaicin; 1–10 μM; Tocris) and the endogenous cannabinoid/vanilloid ligand, AEA (1–10 μM; Tocris) were used to activate TRPV1 and/or CB1R. The CB1R antagonist/inverse agonist, N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM 251; 10 μM; Tocris) and selective TRPV1 antagonist, N-[2-(4-Chlorophenyl)ethyl]-1,3,4,5-tetrahydro-7,8-dihydroxy-2H-2-benzazepine-2-carbothioamide (capsazepine; CPZ; 10μM; Tocris) were used as antagonists to isolate receptors involved in agonist responses. Capsaicin, capsazepine, and AM251 were dissolved in 0.01% DMSO; AEA was dissolved in Tocrisolve®. Vehicle applied alone at identical final concentrations in preliminary analyses (n=5 cells each) was without effect.
Tissue Staining
At the end of the experiment, slices were placed in 0.37% sodium sulfide solution in 0.1M NaHPO4 buffer for 25 minutes and then fixed overnight in 4% paraformaldehyde. The slices were then rinsed with phosphate buffered saline (PBS; 0.01M) and placed in 30% sucrose for cryoprotection. After equilibration with sucrose, 30 μm frozen sections were made using a cryostat, mounted on charged slides (Superfrost Plus; Fisher Scientific) and air-dried overnight. Timm’s and Nissl staining was performed to reveal mossy fiber and cellular distribution patterns using the protocols used previously (Shibley and Smith, 2002).
Western blot analysis
400 μM transverse sections of the hippocampus were made from control and pilocarpine-treated mice that survived status epilepticus using a vibratome, and the dentate gyrus was microdissected away from the hippocampus under a dissection microscope, to include the granule cell and molecular layers. The samples were homogenized at 4°C in lysis buffer (0.5M HEPES, 3M NaCl, 1M MgCl2, 0.5M EDTA, 0.1M DTT, 10% SDS, 10% deoxycholate and 0.3% Triton X-100) and centrifuged at 13000 rpm at 4°C for 10 minutes. The supernatant was collected leaving the pellet behind. Proteins were quantified using a Bradford protein assay. Each lane was loaded with 15 μg of protein in 10% Tris·HCl polyacrylamide gels and electrophoresed at 110 V for 60 to 90 min. Proteins were then transferred at 200 mA for 1h to polyvinylidene difluoride membranes for Western blot analysis. Membranes were rinsed in PBS and blocked in 1:1 Odyssey blocking buffer/PBS (Odyssey, Li-COR Biosciences, Lincoln, NE) for 1h at room temperature. Membranes were then either incubated 1hr at room temperature or overnight at 4°C, with polyclonal VR1 N-terminus (1:2500) (Neuromics, Edina, MN) and α-actin (1:1000) primary antibodies (Sigma, St.Louis, MO) in 1:1 Odyssey blocking buffer/PBS/0.1% Tween 20. Following four 5-min washes (PBS + 0.1% Tween 20), membranes were incubated with fluorescence-conjugated goat anti-rabbit IRDye-680® and goat anti-rabbit IRDye-800® secondary antibodies (1:5000 Odyssey) in Odyssey blocking buffer/PBS/0.1% Tween 20 and 0.01% SDS for 1 hr, followed by four 5-min washes. After a final 10 min PBS wash, the membranes were dried and blots were scanned by a densitometer (Odyssey model 9120, Li-COR Biosciences, Lincoln, NE) to quantify band density. Background density was subtracted from the TRPV1 receptor band density and normalized to α-actin, which was used as the loading control.
Statistical analysis
A value of twice the mean peak-to-peak noise level (typically 3–5 pA) for a given recording in control solutions was used as the detection limit for minimal PSC amplitude. For sEPSCs and mEPSCs, at least 2 min of activity in control and in agonist-containing ACSF was examined to identify agonist effects on amplitude and frequency. Typically, 150–300 events were compared for each condition. The effects of drugs on sEPSC and mEPSC frequencies were analyzed within a recording using the Kolmogorov-Smirnov (K-S) test. Pooled results from responding cells were analyzed using a paired Student’s t-test. Western Blots were analyzed using single factor ANOVA. Results are reported as the mean ± SEM unless indicated otherwise; significance was set at P < 0.05 for all statistical measures.
Results
As in previous analyses of this model of inducible TLE in mice (Shibley and Smith, 2002; Winokur et al., 2004) and rats (Cavalheiro et al., 1996), animals that underwent SE after pilocarpine injection developed spontaneous seizures, mossy fiber sprouting, and showed evidence of recurrent excitatory circuit formation in the dentate gyrus within a few weeks, whereas animals that did not undergo SE did not develop any of these characteristics and were not different from untreated animals with regard to these parameters. Figure 1 shows the dentate gyrus of a control mouse and a pilocarpine-treated mouse that survived SE. Similar to previous reports in this model (Shibley and Smith, 2002; Borges et al., 2003; Winokur et al., 2004), extensive mossy fiber sprouting was seen extending into the inner molecular layer in pilocarpine-treated mice that survived SE, whereas the control mice did not have mossy fiber sprouting.
Figure 1.
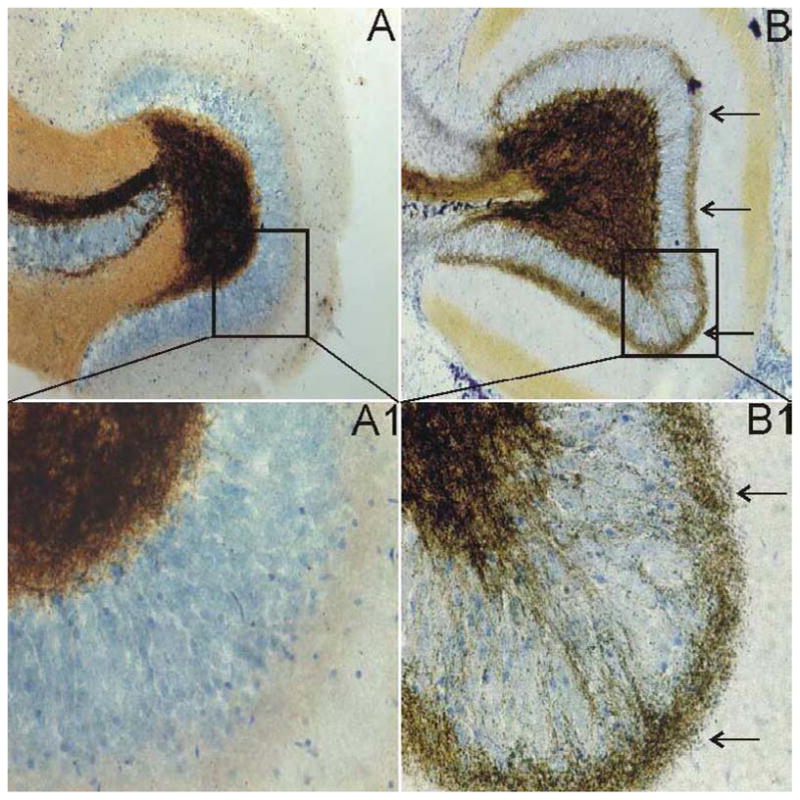
Timm staining showing mossy fiber sprouting into the inner molecular layer of the dentate gyrus in pilocarpine-treated mice. A. Dentate gyrus of a normal mouse. B. Dentate gyrus of a pilocarpine-treated mouse that survived SE showing mossy fiber sprouting. A1 and B1 are enlarged boxed regions of A and B. The arrows in B and B1 point to extensive mossy fiber sprouting in the inner molecular layer of the dentate gyrus.
Whole-cell patch-clamp recordings were made from six untreated animals, three pilocarpine-treated animals that did not undergo SE (injected controls) and 27 pilocarpine-treated animals that survived SE. Preliminary tests were done to detect differences between injected controls and untreated animals. Untreated animals and injected controls did not differ behaviorally, anatomically or electrophysiologically and results from these groups were therefore considered together as control data unless otherwise specified.
In nominally Mg2+-free ACSF that contained bicuculline (30μM), a solution that was meant to expose glutamatergic connections by removing Mg2+-dependent blockade of NMDA receptor-mediated excitation and also reduce recurrent GABAA receptor-mediated synaptic inhibition, patch-clamp recordings from granule cells in control mice voltage-clamped at −70 mV revealed sEPSCs with an average frequency of 1.21 ± 0.15 Hz (n=6 cells). In animals that had experienced SE and developed spontaneous seizures, barrages of sEPSCs were superimposed on the ongoing sEPSC activity. The frequency of sEPSCs in cells from these animals, including those within the sEPSC barrages, was 2.94 ± 0.27 Hz (n=12 cells), which was significantly higher than the control group (P<0.05).
Effect of TRPV1 receptor activation on sEPSCs
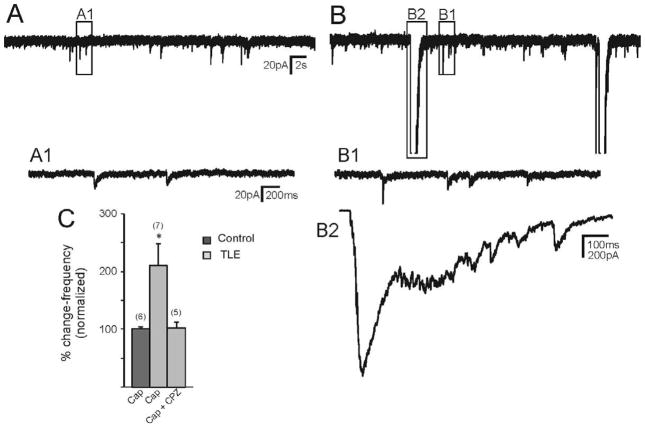
In Mg2+-free ACSF containing bicuculline, application of capsaicin (1 μM) to recordings from granule cells in normal animals resulted in no change in frequency of sEPSCs (1.21 ± 0.15 Hz control; 1.23 ± 0.17 Hz capsaicin; n=6 cells; P>0.05; paired t-test; Fig. 2). The amplitude of sEPSCs was also unchanged (P>0.05). In granule cells from mice that survived pilocarpine-induced SE, application of capsaicin increased the frequency of sEPSCs from a control value of 2.83 ± 0.46 Hz to 6.13 ± 1.33 Hz in the presence of capsaicin (Fig. 2; P<0.05; n=7 cells). The increase in sEPSCs was blocked by pre-application of the TRPV1 antagonist CPZ (10 μM; 3.09 ± 0.11 Hz control; 3.17 ± 0.30 Hz capsaicin; p>0.05; n=5 cells; Fig. 2C) indicating the involvement of TRPV1 in the response. No effect of CPZ alone was observed. In addition, no capsaicin-mediated increase in sEPSC frequency was observed in two cells pre-treated with another TRPV1 antagonist, 5′-iodoresiniferatoxin (1 μM). Capsaicin increased the frequency of sEPSCs through the activation of TRPV1 in pilocarpine-treated mice that survived SE, but not in controls.
Figure 2.
Effect of capsaicin (Cap) on sEPSCs recorded from pilocarpine-treated mice with TLE. A. control trace recorded from a granule cell of a pilocarpine-treated mouse that survived SE. B. Addition of capsaicin increased the frequency of sEPSCs. A1, B1 and B are 2 expanded regions of the boxed areas of A and B. C. Normalized graph showing cap did not have effect on sEPSCs of control mice (p<0.05). In mice with TLE, cap significantly increased sEPSC frequency (asterisk indicates p<0.05) and this effect was blocked by pre-application of capsazepine (CPZ). Number of neurons is indicated in parentheses for each condition.
Action potential-independent glutamate release was also compared between the two groups by measuring miniature EPSC (mEPSC) frequency and amplitude. In the presence of TTX, mEPSC frequency in controls was 1.33 ± 0.16 Hz (n=11 cells). In pilocarpine-treated mice that survived SE, it was significantly higher at 1.93 ± 0.15 Hz (n=27 cells; p<0.05). The amplitude of mEPSCs was not different between the two groups (p>0.05). These data indicated that granule cells from pilocarpine-treated mice that survived SE received enhanced spontaneous glutamatergic synaptic input versus control mice or mice that did not experience SE after pilocarpine injection.
Effect of TRPV1 activation by capsaicin on mEPSCs
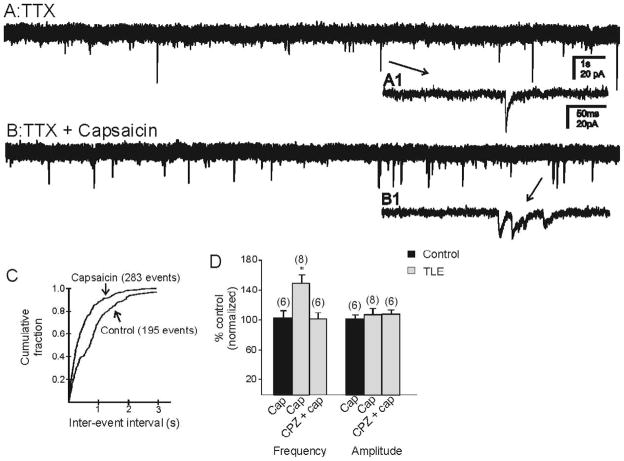
Capsaicin (1 μM) had no significant effect on the frequency of mEPSCs in granule cells from control mice (1.31 ± 0.22 Hz control; 1.42 ± 0.38 Hz capsaicin; n=6 cells; p>0.05; Fig. 3D). In cells from pilocarpine-treated mice that survived SE, capsaicin increased the frequency of mEPSCs from 1.63 ± 0.17 Hz to 2.35 ± 0.23 Hz (Fig. 3; n=8 cells; p<0.05). The capsaicin-induced increase in mEPSC frequency was blocked by the addition of CPZ (10μM; 2.19 ± 0.33 Hz control; 2.09 ± 0.37 capsaicin; n=6 cells; p>0.05). Capsaicin did not alter the amplitude of mEPSCs in either group (p>0.05). These data suggest that capsaicin acts through TRPV1 activation to increase action potential-independent release of glutamate in the dentate gyrus of animals that survived SE but not in controls.
Figure 3.
Capsaicin increased frequency of mEPSCs in pilocarpine-treated mice. A. mEPSCs recorded from granule cell of a pilocarpine-treated mouse in the presence of TTX (1μM). B. mEPSC frequency increased with the application of capsaicin. A1 and B1 are expanded regions of A and B. C. Kolmogrov-Smirnov analysis showing reduction of inter-event interval after application of capsaicin in the same recording. D. Cumulative normalized data from control mice and pilocarpine-treated mice showing that capsaicin increased frequency of mEPSCs in pilocarpine-treated mice but not in controls. * indicates significance (P<0.05) versus control conditions. Number of neurons is indicated in parentheses for each condition.
Effect of endocannabinoid/endovanilloid agonist anandamide (AEA) on mEPSCs
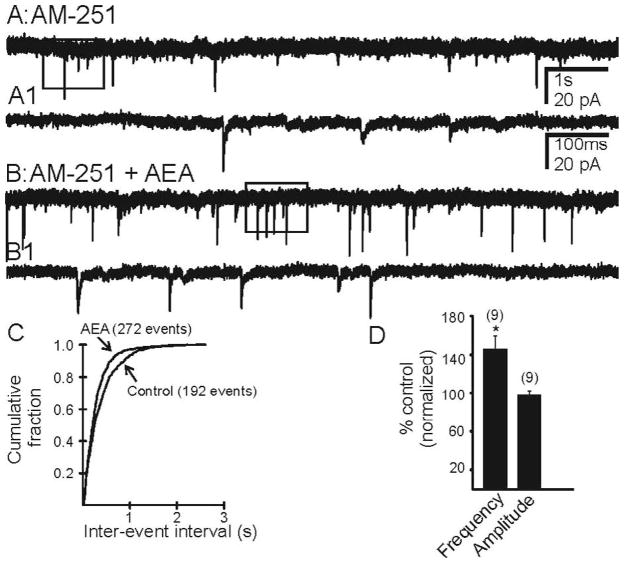
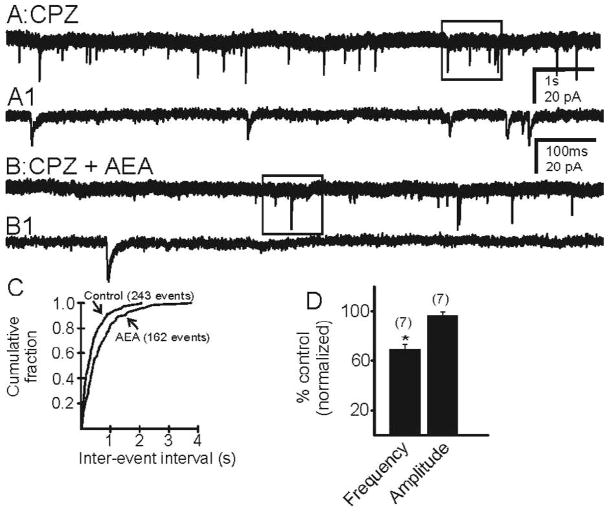
AEA (10 μM) was applied in the presence of the CB1R antagonist AM 251 (10 μM), to isolate any TRPV1-mediated effects or in the presence of TRPV1 antagonist CPZ (10 μM), to isolate any effects of CB1R activation. Neither antagonist had any effect alone on mEPSC frequency. In the presence of AM 251, AEA (10 μM) increased the frequency of mEPSCs from 1.62 ± 0.26 Hz to 2.27 ± 0.44 Hz (Fig. 4; n=9 cells; p<0.05) in pilocarpine-treated mice that survived SE. No subsequent decrease in frequency was observed. In the presence CPZ, AEA (10 μM) decreased the frequency of mEPSCs from 2.03 ± 0.4 Hz to 1.35 ± 0.24 Hz (Fig. 5; n=7 cells; p<0.05), with no transient increase in EPSC frequency. AEA did not have any effect of the amplitude of mEPSCs (p>0.05). There was no effect of AEA on mEPSCs in neurons from control animals (p>0.05). These data suggest that AEA is capable of activating both CB1R and TRPV1 in the dentate gyrus of animals that had survived SE and undergone mossy fiber sprouting.
Figure 4.
AEA increased the frequency of mEPSCs in the presence of CB1R antagonist, AM 251. A. Control trace recorded from a granule cell of a pilocarpine-treated mouse showing mEPSCs in the presence of AM 251. B. Trace showing increase in frequency after addition of AEA. A1 and B1 are expanded segments indicated by the boxed areas in A and B respectively. C. Kolmogrov-Smirnov analysis of the same recording showing reduction in the inter-event interval after the addition of AEA. D. Cumulative normalized data showing an increase in mEPSC frequency induced by AEA when CB1R were blocked in neurons from seven pilocarpine-treated mice with TLE. * indicates significant change (p<0.05) versus control conditions. Number of neurons is indicated in parentheses for each condition.
Figure 5.
AEA decreased the frequency of mEPSCs in the presence of the TRPV1 receptor antagonist, CPZ. A. Control trace recorded from a granule cell of a pilocarpine-treated mouse showing mEPSCs in the presence of CPZ. B. Trace illustrating a decrease in the frequency of mEPSCs after the addition of AEA. A1 and B1 are expanded segments indicated by the boxed areas in A and B respectively. C. Kolmogrov-Smirnov analysis showing an increase in the inter-event interval after the addition of AEA. D. Cumulative normalized data indicating a decrease in mEPSC frequency induced by AEA when TRPV1 receptors were blocked in neurons from six mice with TLE. * indicates significant change (p<0.05) versus control conditions. Number of neurons is indicated in parentheses for each condition.
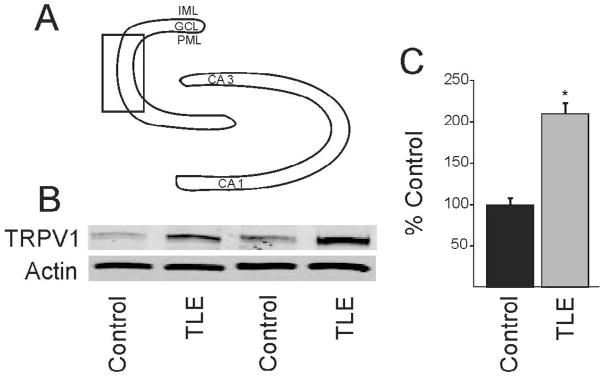
Since effects were seen in animals with mossy fiber sprouting but not control animals, we reasoned that the newly sprouted axon terminals express TRPV1 receptors and this may be reflected in increased TRPV1 receptor protein expression in the dentate gyrus of pilocarpine-treated mice that survived SE. The dentate gyrus was microdissected to include the granule cell layer and the molecular layer in controls and pilocarpine-treated mice that survived SE. A discrete band at 95 kDa, corresponding to the molecular weight of the TRPV1 receptor was identified by Western blot, consistent with previous studies using this antibody (Stein et al., 2006). Western blot analysis showed that the TRPV1 receptor protein labeling was significantly increased by 111% over control levels in pilocarpine-treated mice (n=4 mice per group; p<0.05; Fig. 6).
Figure 6.
Western blot detection of TRPV1 receptor expression in the dentate gyrus. A. Diagram of dentate gyrus showing the micro-dissected area (box). B. Western blot showing TRPV1 receptor expression in two untreated mice and in two pilocarpine-treated mice that survived SE. Actin was used as the loading control which did not change significantly. C. Graph showing significant (p<0.05; n=4) increase in TRPV1 receptor expression in epileptic mice.
Discussion
Studies from our lab and others have shown that cannabinoids possess potential therapeutic properties for a variety of conditions including TLE. However, the ability of some endocannabinoids to act centrally at cellular targets other than CB1R has not been extensively examined in brain disorder models. This study was initiated to identify the effects of TRPV1 activation in the brains of an animal model of TLE, which is one target for the endocannabinoid agonist, AEA. In the present study, we demonstrate that activation of TRPV1 receptors with an agonist (i.e., capsaicin) increases both action-potential-dependent (sEPSCs) and -independent (mEPSCs) glutamate release onto dentate gyrus granule cells of a mouse model of TLE, but not in controls. There was no effect on the amplitude in the presence of TTX suggesting a pre-synaptic site for the TRPV1 on glutamate terminals. This capsaicin-induced effect was prevented by the pre-application of the selective TRPV1 antagonist CPZ, confirming it was TRPV1 receptor-mediated. No effect of CPZ alone was observed, suggesting that its main effect was to antagonize TRPV1 receptors. The endocannabinoid/endovanilloid agonist AEA activates both CB1R and TRPV1 in the synaptically reorganized neuronal circuitry in the dentate gyrus of mice with TLE. The increase in EPSC frequency mediated by AEA was entirely prevented by TRPV1 blockade, and the AEA-mediated decrease in EPSC frequency was blocked by a CB1R antagonist. Whereas it is possible that AEA may act at additional receptors to alter membrane properties, the effects on EPSC frequency we observed were consistent with binding to TRPV1 or CB1R. Western analysis indicated that the protein band with a molecular weight corresponding to TRPV1 labeling was denser in the dentate gyrus from epileptic animals, consistent with the hypothesis that increased TRPV1 function was associated with increased TRPV1 protein expression in the dentate gyrus of mice with TLE compared to control animals. These receptors and others might be expressed on newly sprouted mossy fiber terminals after synaptic reorganization.
In this and other models of TLE, mossy fiber sprouting is robust and leads to increased glutamatergic recurrent excitatory connections between granule cells (Sutula et al., 1989; Winokur et al., 2004). Sprouted axons from perforant path inputs, CA3, or mossy cells might also contribute to the recurrent excitatory circuit in animals with TLE. However, there is little evidence for these circuits contributing robustly to network excitability in TLE models, whereas evidence for increased recurrent circuitry mediated by sprouted mossy fibers is compelling. In animal models of TLE, there is a change in the neuronal circuitry which results in an imbalance between synaptic excitation and inhibition and renders the brain more susceptible to seizure generation (Ben-Ari et al., 1981; Babb et al., 1991; Dudek and Spitz, 1997). Hippocampal sclerosis, selective neuron loss, and synaptic reorganization of dentate granule cells are some of the well characterized changes in human TLE (de Lanerolle et al., 1989; Sutula et al., 1989; Houser et al., 1990; Sloviter et al., 1991; Mathern et al., 1995; Zhu et al., 1997; Magloczky et al., 2000). We used pilocarpine-induced SE in mice to induce TLE for our experiments. This model has many of the same characteristic features as seen in human tissues, and is very similar to pilocarpine-induced TLE models in rats (Shibley and Smith, 2002; Borges et al., 2003; Winokur et al., 2004). Several other TLE models share these features, which result in increased network excitability in multiple brain areas and in seizure generation (Ben-Ari, 1985; Sutula et al., 1989; Wuarin and Dudek, 1996, 2001; Smith and Dudek, 1997,2001; Patrylo and Dudek, 1998). In this mouse model, extensive mossy fiber sprouting into the inner molecular layer of the dentate gyrus is observed (Fig. 1), suggesting the existence of new functional excitatory synapses between granule cells (Winokur et al., 2004). Such mossy fiber sprouting was not seen in control mice that did not experience SE. Moreover, we and others have found that selective stimulation of granule cells, but not hilar or CA3 areas, activates the recurrent excitatory circuit in TLE models (Molnar and Nadler, 1999; Winokur et al., 2004). New connections between granule cells likely account for the increased excitability observed in the dentate gyrus under the experimental conditions studied here.
The TRPV1 (also called vanilloid type 1) receptors, cloned in 1997 (Caterina et al., 1997), are non-specific ion channels, permeable to Ca2+ and Na+. When activated, they primarily increase neuronal excitability (Van Der Stelt and Di Marzo, 2004). The receptors are activated by a variety of noxious stimuli including heat, pH, capsaicin (the pungent ingredient in hot chili peppers), plant toxins like resiniferatoxin, and by a subset of endogenous cannabinoid ligands, including AEA, which has been identified in the dentate gyrus (Tominaga et al., 1998; Caterina et al., 1999; Smart et al., 2000). Physiological functions of the receptors have been well characterized in peripheral sensory neurons (Szallasi and Blumberg, 1999). Initially believed to be absent in the brain (Caterina et al., 1997), subsequent studies have shown TRPV1 mRNA to be present in various brain regions including the dentate gyrus and the hippocampus (Mezey et al., 2000; Roberts et al., 2004; Toth et al., 2005; Cristino et al., 2006). Although speculated to be present on the GABAergic terminals in the hippocampus of normal animals and to increase GABA release in CA1 region when activated (Al-Hayani et al., 2001; Huang et al., 2002), their function in the brain is still not well defined (Kofalvi et al., 2006). Evidence also exists to demonstrate the presence of TRPV1-mediated effects on glutamatergic terminals in other regions (Hajos and Freund, 2002; Marinelli et al., 2002; Marinelli et al., 2003; Derbenev et al., 2006). Recent evidence also suggest that there could be a non-TRPV1-mediated effect of high concentrations (i.e., 10 μM) of capsaicin on glutamatergic transmission in the dentate gyrus (Benninger et al., 2008). However here, we found that capsaicin- or AEA-induced increases in glutamate release were blocked by the highly selective TRPV1 antagonist, CPZ, implicating TRPV1 receptors in the response.
The effect of TRPV1 activation on the reorganized synaptic circuit associated with the neuropathology of TLE has not been previously studied. Considering that the slice preparation used here excluded the entorhinal cortex and that there was no measurable effect of capsaicin in slices from control mice, it is possible that some functional TRPV1 receptors are expressed de novo on the newly sprouted mossy fiber terminals formed after synaptic reorganization. Capsaicin increased the frequency of sEPSCs by about 115% whereas the frequency of mEPSCs was increased only by about 45%. Synaptic network reorganization and activation of that network by capsaicin may contribute to this difference by causing spike-dependent increases in the frequency and synchronous firing of action potentials in other granule cells by increasing glutamate release. This suggests that in pilocarpine-treated mice that survived SE, activation of TRPV1 receptors could increase synaptic network activity and, by inference, might increase seizure-like activity.
AEA is believed to be a functional agonist at both CB1R and TRPV1 (Devane et al., 1992; Van Der Stelt and Di Marzo, 2004). Endocannabinoids like AEA and 2-AG are increased during seizures or cellular activity (Marsicano et al., 2003; Wallace et al., 2003) and are thought to play a protective role during excitatory conditions. However, native CB1R are most often described on inhibitory GABAergic terminals (Katona et al., 1999; Katona et al., 2000) and mediate depolarization induced suppression of inhibition (DSI) (Alger and Pitler, 1995; Wilson and Nicoll, 2001). Hence the typical action of CB1R activation would be to reduce GABA release and, by inference, increase seizure severity. However, loss of hilar inhibitory interneurons and mossy cells during epileptogenesis (Buckmaster and Dudek, 1997) may be associated with a concomitant loss of CB1R in inhibitory circuits. This would allow released endocannabinoids to act on other molecular targets including CB1R or TRPV1 located on glutamatergic terminals. In human patients (Braakman et al., 2009) or rat models (Wallace et al., 2003) with TLE, treatment with CB1R antagonists exacerbates seizures, suggesting that released endocannabinoids act at CB1R to keep seizures in check. Our data suggest that blocking CB1R not only removes this negative control, but may also allow released eCBs to bind TRPV1 and contribute to seizures. Hence, depending on the site of action, endocannabinoids could either suppress synaptic activity through their action at CB1R, or increase synaptic excitation through their action at TRPV1 receptors.
In our experimental conditions with GABAA receptors blocked with bicuculline, AEA-mediated effects of activating CB1R and TRPV1 on glutamatergic synapses were isolated using the antagonists, CPZ and AM 251, respectively. Effects on GABA release are being studied separately. Consistent with the hypothesis, AEA decreased synaptic activity in the presence of CPZ, but increased synaptic activity in the presence of AM 251. This suggests the presence of functional CB1R and TRPV1 receptors in the reorganized circuit. There was no change in the amplitude of mEPSCs suggesting a presynaptic location for TRPV1, similar to CB1R, which are also typically located on synaptic terminals in the dentate gyrus (Katona et al., 1999; Marinelli et al., 2003). If the receptors are located on robustly-sprouted mossy fiber terminals, this could account for the increased TRPV1 protein levels in the dentate gyrus suggested by Western blot analysis.
It is not clear from the above experiments whether both CB1R and TRPV1 are present in the same or different synaptic terminals but the vast number of potential new synapses associated with mossy fiber sprouting could include both cases, at least in this model. Activation by AEA of additional receptors not examined here also remains a possibility. This study provides insight to potential side-effects that might be associated with the design of pharmaceutical therapies for conditions like TLE based on the endocannabinoid system. Whereas endocannabinoid ligands might bind CB1R to reduce neurotransmitter release, some of these ligands, including AEA, might increase synaptic excitability by acting at newly expressed TRPV1 on glutamatergic terminals in the dentate gyrus. Because activity-dependent release of endocannabinoids has been demonstrated in the dentate gyrus (Wallace et al., 2003; Isokawa and Alger, 2005), ubiquitous blockade of CB1R in patients with TLE and mesial temporal lobe sclerosis should be considered with caution (Braakman et al., 2009).
Acknowledgments
Funded by grants from the NIH (R21 NS052302) and the Epilepsy Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hayani A, Wease KN, Ross RA, Pertwee RG, Davies SN. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41:1000–1005. doi: 10.1016/s0028-3908(01)00145-9. [DOI] [PubMed] [Google Scholar]
- Alger BE, Pitler TA. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995;18:333–340. doi: 10.1016/0166-2236(95)93923-l. [DOI] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Benninger F, Freund TF, Hajos N. Control of excitatory synaptic transmission by capsaicin is unaltered in TRPV1 vanilloid receptor knockout mice. Neurochem Int. 2008;52:89–94. doi: 10.1016/j.neuint.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran MD, Smith BN. Cannabinoid and vanilloid interactions in the modified dentate gyrus of a pilocapine-induced status epilepticus mouse model of temporal lobe epilepsy. Epilepsia. 2007;48 (S6):118. [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Braakman HMH, van Oostenbrugge RJ, van Kranen-Mastenbroek VHJM, de Krom MCTFM. Rimonabant induces partial seizures in a patient with a history of generalized epilepsy. Epilepsia. 2009;50:2171. doi: 10.1111/j.1528-1167.2009.02203.x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Santos NF, Priel MR. The pilocarpine model of epilepsy in mice. Epilepsia. 1996;37:1015–19. doi: 10.1111/j.1528-1157.1996.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Castillo PE. Input-specific plasticity at excitatory synapses mediated by endocannabinoids in the dentate gyrus. Neuropharmacology. 2008;54:68–78. doi: 10.1016/j.neuropharm.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Monroe MJ, Glatzer NR, Smith BN. Vanilloid-mediated heterosynaptic facilitation of inhibitory synaptic input to neurons of the rat dorsal motor nucleus of the vagus. J Neurosci. 2006;26:9666–9672. doi: 10.1523/JNEUROSCI.1591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Spitz M. Hypothetical mechanisms for the cellular and neurophysiologic basis of secondary epileptogenesis: proposed role of synaptic reorganization. J Clin Neurophysiol. 1997;14:90–101. doi: 10.1097/00004691-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA. Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia. 1995;36:543–558. doi: 10.1111/j.1528-1157.1995.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokawa M, Alger BE. Retrograde endocannabinoid regulation of GABAergic inhibition in the rat dentate gyrus granule cell. J Physiol. 2005;567:1001–1010. doi: 10.1113/jphysiol.2005.094219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci. 2009;32:215–24. doi: 10.1016/j.tins.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kofalvi A, Oliveira CR, Cunha RA. Lack of evidence for functional TRPV1 vanilloid receptors in rat hippocampal nerve terminals. Neurosci Lett. 2006;403:151–156. doi: 10.1016/j.neulet.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Kofalvi A, Pereira MF, Rebola N, Rodrigues RJ, Oliveira CR, Cunha RA. Anandamide and NADA bi-directionally modulate presynaptic Ca2+ levels and transmitter release in the hippocampus. Br J Pharmacol. 2007;151:551–563. doi: 10.1038/sj.bjp.0707252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, Watanabe M, Palkovits M, Magloczky Z, Freund TF, Katona I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloczky Z, Wittner L, Borhegyi Z, Halasz P, Vajda J, Czirjak S, Freund TF. Changes in the distribution and connectivity of interneurons in the epileptic human dentate gyrus. Neuroscience. 2000;96:7–25. doi: 10.1016/s0306-4522(99)00474-1. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Vaughan CW, Christie MJ, Connor M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J Physiol. 2002;543:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, Mercuri NB. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci. 2003;23:3136–3144. doi: 10.1523/JNEUROSCI.23-08-03136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár P, Nadler JV. Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J Neurophysiol. 1999;82:1883–1894. doi: 10.1152/jn.1999.82.4.1883. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol. 1998;79:418–429. doi: 10.1152/jn.1998.79.1.418. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Shibley H, Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002;49:109–120. doi: 10.1016/s0920-1211(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Sollas AL, Barbaro NM, Laxer KD. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J Comp Neurol. 1991;308:381–396. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Enhanced population responses in the basolateral amygdala of kainate-treated, epileptic rats in vitro. Neurosci Lett. 1997;222:1–4. doi: 10.1016/s0304-3940(97)13326-2. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Short- and long-term changes in CA1 network excitability after kainate treatment in rats. J Neurophysiol. 2001;85:1–9. doi: 10.1152/jn.2001.85.1.1. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Network interactions mediated by new excitatory connections between CA1 pyramidal cells in rats with kainate-induced epilepsy. J Neurophysiol. 2002;87:1655–1658. doi: 10.1152/jn.00581.2001. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Cristino L, Di Marzo V. TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr Pharm Des. 2008;14:42–54. doi: 10.2174/138161208783330790. [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–22. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol. 2002;452:295–301. doi: 10.1016/s0014-2999(02)02331-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Winokur RS, Kubal T, Liu D, Davis SF, Smith BN. Recurrent excitation in the dentate gyrus of a murine model of temporal lobe epilepsy. Epilepsy Res. 2004;58:93–105. doi: 10.1016/j.eplepsyres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol. 2001;85:1067–1077. doi: 10.1152/jn.2001.85.3.1067. [DOI] [PubMed] [Google Scholar]
- Zhu ZQ, Armstrong DL, Hamilton WJ, Grossman RG. Disproportionate loss of CA4 parvalbumin-immunoreactive interneurons in patients with Ammon’s horn sclerosis. J Neuropathol Exp Neurol. 1997;56:988–998. doi: 10.1097/00005072-199709000-00004. [DOI] [PubMed] [Google Scholar]