Abstract
Background
Rapid identification of diverse fusion genes with involvement of PDGFRA or PDGFRB in eosinophilia-associated myeloproliferative neoplasms is essential for adequate clinical management but is complicated by the multitude and heterogeneity of partner genes and breakpoints.
Design and Methods
We established a generic quantitative reverse transcriptase polymerase chain reaction to detect overexpression of the 3′-regions of PDGFRA or PDGFRB as a possible indicator of an underlying fusion.
Results
At diagnosis, all patients with known fusion genes involving PDGFRA (n=5; 51 patients) or PDGFRB (n=5; 7 patients) showed significantly increased normalized expression levels compared to 191 patients with fusion gene-negative eosinophilia or healthy individuals (PDGFRA/ABL: 0.73 versus 0.0066 versus 0.0064, P<0.0001; PDGFRB/ABL: 196 versus 3.8 versus 5.85, P<0.0001). The sensitivity and specificity of the activation screening test were, respectively, 100% and 88.4% for PDGFRA and 100% and 94% for PDGFRB. Furthermore, significant overexpression of PDGFRB was found in a patient with an eosinophilia-associated myeloproliferative neoplasm with uninformative cytogenetics and an excellent response to imatinib. Subsequently, a new SART3-PDGFRB fusion gene was identified by 5′-rapid amplification of cDNA ends polymerase chain reaction (5′-RACE-PCR).
Conclusions
Quantitative reverse transcriptase polymerase chain reaction analysis is a simple and useful adjunct to standard diagnostic assays to detect clinically significant overexpression of PDGFRA and PDGFRB in eosinophilia-associated myeloproliferative neoplasms or related disorders.
Keywords: myeloproliferative neoplasm, PDGFRA, PDGFRB, RQ-PCR
Introduction
The identification of the BCR-ABL fusion gene and variable point and length mutations of JAK2 and MPL have highlighted the fundamental role of constitutively activated tyrosine kinases in the pathogenesis of myeloproliferative neoplasms such as chronic myeloid leukemia, polycythemia vera, essential thrombocythemia and primary myelofibrosis.1,2 In contrast, the majority of underlying molecular aberrations in other and less frequent subtypes of myeloproliferative neoplasms, such as atypical chronic myeloid leukemia, overlap syndromes between myelodysplastic syndrome and myeloproliferative neoplasms, chronic eosinophilic leukemia, hypereosinophilic syndrome, chronic myelomonocytic leukemia and chronic neutrophilic leukemia are largely unknown.
A minority of cases present with acquired chromosomal aberrations or cytogenetically invisible deletions leading to constitutive activation of related tyrosine kinases such as PDGFRA, PDGFRB, FGFR1, JAK2, and FLT3 through fusion to a variety of unrelated partner genes.1,3 The new World Health Organization classification now includes patients with fusion genes and involvement of PDGFRA, PDGFRB and FGFR1 in a separate category.4 At present, the most common abnormalities are FIP1L1-PDGFRA fusions in chronic eosinophilic leukemia resulting from a cytogenetically invisible deletion on 4q12 and the ETV6-PDGFRB fusion in chronic myelomonocytic leukemia with a t(5;12)(p12;q31–33).5,6 However, five other PDGFRA fusion partners and more than 20 PDGFRB fusion partners have been reported to be associated with eosinophilia-associated myeloproliferative neoplasms including chronic eosinophilic leukemia, chronic myelomonocytic leukemia, atypical chronic myeloid leukemia, and myelodysplastic/myeloproliferative neoplasms.3 Although these abnormalities are very uncommon, they are associated with excellent responses to imatinib and thus their detection is critical for optimal management of patients.7–12 Accurate detection is, however, complicated by several factors: (i) bone marrow cytogenetic assessment, which is critical to the detection of 4q (PDGFRA) or 5q (PDGFRB) rearrangements for all fusions apart from FIP1L1-PDGFRA, may fail to yield adequate metaphases; (ii) the size of the clone in peripheral blood may be very small and the abnormality thus escapes detection by cytogenetics; (iii) split apart fluorescence in situ hybridization (FISH) may fail to detect small clones or cases with complex rearrangements;13,14 (iv) the heterogeneity of fusion partners and breakpoints makes it difficult and expensive to develop comprehensive and specific reverse transcriptase polymerase chain reaction (RT-PCR) assays.15 Although some clinicians consider that a short trial of imatinib might be the best way to identify sensitive cases, this is simply not possible in many countries due to budgetary and prescribing restrictions.
We describe here the development of technically straightforward generic quantitative RT-PCR (RQ-PCR) assays that enable rapid screening of patients with eosinophilia-associated myeloproliferative neoplasms, hypereosinophilic syndrome and reactive eosinophilia for the potential constitutive activation of PDGFRA and PDGFRB by fusion genes as adjuncts to standard diagnostic tests.
Design and Methods
Patients and samples
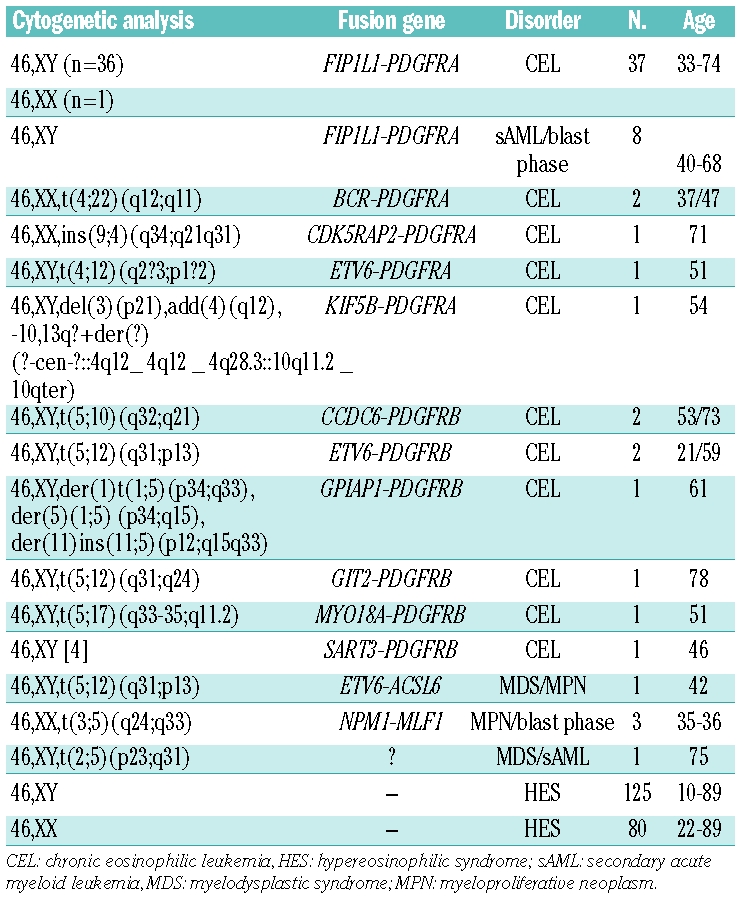
A total of 542 peripheral blood samples from 249 patients (170 males, 79 females) and 35 healthy individuals were investigated. The study included diagnostic samples from 45 patients (44 males, 1 females; median age 54 years, range 33–75) with a FIP1L1-PDGFRA fusion gene (chronic phase: n=37; blast phase: n=8), six patients (4 males, 2 females; median age 51 years, range 37–71) with diverse PDGFRA fusion genes (BCR-PDGFRA,16 n=3; ETV6-PDGFRA,17 n=1; CDK5RAP2-PDGFRA,18 n=1 or KIF5B-PDGFRA,19 n=1) and seven patients (7 males; median age 57 years, range 21–78) with diverse PDGFRB fusion genes (ETV6-PDGFRB,6 n=2, CCDC6-PDGFRB,14,20 n=2; GIT2-PDGFRB,21 n=1 GPIAP1-PDGFRB,21 n=1 and MYO18A-PDGFRB,22 n=1). In addition, five patients (3 males, 2 females; median age 46 years, range 36–75) with reciprocal translocations and involvement of chromosome bands 5q31–32 (Table 1) were analyzed, as were 191 diagnostic samples from patients with eosinophilia-associated myeloproliferative neoplasms, hypereosinophilic syndrome or eosinophilia of unknown origin (115 males, 76 females; median age 56 years, range 5–89), which had tested negative for FIP1L1-PDGFRA. Seven of these patients without known molecular aberrations had a sustained response to treatment with imatinib and were screened within this series. Informed consent was obtained from all patients according to the Declaration of Helsinki.
Table 1.
Patients’ characteristics.
Cytogenetic analysis
Bone marrow cells were cultured for 24 or 48 h. Metaphases were analyzed after G-banding or R-banding and karyotypes are described according to the International System for Human Cytogenetic Nomenclature (2005).
Cell and mRNA dilutions
FIP1L1-PDGFRA positive EOL-1 cells were serially diluted in HL-60 cells (both obtained from DSMZ, Braunschweig, Germany). In addition, serial dilutions of RNA from a FIP1L1-PDGFRA-positive patient and a CCDC6-PDGFRB-positive patient were made with RNA from a healthy donor (Peripheral Leukocytes Total RNA, Clontech, Mountain View, CA, USA).
RNA extraction, cDNA synthesis and reverse transcriptase polymerase chain reaction
Following red cell lysis for the isolation of total leukocytes from peripheral blood using standard procedures, RNA was extracted using TrizolTM reagent (Invitrogen, Karlsruhe, Germany), the RNeasy extraction kit (Qiagen, Hilden, Germany) or cesium chloride gradient ultracentrifugation, as described elsewhere.23 RNA was reverse transcribed using random hexamer priming and MMLV reverse transcriptase (Invitrogen). Single-step and nested RT-PCR for the detection of FIP1L1-PDGFRA,5 BCR-PDGFRA,16 ETV6-PDGFRA,17 CDK5RAP2-PDGFRA,18 KIF5B-PDGFRA,19 ETV6-PDGFRB,11 H4-PDGFRB,14,20 GIT2-PDGFRB,21 GIPIAP1-PDGFRB,21 and MYO18A-PDGFRB22 fusion genes was performed as previously described. Single-step primers for the detection of the SART3-PDGFRB fusion gene were SART2F: 5'′ CTGATTATGTGGAGATTTGGCA-3′, and PDGFR-C 5′-TGGCTTCTTCTGCCAAAGCA-3′. The reciprocal fusion transcript was amplified with PDB-9F 5′-AGACCTCAAAAGGT-GTCCACG-3′ and SART19R 5′-TAGAGACAGCTGCGTCCTTC-3′. ETV6-ACSL6 and NPM1-MLF1 fusion transcripts were amplified as previously described.24,25 Amplification reactions were undertaken for 32 cycles with an annealing temperature of 60°C.
Real time quantitative reverse transcriptase polymerase chain reaction
The expression of PDGFRA and PDGFRB was analyzed using the LightCycler instrument 1.5 (Roche Diagnostics, Mannheim, Germany). Each 20 μL reaction mix for PDGFRA RQ-PCR contained 4 μL LightCycler Faststart DNA Masterplus Hyb Probes Master Mix (Roche Diagnostics), 2 μL cDNA template or plasmid dilution, 0.5 μM forward primer PDA12F: 5′-CCAAGAGATGGACTAGTGCTTG-3′, 0.5 μM reverse primer PDA15R: 5′-TAGCTCCGTGTGCTTTCATCAG-3′, 0.25 μM anchor probe PDA15FL: GAATAGGGATAGCTTCCTGAGCCACCA-fluorescein, 0.25 μM sensor probe PDA15LC: LCred640-CCAGAGAAGCCAAA-GAAAGAGCTGGA-P, (TIB Molbiol, Berlin, Germany). The PDGFRB RQ-PCR reaction mix contained 4 μL LightCycler Faststart DNA Masterplus Hyb Probes Master Mix, 2 μL cDNA template or plasmid dilution, 0.5 μM forward primer PDB13F: 5′-CGTCAAGATGCTTAAATCCACAGC-3′, 0.5 μM reverse primer PDB15R: 5′-TGATGATATAGATGGGTCCTCCTTTG, 0.25 μM anchor probe PDB14FL: 5′-GCTGAAGATCATGAGTCACCTTGGGC-fluorescein, 0.25 μM sensor probe PDB14LC: LCRed640-CCACCTGAACGTGGTCAACCTGTTG-P. Cycler conditions were the following: 10 min denaturation at 95°C, 50 cycles of 10 sec at 59°C/60°C (annealing PDGFRA/PDGFRB) and 26 sec at 72°C (elongation). A 5 log series of plasmid dilutions (see below) was amplified within the PCR runs for quantification of PDGFRA and PDGFRB. ABL mRNA was quantified as an internal control as previously described.26 The LightCycler software prepares standard curves using linear regression analysis of the plasmid dilutions and calculates copy numbers of the unknown sample.27 Values below the lowest standard dilution for PDGFRA (4 copies) and for PDGFRB (400 copies) were assigned as negative.
Cloning of quantification standards
For plasmid preparation, nested RT-PCR products from sequences of PDGFRA and PDGFRB were amplified from cell lines (HL-60, PDGFRA; SW480, PDGFRB) with the following primers (PDGFRA - PDA11F1: 5′-TGGCTGCTGCAGTCCTGGTGCT-3′, PDA16R1: 5′-CTGTGTAGTATCAGCCTGCTTC-3′, PDA11F2: 5′-AGTCCTGGTGCTGTTGGTGATTGTGA-3′, PDA16R2: 5′-AGTATCAGCCTGCTTCATGTCCATGT-3′; PDGFRB - PDB1F: 5′-TGTCAGAGCTGACACTGGTTCG-3′, PDB1R: 5′-CCATGTAGTTGGAGGACTCGATG-3′, PDB2F: 5′-GCTGACACTGGTTCGCGTGAA-3′, PDB2R: 5′-GTTGGAGGACTCGATGTCTGCAT-3′). The Expand high fidelity plus PCR system (Roche Diagnostics) was used. PCR transcripts were cloned into the PCR2.1-TOPO vector and introduced into E. coli TOP10F’ according to the manufacturer’s instructions (Invitrogen). Plasmid DNA containing the desired construct was isolated using the Plasmid Midi and Maxi Kit (Qiagen) and inserts were confirmed by bidirectional direct sequencing. The resulting plasmid was linearized by XbaI digestion at 37°C for 2 h followed by heat inactivation at 65°C for 20 min. ABL mRNA transcripts were measured as an internal control using a standard plasmid (pME-2) containing BCR-ABL, ABL, and GUS sequences.28 Dilutions of the linearized plasmid were prepared in 10 mM Tris-HCl pH 8.0; 1 mM EDTA containing 20 μg/mL tRNA (Roche Diagnostics).
5′-rapid amplification of cDNA ends polymerase chain reaction and bubble polymerase chain reaction
Screening for potential PDGFRA and PDGFRB fusion genes was performed by 5′-rapid amplification of cDNA ends polymerase chain reaction (5′-RACE-PCR) according to the manufacturer’s instructions (5′/3′RACE Kit, Roche Diagnostics) as recently described.21 Bubble-PCR for PDGFRA fusion genes was also performed as recently described.19
Statistical analysis
Comparisons between two groups of variables were performed using the non-parametric Mann-Whitney U test. Correlations between continuous variables were calculated using the Spearman’s rank test. P values below 0.05 were considered statistically significant. The diagnostic sensitivity and specificity of the RQ-PCR assay were calculated (GraphPad prism, Version 5.0 software, San Diego, CA, USA).
Results
The generic RQ-PCR assays target the 3′-sequences of PDGFRA and PDGFRB which are retained in all known fusion genes and which may, therefore, be overexpressed, as described previously.19
Precision analysis of quantitative reverse transcriptase polymerase chain reaction assays
Intra- and inter-assay coefficients of variation (CV) were calculated to test the reproducibility of the assays. Two FIP1L1-PDGFRA cDNA samples with high and low level PDGFRA expression were tested ten times within one assay (intra-assay variability, “high level” CV 7%, “low level” CV 10%). In addition, the samples were tested ten times in different assays (inter-assay variability, “high level” CV 25%, “low level” CV 44%). Identical testing was undertaken with a CCDC6-PDGFRB sample (intra-assay variability, CV 6%, inter-assay variability CV 29%).
Detection level of quantitative reverse transcriptase polymerase chain reaction assays
The RQ-PCR assays were linear over six orders of magnitude as assessed by analysis of plasmid dilutions. Cell and RNA dilutions were processed and analyzed for the calculation of the maximum detection level over the background. In healthy individuals, PDGFRA is only expressed at very low levels (median PDGFRA/ABL 0.00068; range, 0–0.0027) while PDGFRB is expressed at significantly higher levels (median PDGFRB/ABL 5.85; range, 0.97–22.2). A cutoff point for overexpression of PDGFRA and PDGFRB (mean+3SD) was determined by analysis of a series of 35 healthy volunteers and set at 0.0030 for PDGFRA/ABL and 23 for PDGFRB/ABL. For FIP1L1-PDGFRA, the levels detectable over the background were between 10−3 and 10−4 for cell line dilutions and between 10−2 and 10−3 for RNA dilutions. The detection limit of FIP1L1-PDGFRA and CCDC6-PDGFRB fusion transcripts by nested RT-PCR was between 10−4 and 10−5.
Diagnosis of PDGFR overexpression by quantitative reverse transcriptase polymerase chain reaction
The RQ-PCR assay for overexpression of PDGFRA was validated in 15 FIP1L1-PDGFRA positive samples by comparison with specific RQ-PCR for FIP1L1-PDGFRA (r=−0.66, P=0.0004).10 Overall, patients with PDGFRA fusion genes (n=51) showed significantly increased expression of PDGFRA/ABL at diagnosis without the levels overlapping with those of normal controls (n=35). The median PDGFRA/ABL ratio was 0.73 (range, 0.31–7.77) in patients compared to 0.00068 in healthy controls (P<0.0001). The level of expression of PDGFRA was not different between patients with a FIP1L1-PDGFRA fusion gene and those with other PDGFRA fusion genes (PDGFRA/ABL 0.73 versus 0.88, P=0.26, Figure 1). At diagnosis and during the first 12 months on imatinib, the normalized PDGFRA ratio was not statistically different in patients in chronic phase (n=37) compared to patients in blast phase or with secondary acute myeloid leukemia (n=8) (Figure 2).
Figure 1.
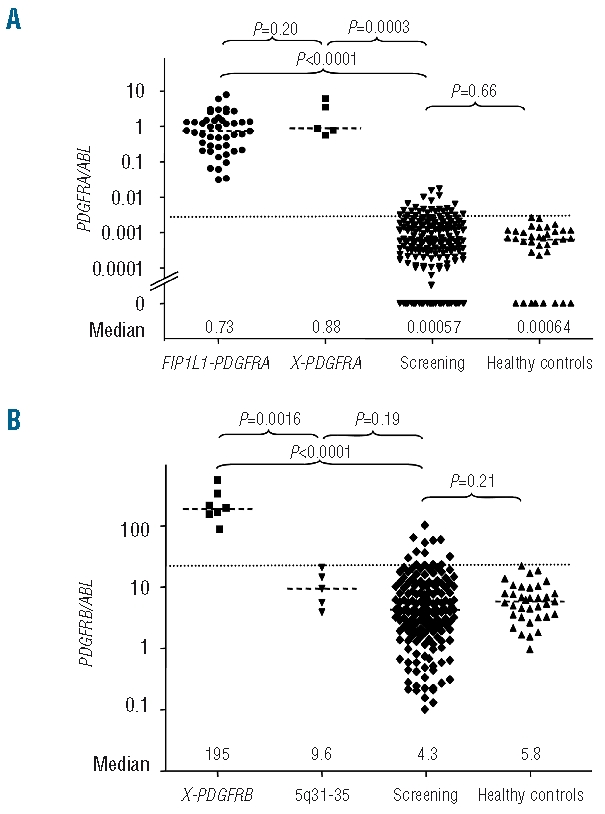
(A) Patients with PDGFRA fusion genes showed significantly increased PDGFRA expression levels (PDGFRA/ABL) as compared to patients with non-reactive eosinophilia without known molecular aberrations (n=191) and healthy controls (n=35). The expression level was not different between patients with FIP1L1-PDGFRA (n=45) and alternative PDGFRA fusion genes (X-PDGFRA: BCR-PDGFRA, CDK5RAP2-PDGFRA, ETV6-PDGFRA, KIF5B-PDGFRA). The cut-off point for overexpression of PDGFRA was determined for PDGFRA at a ratio of 0.030 PDGFRA/ABL (35 healthy controls, mean + 3 SD, dotted line). PDGFRA expression levels were not different between patients with non-reactive eosinophilia and healthy controls. (B) Patients with different PDGFRB fusion genes (X-PDGFRB [n=7]: ETV6-PDGFRB, CCDC6-PDGFRB, GIT2-PDGFRB, GPIAP1-PDGFRB and MYO18A-PDGFRB showed significantly increased PDGFRB expression levels compared to patients with non-reactive eosinophilia without known molecular aberrations and healthy controls. In the screening group, 13 patients showed significant overexpression of PDGFRB. In one of these patients, with uninformative cytogenetics and an excellent response to imatinib a new SART3-PDGFRB fusion gene was identified by 5′-RACE-PCR. No increased PDGFRB expression was found in five patients with chromosomal aberrations and involvement of chromosome bands 5q31–32. In four of these cases, alternative fusion genes with involvement of ETV6, NPM1, MLF1 and ACSL6 could be confirmed by RT-PCR. The cut-off point for overexpression was determined at a ratio of 23 PDGFRB/ABL (35 healthy controls, mean+ 3 SD, dotted line). PDGFRB expression levels were not different between patients with non-reactive eosinophilia and healthy controls.
Figure 2.
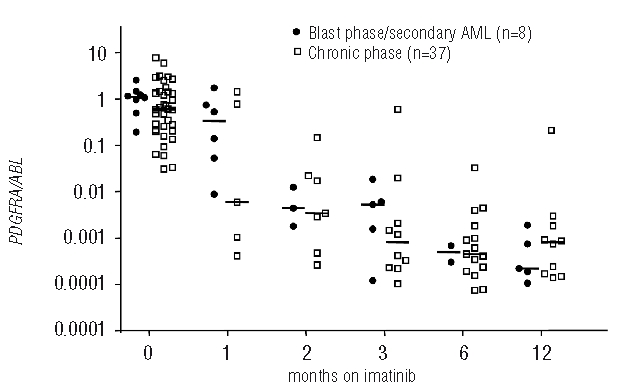
At diagnosis and during the first 12 months on imatinib the normalized PDGFRA ratio was not statistically different in patients in chronic phase (n=37) compared to patients in blast phase or with secondary AML (n=8).
The median PDGFRB/ABL ratio in seven patients with PDGFRB fusion genes was 195 (range, 68–581) compared to 5.85 in healthy controls (P<0.0001). The PDGFRB/ABL ratios in five patients with breakpoints at chromosome band 5q31–35 but without a rearrangement of PDGFRB were not different from those in normal controls (median 5.8, range 0.97–22.2 versus median 9.6, range 0.91–22.1, respectively; P=0.19). Specific RT-PCR revealed NPM1-MLF1 fusion genes (n=3) and an ETV6-ACSL6 fusion (n=1). The underlying fusion gene in the remaining patient could not be identified.
The ratios of PDGFRA/ABL and PDGFRB/ABL in patients with hypereosinophilic syndrome/chronic eosinophilic leukemia (n=191) without known molecular aberrations were comparable to those of healthy controls in the vast majority of cases. Twenty-five patients (13%) showed a significantly elevated PDGFRA/ABL (median 0.043, 0.0030–0.017 versus 0.00068, P<0.0001) and 13 (6.8%) showed elevated PDGFRB/ABL (median 36.3, 23–102 versus 5.8, P<0.0001) over the cut-off level determined in healthy controls. None of the patients showed simultaneous overexpression of PDGFRA and PDGFRB. For PDGFRA, none of the patients with elevated expression levels showed levels comparable to cases with PDGFRA fusions. For PDGFRB, three of the patients showed expression levels comparable to those of patients with PDGFRB fusions. In one of these, a novel fusion transcript was identified. Thus, the diagnostic sensitivity and specificity of the described screening test were, respectively, 100% and 88.4% for PDGFRA and 100% and 94% for PDGFRB.
A SART3-PDGFRB fusion gene
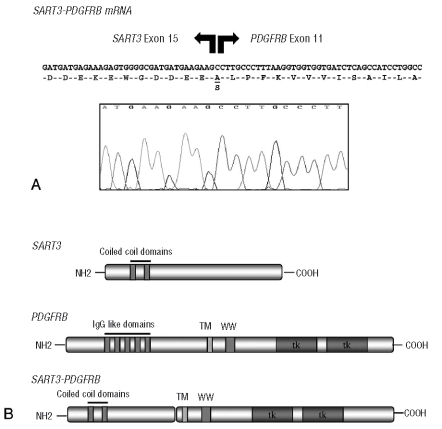
Selected cases (based on availability of suitable samples) were analyzed in more detail to determine whether the observed overexpression was a consequence of previously unrecognized PDGFRA or PDGFRB fusions. Because the genomic breakpoint region within PDGFRA is known to be highly restricted to PDGFRA exon 12, bubble-PCR with DNA and reverse primers located immediately downstream of the breakpoint cluster region19 was employed in five patients with significant overexpression of PDGFRA, but no new PDGFRA fusions were identified. Genomic breakpoint regions within PDGFRB are more heterogeneous and, therefore, 5′-RACE-PCR was used with cDNA samples derived from eight patients with significant over-expression of PDGFRB, including two patients who achieved complete clinical and hematologic remission on imatinib. A male patient with an eosinophilia-associated myeloproliferative neoplasm and uninformative cytogenetic analysis due to myelofibrosis (normal karyotype in 4 of 4 metaphases) but rapid achievement of complete remission following treatment with 100 mg imatinib revealed the fusion of a novel sequence to PDGFRB exon 12. Sequencing of the RACE-PCR products revealed an in-frame fusion between SART3 exon 15 (squamous cell carcinoma antigen recognized by T-cells 3, Gene Bank accession number: AB020880) and PDGFRB exon 12 (Figure 3). A reciprocal fusion gene could be amplified by RT-PCR and revealed a fusion between PDGFRB exon 11 and SART3 exon 16. No cytogenetic or molecular aberration could be identified in the second patient with response to imatinib and PDGFRB overexpression.
Figure 3.
(A) Junction sequence and corresponding amino acids of the SART3-PDGFRB fusion protein. SART3-PDGFRB splice variants contain intron-derived sequences which are spliced in-frame between SART3 exon 14 and PDGFRB exon 10. (B) Structure of SART3, PDGFRB and the predicted fusion protein. TM: transmembrane domain, WW: WW-like domain, TK: tyrosine kinase domain.
Discussion
For patients with non-reactive eosinophilia, a major diagnostic and therapeutic breakthrough was achieved by the identification of the FIP1L1-PDGFRA fusion gene. Virtually all patients with this fusion gene achieve rapid and sustained complete clinical and hematologic remissions, and the majority even obtain complete molecular remissions, at low toxicity. However, this fusion is only seen in approximately 5–15% of cases with non-reactive eosinophilia and, furthermore, may be difficult to detect in some diagnostic cases.7,9,13 In contrast, the clinical phenotype of FIP1L1-PDGFRA-negative cases is frequently indistinguishable from that in patients in whom the fusion is present, suggesting the presence of alternative fusion genes or as yet unknown molecular mechanisms leading to constitutive tyrosine kinase activation.
In FIP1L1-PDGFRA negative cases, cytogenetic analysis from bone marrow cells is recommended since all other known PDGFRA or PDGFRB fusions are associated with abnormalities involving chromosome bands 4q12 (PDGFRA) and 5q31–33 (PDGFRB). Overall, 28 fusion genes are currently known with involvement of PDGFRA or PDGFRB.3 Response rates are similar to those seen in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia and importantly, primary or secondary resistance is very rare. In contrast, fusion genes with involvement of FGFR1 and JAK2 are imatinib-resistant and associated with an aggressive clinical course. Transformation to blast phase/secondary acute leukemia, usually of myeloid phenotype, regularly occurs within 1 or 2 years of diagnosis. For these patients, allogeneic stem cell transplantation remains the only potentially curative treatment option as long as selective inhibitors of FGFR1 and JAK2 are not widely available.
Detection of variant PDGFR fusions remains a significant diagnostic challenge. We sought to address this by designing and validating generic quantitative RT-PCR assays which allow rapid screening of fresh or stored peripheral blood or bone marrow material for the possible presence of fusion genes involving PDGFRA or PDGFRB. All positive controls were correctly identified and significant overexpression of PDGFRA or PDGFRB was found in 13% and 7%, respectively, of cases with non-reactive eosinophilia lacking FIP1L1-PDGFRA or cytogenetic indicators of other PDGFR fusions. Among the overexpressors, a single case was found to harbor a PDGFR fusion and thus the sensitivity and specificity of the screening test were, respectively, 100% and 88.4% for PDGFRA and 100% and 94% for PDGFRB.
This screening strategy may be particularly useful as a means to select candidates for exploratory imatinib treatment. Seven patients with hypereosinophilic syndrome without known molecular aberrations and sustained response to treatment with imatinib were screened within this series. None of them showed significant overexpression of PDGFRA while two patients showed overexpression of PDGFRB comparable to PDGFRB fusions. 5′-RACE-PCR was performed in both patients and identified a new SART3-PDGFRB fusion gene corresponding to a t(5;12)(q31–32;q23–24) in one of these cases. This reciprocal translocation was not picked up by routine cytogenetic analysis; however, it had only been possible to investigate four metaphases because of myelofibrosis. The lack of overexpression of PDGFRA or PDGFRB in five imatinib-responsive patients highlights the fact that assays may miss some patients with potential response due to yet unknown targets or off-target activity of imatinib. We screened these patients for abnormalities of PDGFRA, PDGFRB, KIT, FMS and also by array comparative genome hybridization but did not find anything.
SART3 (squamous cell carcinoma antigen recognized by T-cells 3, synonym: KIAA0156, RP11-13G14, p110) maps to chromosome 12q23–24. It encodes for a RNA-binding nuclear protein that was initially identified from cDNA clones of the myeloid cell line KG-1.29 The protein is present in almost all tissues analyzed but seems to be highly expressed in tumor tissue and cancer cells.30 This antigen possesses tumor epitopes capable of inducing HLA-A24-restricted and tumor-specific cytotoxic T lymphocytes in cancer patients suggesting that these proteins could be useful for specific immunotherapy, as shown for colorectal, bladder and breast cancer.31–33 SART3 associates transiently with U6 and U4/U6 small nuclear ribonucleoproteins during the recycling phase of the spliceosome cycle and is involved in regulation of mRNA splicing.34,35 The genomic PDGFRB breakpoint is located within intron 10, and thus the transmembrane and juxtamembrane domains of PDGFRB are retained in the SART3-PDGFRB fusion protein. Coiled-coil domains are present in the N-terminal region of SART3 suggesting that dimerization of the fusion protein causes constitutive activation of the PDGFRB kinase domain.3 The majority of known PDGFRB fusion genes have genomic breakpoints leading to disruption of the autoinhibitory WW-like domain within the juxtamembrane region that may enhance transformation properties of the chimeric fusion protein.36,37
Our data indicate that expression analysis of PDGFRA or PDGFRB is helpful in cases of eosinophilia-associated myeloproliferative neoplasms which are negative for FIP1L1-PDGFRA and which have a normal, insufficient or missing karyotype. It should be emphasized that screening for overexpression of PDGFRA and PDGFRB may also be useful for patients with eosinophilia-associated primary and secondary acute leukemias which are negative for core-binding factor fusions genes. We have recently reported rapid and sustained complete hematologic and complete molecular remissions in patients with FIP1L1-PDGFRA-positive blast phase disease on imatinib as monotherapy or as maintenance after intensive chemotherapy.9 Of interest, the decline of expression levels was no different between patients in chronic phase or with blast phase/secondary acute leukemia. In addition, this assay is useful in cases presenting with reciprocal translocations and involvement of 5q31–33. PDGFRB is clearly not involved in all these cases38,39 and FISH analysis alone might occasionally miss a rearrangement of PDGFRB.14
The identification of more than 40 different fusion genes as the consequence of diverse chromosomal abnormalities in eosinophilia-associated myeloproliferative neoplasms has highlighted the fundamental role of constitutively activated tyrosine kinases in the pathogenesis of these disorders. We have shown here that the universal quantification of regions that are retained in all known PDGFRA and PDGFRB fusion genes is a sensitive assay for the screening of potential fusion genes and serves as a useful adjunct to standard diagnostic procedures, particularly for laboratories that are familiar with routine RQ-PCR analysis. Expanding these techniques to other tyrosine kinases or translocation partners might help to define the molecular pathogenesis of the vast majority of eosinophilia-associated myeloproliferative neoplasms for whom the causative lesion remains unknown.
Footnotes
Funding: this work was supported by the ‘Deutsche José Carreras Leukämie-Stiftung e.V.’ (AR, AH - DJCLS R06/02 and H03/01), Germany, the Leukaemia Research Fund, United Kingdom, the Competence Network ‘Acute and Chronic Leukemias’, sponsored by the German Bundesministerium für Bildung und Forschung (Projektträger Gesundheitsforschung; DLR e.V. - 01GI9980/6) and the ‘European LeukemiaNet’ within the 6th European Community Framework Programme for Research and Technological Development.
Authorship and Disclosures
AR was the principal investigator, takes primary responsibility for the paper and co-ordinated the research. AR, FV, GM recruited the patients. PE, MM, AH and NCBC developed the PCR methodology. PE, DG, MM, JR, CW, JM, JS, TE, and CH performed the laboratory work for this study. PE and AR wrote the paper. AH and NCPC revised the manuscript.
AR and AH have received research funding from Novartis and also served as consultants for Novartis. The remaining authors have no disclosures.
References
- 1.Tefferi A. Molecular drug targets in myeloproliferative neoplasms: mutant ABL1, JAK2, MPL, KIT, PDGFRA, PDGFRB and FGFR1. J Cell Mol Med. 2009;13(2):215–37. doi: 10.1111/j.1582-4934.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walz C, Cross NC, Van Etten RA, Reiter A. Comparison of mutated ABL1 and JAK2 as oncogenes and drug targets in myeloproliferative disorders. Leukemia. 2008;22(7):1320–34. doi: 10.1038/leu.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiter A, Walz C, Cross NC. Tyrosine kinases as therapeutic targets in BCR-ABL negative chronic myeloproliferative disorders. Curr Drug Targets. 2007;8(2):205–16. doi: 10.2174/138945007779940124. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 5.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 6.Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77(2):307–16. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 7.Baccarani M, Cilloni D, Rondoni M, Ottaviani E, Messa F, Merante S, et al. The efficacy of imatinib mesylate in patients with FIP1L1-PDGFRα-positive hypereosinophilic syndrome. Results of a multi-center prospective study. Haematologica. 2007;92(9):1173–9. doi: 10.3324/haematol.11420. [DOI] [PubMed] [Google Scholar]
- 8.David M, Cross NC, Burgstaller S, Chase A, Curtis C, Dang R, et al. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood. 2007;109(1):61–4. doi: 10.1182/blood-2006-05-024828. [DOI] [PubMed] [Google Scholar]
- 9.Metzgeroth G, Walz C, Score J, Siebert R, Schnittger S, Haferlach C, et al. Recurrent finding of the FIP1L1-PDGFRA fusion gene in eosinophilia-associated acute myeloid leukemia and lymphoblastic T-cell lymphoma. Leukemia. 2007;21(6):1183–8. doi: 10.1038/sj.leu.2404662. [DOI] [PubMed] [Google Scholar]
- 10.Jovanovic JV, Score J, Waghorn K, Cilloni D, Gottardi E, Metzgeroth G, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood. 2007;109(11):4635–40. doi: 10.1182/blood-2006-10-050054. [DOI] [PubMed] [Google Scholar]
- 11.Apperley JF, Gardembas M, Melo JV, Russell-Jones R, Bain BJ, Baxter EJ, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med. 2002;347(7):481–7. doi: 10.1056/NEJMoa020150. [DOI] [PubMed] [Google Scholar]
- 12.Metzgeroth G, Walz C, Erben P, Popp H, Schmitt-Graeff A, Haferlach C, et al. Safety and efficacy of imatinib in chronic eosinophilic leukaemia and hypereosinophilic syndrome: a phase-II study. Br J Haematol. 2008;143(5):707–15. doi: 10.1111/j.1365-2141.2008.07294.x. [DOI] [PubMed] [Google Scholar]
- 13.Score J, Walz C, Jovanovic JV, Jones AV, Waghorn K, Hidalgo-Curtis C, et al. Detection and molecular monitoring of FIP1L1-PDGFRA-positive disease by analysis of patient-specific genomic DNA fusion junctions. Leukemia. 2009;23(2):332–9. doi: 10.1038/leu.2008.309. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni S, Heath C, Parker S, Chase A, Iqbal S, Pocock CF, et al. Fusion of H4/D10S170 to the platelet-derived growth factor receptor beta in BCR-ABL-negative myeloproliferative disorders with a t(5;10)(q33;q21) Cancer Res. 2000;60(13):3592–8. [PubMed] [Google Scholar]
- 15.Walz C, Score J, Mix J, Cilloni D, Roche-Lestienne C, Yeh RF, et al. The molecular anatomy of the FIP1L1-PDGFRA fusion gene. Leukemia. 2009;23(2):271–8. doi: 10.1038/leu.2008.310. [DOI] [PubMed] [Google Scholar]
- 16.Baxter EJ, Hochhaus A, Bolufer P, Reiter A, Fernandez JM, Senent L, et al. The t(4;22)(q12;q11) in atypical chronic myeloid leukaemia fuses BCR to PDGFRA. Hum Mol Genet. 2002;11(12):1391–7. doi: 10.1093/hmg/11.12.1391. [DOI] [PubMed] [Google Scholar]
- 17.Curtis CE, Grand FH, Musto P, Clark A, Murphy J, Perla G, et al. Two novel imatinib-responsive PDGFRA fusion genes in chronic eosinophilic leukaemia. Br J Haematol. 2007;138(1):77–81. doi: 10.1111/j.1365-2141.2007.06628.x. [DOI] [PubMed] [Google Scholar]
- 18.Walz C, Curtis C, Schnittger S, Schultheis B, Metzgeroth G, Schoch C, et al. Transient response to imatinib in a chronic eosinophilic leukemia associated with ins(9;4)(q33;q12q25) and a CDK5RAP2-PDGFRA fusion gene. Genes Chromosomes Cancer. 2006;45(10):950–6. doi: 10.1002/gcc.20359. [DOI] [PubMed] [Google Scholar]
- 19.Score J, Curtis C, Waghorn K, Stalder M, Jotterand M, Grand FH, et al. Identification of a novel imatinib responsive KIF5B-PDGFRA fusion gene following screening for PDGFRA overexpression in patients with hypereosinophilia. Leukemia. 2006;20(5):827–32. doi: 10.1038/sj.leu.2404154. [DOI] [PubMed] [Google Scholar]
- 20.Schwaller J, Anastasiadou E, Cain D, Kutok J, Wojiski S, Williams IR, et al. H4(D10S170), a gene frequently rearranged in papillary thyroid carcinoma, is fused to the platelet-derived growth factor receptor beta gene in atypical chronic myeloid leukemia with t(5;10)(q33;q22) Blood. 2001;97(12):3910–8. doi: 10.1182/blood.v97.12.3910. [DOI] [PubMed] [Google Scholar]
- 21.Walz C, Metzgeroth G, Haferlach C, Schmitt-Graeff A, Fabarius A, Hagen V, et al. Characterization of three new imatinib-responsive fusion genes in chronic myeloproliferative disorders generated by disruption of the platelet-derived growth factor receptor beta gene. Haematologica. 2007;92(2):163–9. doi: 10.3324/haematol.10980. [DOI] [PubMed] [Google Scholar]
- 22.Walz C, Haferlach C, Hanel A, Metzgeroth G, Erben P, Gosenca D, et al. Identification of a MYO18A-PDGFRB fusion gene in an eosinophilia-associated atypical myeloproliferative neoplasm with a t(5;17)(q33–34;q11.2) Genes Chromosomes Cancer. 2008;48:179–83. doi: 10.1002/gcc.20629. [DOI] [PubMed] [Google Scholar]
- 23.Cross NC, Melo JV, Feng L, Goldman JM. An optimized multiplex polymerase chain reaction (PCR) for detection of BCR-ABL fusion mRNAs in haematological disorders. Leukemia. 1994;8(19):186–9. [PubMed] [Google Scholar]
- 24.Yagasaki F, Jinnai I, Yoshida S, Yokoyama Y, Matsuda A, Kusumoto S, et al. Fusion of TEL/ETV6 to a novel ACS2 in myelodysplastic syndrome and acute myelogenous leukemia with t(5;12)(q31;p13) Genes Chromosomes Cancer. 1999;26(3):192–202. doi: 10.1002/(sici)1098-2264(199911)26:3<192::aid-gcc2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Arber DA, Chang KL, Lyda MH, Bedell V, Spielberger R, Slovak ML. Detection of NPM/MLF1 fusion in t(3;5)-positive acute myeloid leukemia and myelodysplasia. Human Pathology. 2003;34(8):809–13. doi: 10.1016/s0046-8177(03)00251-x. [DOI] [PubMed] [Google Scholar]
- 26.Muller MC, Gattermann N, Lahaye T, Deininger MW, Berndt A, Fruehauf S, et al. Dynamics of BCR-ABL mRNA expression in first-line therapy of chronic myelogenous leukemia patients with imatinib or interferon alpha/ara-C. Leukemia. 2003;17(12):2392–400. doi: 10.1038/sj.leu.2403157. [DOI] [PubMed] [Google Scholar]
- 27.van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003;17(6):1013–34. doi: 10.1038/sj.leu.2402922. [DOI] [PubMed] [Google Scholar]
- 28.Muller MC, Erben P, Saglio G, Gottardi E, Nyvold CG, Schenk T, et al. Harmonization of BCR-ABL mRNA quantification using a uniform multifunctional control plasmid in 37 international laboratories. Leukemia. 2008;22(1):96–102. doi: 10.1038/sj.leu.2404983. [DOI] [PubMed] [Google Scholar]
- 29.Nagase T, Seki N, Tanaka A, Ishikawa K-i, Nomura N. Prediction of the coding sequences of unidentified human genes. IV. The coding sequences of 40 new genes (KIAA0121–KIAA0160) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 1995;2(4):167–74. doi: 10.1093/dnares/2.4.167. [DOI] [PubMed] [Google Scholar]
- 30.Bell M, Schreiner S, Damianov A, Reddy R, Bindereif A. p110, a novel human U6 snRNP protein and U4/U6 snRNP recycling factor. Embo J. 2002;21(11):2724–35. doi: 10.1093/emboj/21.11.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasatomi T, Suefuji Y, Matsunaga K, Yamana H, Miyagi Y, Araki Y, et al. Expression of tumor rejection antigens in colorectal carcinomas. Cancer. 2002;94(6):1636–41. doi: 10.1002/cncr.10421. [DOI] [PubMed] [Google Scholar]
- 32.Suefuji Y, Sasatomi T, Shichijo S, Nakagawa S, Deguchi H, Koga T, et al. Expression of SART3 antigen and induction of CTLs by SART3-derived peptides in breast cancer patients. Br J Cancer. 2001;84(7):915–9. doi: 10.1054/bjoc.2000.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komohara Y, Harada M, Arima Y, Suekane S, Noguchi M, Yamada A, et al. Anti-cancer vaccine candidates in specific immunotherapy for bladder carcinoma. Int J Oncol. 2006;29(6):1555–60. [PubMed] [Google Scholar]
- 34.Medenbach J, Schreiner S, Liu S, Luhrmann R, Bindereif A. Human U4/U6 snRNP recycling factor p110: mutational analysis reveals the function of the tetratricopeptide repeat domain in recycling. Mol Cell Biol. 2004;24(17):7392–401. doi: 10.1128/MCB.24.17.7392-7401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hidalgo-Curtis C, Chase A, Drachenberg M, Roberts MW, Finkelstein JZ, Mould S, et al. The t(1;9)(p34;q34) and t(8;12)(p11;q15) fuse pre-mRNA processing proteins SFPQ (PSF) and CPSF6 to ABL and FGFR1. Genes Chromosomes Cancer. 2008;47(5):379–85. doi: 10.1002/gcc.20541. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Williams IR, Kutok JL, Duclos N, Anastasiadou E, Masters SC, et al. Positive and negative regulatory roles of the WW-like domain in TEL-PDGFβR transformation. Blood. 2004;104(2):535–42. doi: 10.1182/blood-2004-01-0169. [DOI] [PubMed] [Google Scholar]
- 37.Irusta PM, Luo Y, Bakht O, Lai C-C, Smith SO, DiMaio D. Definition of an inhibitory juxtamembrane WW-like domain in the platelet-derived growth factor beta receptor. J Biol Chem. 2002;277(41):38627–34. doi: 10.1074/jbc.M204890200. [DOI] [PubMed] [Google Scholar]
- 38.Cools J, Mentens N, Odero MD, Peeters P, Wlodarska I, Delforge M, et al. Evidence for position effects as a variant ETV6-mediated leukemogenic mechanism in myeloid leukemias with a t(4;12)(q11–q12;p13) or t(5;12)(q31;p13) Blood. 2002;99(5):1776–84. doi: 10.1182/blood.v99.5.1776. [DOI] [PubMed] [Google Scholar]
- 39.Murati A, Adelaide J, Gelsi-Boyer V, Etienne A, Remy V, Fezoui H, et al. t(5;12)(q23–31;p13) with ETV6-ACSL6 gene fusion in polycythemia vera. Leukemia. 2006;20(6):1175–8. doi: 10.1038/sj.leu.2404194. [DOI] [PubMed] [Google Scholar]