Abstract
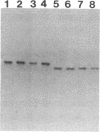
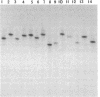
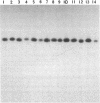
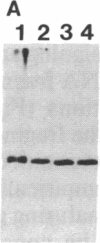
Denaturing gradient gel electrophoresis (DGGE) can be used to distinguish two DNA molecules that differ by as little as a single-base substitution. This method detects approximately 50% of all possible single-base changes in DNA fragments ranging from 50 to approximately 1000 base pairs. To increase the number of single-base changes that can be distinguished by DGGE, we used the polymerase chain reaction to attach a 40-base-pair G + C-rich sequence, designated a GC-clamp, to one end of amplified DNA fragments that encompass regions of the mouse and human beta-globin genes. We show that this GC-clamp allows the detection of mutations, including the hemoglobin sickle (HbS) and hemoglobin C (HbC) mutations within the human beta-globin gene, that were previously indistinguishable by DGGE. In addition to providing an easy way to attach a GC-clamp to genomic DNA fragments, the polymerase chain reaction technique greatly increases the sensitivity of DGGE. With this approach, DNA fragments derived from less than 5 ng of human genomic DNA can be detected by ethidium bromide staining of the gel, obviating the need for radioactive probes. These improvements extend the applicability of DGGE for the detection of polymorphisms and mutations in genomic and cloned DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. A sensitive new prenatal test for sickle-cell anemia. N Engl J Med. 1982 Jul 1;307(1):30–32. doi: 10.1056/NEJM198207013070105. [DOI] [PubMed] [Google Scholar]
- Embury S. H., Scharf S. J., Saiki R. K., Gholson M. A., Golbus M., Arnheim N., Erlich H. A. Rapid prenatal diagnosis of sickle cell anemia by a new method of DNA analysis. N Engl J Med. 1987 Mar 12;316(11):656–661. doi: 10.1056/NEJM198703123161103. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. Length-independent separation of DNA restriction fragments in two-dimensional gel electrophoresis. Cell. 1979 Jan;16(1):191–200. doi: 10.1016/0092-8674(79)90200-9. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Kooter J. M., De Boer E., Little P. F., Williamson R. Analysis of the beta-delta-globin gene loci in normal and Hb Lepore DNA: direct determination of gene linkage and intergene distance. Cell. 1978 Sep;15(1):25–41. doi: 10.1016/0092-8674(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Geever R. F., Wilson L. B., Nallaseth F. S., Milner P. F., Bittner M., Wilson J. T. Direct identification of sickle cell anemia by blot hybridization. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5081–5085. doi: 10.1073/pnas.78.8.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofker M. H., Wapenaar M. C., Goor N., Bakker E., van Ommen G. J., Pearson P. L. Isolation of probes detecting restriction fragment length polymorphisms from X chromosome-specific libraries: potential use for diagnosis of Duchenne muscular dystrophy. Hum Genet. 1985;70(2):148–156. doi: 10.1007/BF00273073. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K. Computational simulation of DNA melting and its application to denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:482–501. doi: 10.1016/0076-6879(87)55032-7. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K., Grinfeld E. Searching for gene defects by denaturing gradient gel electrophoresis. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):285–297. doi: 10.1101/sqb.1986.051.01.034. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Maniatis T., Lerman L. S. Modification of the melting properties of duplex DNA by attachment of a GC-rich DNA sequence as determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3111–3129. doi: 10.1093/nar/13.9.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Lerman L. S., Maniatis T. A general method for saturation mutagenesis of cloned DNA fragments. Science. 1985 Jul 19;229(4710):242–247. doi: 10.1126/science.2990046. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lumelsky N., Lerman L. S., Maniatis T. Detection of single base substitutions in total genomic DNA. Nature. 1985 Feb 7;313(6002):495–498. doi: 10.1038/313495a0. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T. Recent advances in the development of methods for detecting single-base substitutions associated with human genetic diseases. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):275–284. doi: 10.1101/sqb.1986.051.01.033. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Little P. F., Kazazian H. H., Jr, Boehm C. D. Improved detection of the sickle mutation by DNA analysis: application to prenatal diagnosis. N Engl J Med. 1982 Jul 1;307(1):32–36. doi: 10.1056/NEJM198207013070106. [DOI] [PubMed] [Google Scholar]
- Pirastu M., Kan Y. W., Cao A., Conner B. J., Teplitz R. L., Wallace R. B. Prenatal diagnosis of beta-thalassemia. Detection of a single nucleotide mutation in DNA. N Engl J Med. 1983 Aug 4;309(5):284–287. doi: 10.1056/NEJM198308043090506. [DOI] [PubMed] [Google Scholar]
- Päbo S., Wilson A. C. Polymerase chain reaction reveals cloning artefacts. Nature. 1988 Aug 4;334(6181):387–388. doi: 10.1038/334387b0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Solomon E., Bodmer W. F. Evolution of sickle variant gene. Lancet. 1979 Apr 28;1(8122):923–923. doi: 10.1016/s0140-6736(79)91398-9. [DOI] [PubMed] [Google Scholar]