Abstract
Background
T cells from patients with chronic lymphocytic leukemia may play an important role in contributing to the onset, sustenance, and exacerbation of the disease by providing survival and proliferative signals to the leukemic clone within lymph nodes and bone marrow.
Design and Methods
By performing chemotaxis assays towards CXCL12, CCL21 and CCL19, we sought to evaluate the migratory potential of T cells from chronic lymphocytic leukemia patients. We next analyzed the chemokine-induced migration of T cells, dividing the chronic lymphocytic leukemia samples according to their expression of the poor prognostic factors CD38 and ZAP-70 in leukemic cells determined by flow cytometry.
Results
We found that T cells from patients with chronic lymphocytic leukemia are less responsive to CXCL12, CCL21 and CCL19 than T cells from healthy adults despite similar CXCR4 and CCR7 expression. Following separation of the patients into two groups according to ZAP-70 expression, we found that T cells from ZAP-70-negative samples showed significantly less migration towards CXCL12 compared to T cells from ZAP-70-positive samples and that this was not due to defective CXCR4 down-regulation, F-actin polymerization or to a lesser expression of ZAP-70, CD3, CD45, CD38 or CXCR7 on these cells. Interestingly, we found that leukemic cells from ZAP-70-negative samples seem to be responsible for the defective CXCR4 migratory response observed in their T cells.
Conclusions
Impaired migration towards CXCL12 may reduce the access of T cells from ZAP-70-negative patients to lymphoid organs, creating a less favorable microenvironment for leukemic cell survival and proliferation.
Keywords: chronic lymphocytic leukemia, T-cell chemotaxis, CXCL12, CXCR4
Introduction
B-cell chronic lymphocytic leukemia (CLL) is characterized by a lymphocytosis of small mature clonal CD5+ B lymphocytes in blood, peripheral lymphoid organs and bone marrow. The clinical course of CLL is very heterogeneous and a number of prognostic factors may help to predict the outcome of the disease. Thus, patients with poor prognosis, who develop aggressive disease requiring early therapy, typically display leukemic cells with B-cell receptors encoded by unmutated immunoglobulin variable heavy-chain genes (IgVH) and express the protein tyrosine kinase ZAP-70 and the type II transmembrane glycoprotein CD38.1–4 By contrast, mutated IgVH genes and the absence of ZAP-70 and CD38 are mostly found in leukemic cells from patients with a good prognosis, who have a more stable, indolent disease, with no benefit from palliative chemotherapy.5
CLL is now widely viewed as a dynamic lymphoid neoplasm comprising leukemic cells that multiply and die at measurable rates.6 Although 99% of circulating malignant B cells are in the G0/G1 phase of the cell cycle, clusters of larger leukemic cells can be found in lymph nodes and bone marrow. These clusters of CLL cells expressing cell-cycle markers and particular anti-apoptotic molecules are arranged in particular areas termed proliferation centers7,8 where the selected microenvironmental signals delivered by non-leukemic cells, such as stromal and T cells, appear to confer CLL lymphocytes with a growth advantage and extended survival.9,10 In vitro findings suggest that T cells, particularly CD4+CD40L+ cells, provide short-term support which influences malignant B-cell proliferation through secretion of cytokines (e.g. interleukin-4 or interferon-γ) and CD40/CD40L interactions,10–12 while stromal cells and accessory cells provide long-term support favoring prolonged survival and accumulation of leukemic cells.
Various studies have focused on chemokine-chemokine receptor interactions implicated in malignant B-cell homing to lymph nodes and bone marrow.13–18 Leukemic B cells from CLL patients express high levels of CCR7, CXCR4 and CXCR5,14–16 which are the main chemokine receptors that mediate B-cell entry into secondary lymphoid organs and their positioning in T- and B-cell zones. Of note, ZAP-70 and CD38 expression in leukemic cells is associated with an enhanced ability to respond through CCR7 and CXCR4.17,18 In contrast to the well-known role of chemokines in CLL B-cell migration, there is no information about the ability of T cells from CLL patients to respond to lymphoid organ chemokines. This is not a trivial issue as the T-cell compartment in CLL patients presents numerous qualitative and quantitative abnormalities,19–21 some of which could be directly related to its interaction with the leukemic clone itself.22 The aim of this study was, therefore, to evaluate the responsiveness of T cells from good and bad prognosis CLL patients to CXCL12, CCL21 and CCL19, the central chemokines involved in T-cell recruitment to lymphoid organs.23–25
Design and Methods
All reagents and antibodies used, the preparation of the samples from CLL patients and healthy donors, cell separation procedures and cultures are described in detail in the Online Supplementary Appendix.
The expression of CD38, CD19, CD3, CD56, CD19 and ZAP-70 was determined by flow cytometry. Patients were considered ZAP-70+ when 20% or more of their CD19+ cells expressed ZAP-70. CXCR4 and CCR7 surface expression on T cells from CLL patients or healthy donors was performed by direct staining of whole blood samples in order to avoid Ficoll purification, which can transiently affect chemokine receptor expression, followed by flow cytometry.
Chemotaxis assays towards CXCL12, CCL21 and CCL19 were conducted to evaluate the migratory potential of T cells from CLL patients and healthy controls. The migration index was calculated by determining the ratio of migrated CD3+CD56− (CD4+ or CD8+) cells in chemokine-treated wells versus control wells, taking the spontaneous migration in control wells as 100%.
The time course and dose-dependency of CXCR4 endocytosis in T cells from CLL patients in response to CXCL12 were examined and F-actin polymerization was evaluated. Finally, co-culture experiments were conducted in which T cells were cultured in complete medium alone (pT cultures) or at a 1:4 ratio with autologous pCLL (pT+pCLL cultures). Chemotaxis assays towards CXCL12 (1 μg/mL) were performed as previously described with freshly purified and 48 h cultured cells. In these experiments, the relative migration of CD3+ cells was calculated by taking the migration index of T cells in pT+pCLL cultures as 100%.
The design and methods of the study are described more extensively in the Online Supplementary Appendix.
Statistical analysis
For statistical comparisons between groups the non-parametric Mann-Whitney test or one way ANOVA, Bonferroni’s multiple comparison test was used. The significance of migration indices was analyzed using a one sample t test or the Wilcoxon’s signed rank test.
Results
T cells from chronic lymphocytic leukemia patients are less responsive to CXCL12, CCL21 and CCL19 than T cells from healthy adults
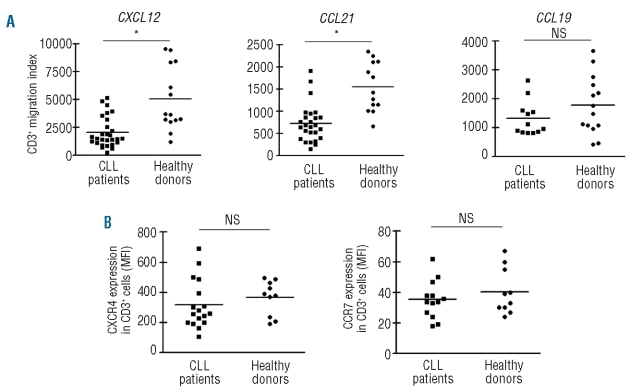
In order to evaluate the capacity of T cells from CLL patients to migrate towards CXCL12, CCL21 and CCL19, we performed transwell chemotaxis assays. We first determined the optimal chemokine concentration for T cells from CLL patients and healthy adults by performing dose-response chemotaxis assays (Online Supplementary Figure S2) and chose 1 μg/mL of CXCL12, 1 μg/mL of CCL21 and 5 μg/mL of CCL19 for further assays. The chemotactic response of T cells, expressed as CD3+ migration index, is depicted in Figure 1A. T cells from CLL patients were less responsive to CXCL12, CCL21 and CCL19 than T cells from healthy donors, with the differences being statistically significant for CXCL12 and CCL21. The migration of CD4+ T cells and CD8+ T cells towards the chemokines was comparable (data not shown). As expected, treatment of the cells with CXCL12, CCL21 or CCL19 prior to the chemotaxis assay completely abrogated their migratory response to CXCL12, CCL21 and CCL19, respectively (Online Supplementary Figure S3) due to down-regulation of the corresponding receptor. The different migration of T cells from CLL patients and healthy adults could not be ascribed to differences in CXCR4 and CCR7 expression (Figure 1B). In addition, the expression of CXCR4 and CCR7 was similar in CD4+ T cells and CD8+ T cells (Online Supplementary Figure S4).
Figure 1.
T-cell migration towards CXCL12, CCL21 and CCL19 and the expression of CXCR4 and CCR7 in T cells from healthy donors and CLL patients. (A) PBMC from CLL patients or healthy adults were placed in the upper chamber of a 24-transwell plate; medium alone (control wells) or with the optimal dose of CXCL12 (1 μg/mL), CCL21 (1 μg/mL) or CCL19 (5 μg/mL) as chemoattractant was placed in the lower chamber. After 2 h of culture the migrating cells were suspended and divided into aliquots for counting with a FACSCalibur or for immunophenotyping. The migration index for each sample was calculated by determining the ratio of migrated T cells in chemokine-treated wells versus control wells, taking the spontaneous migration in control wells as 100%. NS: not significant; *P<0.01 Mann-Whitney test. (B) Freshly collected whole blood from CLL patients or healthy donors was incubated with saturating concentrations of PerCP-conjugated anti-CD3 antibodies and PE-conjugated anti-CXCR4 (clone 12G5) or anti-CCR7 (clone 3D12), or the appropriate isotype control antibody for 30 min at 4ºC. After selective red blood cell lysis the white cell pellet was evaluated by flow cytometry. Results are expressed as the mean fluorescence intensity (MFI) of CXCR4 and CCR7 expression on CD3+ cells for each sample analyzed. NS: not significant, Mann Whitney test.
T cells from ZAP-70− chronic lymphocytic leukemia patients are less responsive to CXCL12 than T cells from ZAP-70+ patients and healthy adults
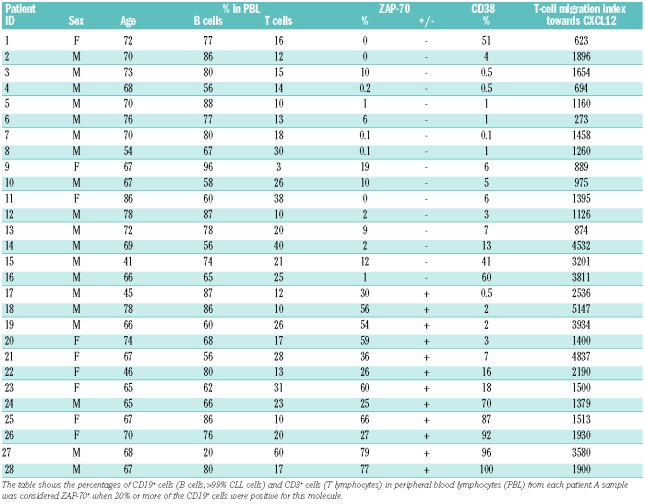
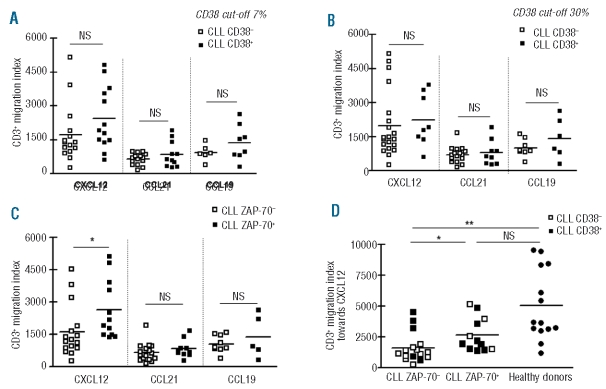
We next analyzed the chemokine-induced migration of T cells according to whether the cells came from patients with high or low risk CLL, defined by flow cytometrically determined expression of the poor prognostic factors CD38 and ZAP-70 in CLL cells. Table 1 shows the main characteristics of the 28 CLL patients enrolled in our study, including the percentages of CD19+ cells positive for ZAP-70 and CD38. Although CD38 expression is undisputedly a reliable negative prognostic marker in CLL, since there are discrepancies in defining the cut-off for positive values,26–28 we decided to separate our CLL samples using both the 7%27 and 30%28 cut-off values. As shown in Figure 2 AB, in both cases T cells from CD38− patients were less responsive to CXCL12 and CCL19 than T cells from CD38+ patients, although the differences were not statistically significant, while comparable responses were found for CCL21 in CD38− and CD38+ samples. No differences in CXCR4 and CCR7 expression in T cells were found between CD38+ and CD38− CLL patients (Online Supplementary Table S1).
Table 1.
Age, sex and main cellular characteristics of the CLL patients enrolled in the study.
Figure 2.
T-cell migration towards CXCL12, CCL21 and CCL19 in CLL patients separated according to CD38 and ZAP-70 expression. The figure shows the migration indices of T cells from CLL patients divided according to CD38 expression using cut-off values of 7% (A) and 30% (B) and according to ZAP-70 expression (C). NS: not significant, *P<0.01 Mann Whitney test. (D) Migration towards CXCL12 of T cells from ZAP-70− and ZAP-70+ CLL patients and healthy donors. Black boxes correspond to CD38+ CLL patients and white boxes represent CD38− CLL patients using the 7% cut-off value. T cells from ZAP-70− CLL patients had lower migration indices towards CXCL12 than T cells from ZAP- 70+ CLL patients and healthy donors. **P<0.001; *P<0.05; NS: not significant; one-way ANOVA, Bonferroni’s multiple comparison test.
Of note, when we analyzed T-cell migration in ZAP-70+ and ZAP-70− subsets, we found that the migratory capacity towards CXCL12 was lower in ZAP-70− samples than in ZAP-70+ samples (Figure 2C). It should be mentioned that this difference in migratory capacity was highly significant (P=0.009) even though the T cells from both groups of CLL patients expressed similar surface levels of CXCR4 (Online Supplementary Table S1). As shown in Figure 2D, when we compared the migration indices towards CXCL12 of T cells from healthy donors and CLL patients separated according to ZAP-70 expression, we found, surprisingly, that only T cells from ZAP-70− samples showed a significantly defective migratory capacity compared to cells from healthy donors, while no statistically significant differences were found between T cells from ZAP-70+ CLL patients and healthy donors. In addition, when CD38+ cases were distinguished from CD38− cases within each group, T cells from the ZAP-70-CD38− samples seemed to have the lowest migratory capacity towards CXCL12. Comparable migratory capacity was found between CD4+ T cells and CD8+ T cells within each group (data not shown).
CXCL12 induces similar CXCR4 endocytosis and F-actin polymerization in T cells from ZAP-70+ and ZAP-70−patients
Receptor internalization by endocytosis is characteristic of chemokine receptors and may allow for continuous sampling of chemoattractants, enabling the cells to follow a chemotactic gradient. We, therefore, investigated whether the different migratory capacity of T cells from ZAP-70+ and ZAP-70− CLL patients towards CXCL12 was due to different CXCR4 down-regulation. Figure 3A shows the percentage of CXCR4 on T cells from healthy donors, ZAP-70+ and ZAP-70− CLL patients after incubation for various times with CXCL12 (1 μg/mL) relative to control cells (without CXCL12). T cells from both groups of CLL patients and healthy donors down-regulated CXCR4 comparably in a time-dependent fashion after incubation with this optimal concentration of CXCL12. In order to determine the dose-dependency of CXCR4 endocytosis, peripheral blood mononuclear cells (PBMC) from healthy donors and CLL patients were incubated with increasing concentrations of CXCL12 or medium alone and surface CXCR4 expression was evaluated after 1 h of culture. CXCL12 induced similar dose-dependent down-modulation of CXCR4 on T cells from each group (Figure 3B).
Figure 3.
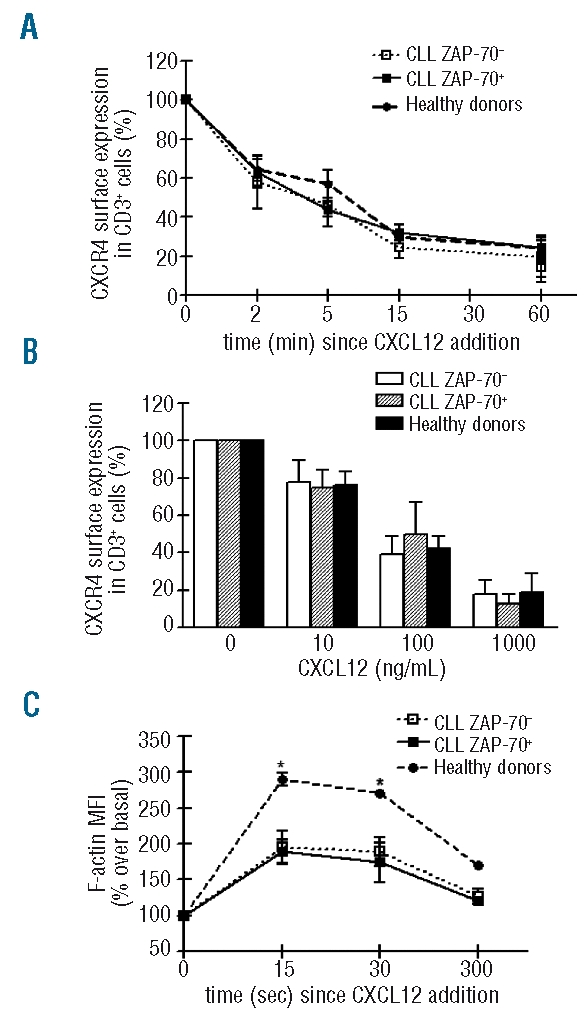
The impaired migratory capacity of T cells from ZAP-70−CLL patients was not related to defective CXCR4 down-regulation or a particular actin polymerization defect. (A) PBMC from six ZAP-70−(dotted line), six ZAP-70+ CLL patients (dashed line) and six healthy donors (solid line) were incubated with medium alone (control) or with CXCL12 (1 μg/mL) at 37ºC for 0, 2, 5, 15 and 60 min and the remaining CXCR4 on CD3+ cells was assessed by flow cytometry. The graph shows the percentage of CXCR4 on the surface of CD3+ cells treated with CXCL12 relative to the control. (B) PBMC from ZAP-70− and ZAP-70+ CLL patients and healthy donors were incubated for 60 min with different concentrations of CXCL12 (0, 10, 100, 1000 ng/mL) at 37ºC. The expression of remaining CXCR4 on the surface of T cells was then analyzed by flow cytometry. The data shown represent the mean±SEM (n=6) for the percentage of CXCR4 expression on the surface of CXCL12-treated T cells relative to control T cells (medium alone). (C) Intracellular F-actin was measured using FITC-labeled phalloidin in purified T cells from three ZAP-70−CLL patients, three ZAP-70+ CLL patients and three healthy donors after the addition of CXCL12 (1000 ng/mL) for 15, 30 and 300 sec. Results are shown as the mean±SEM of the percent of intracellular F-actin relative to the value before the addition of CXCL12. *P<0.05 Mann-Whitney test (CLL versus healthy donors).
Reorganization of the actin cytoskeleton is an early, pivotal event in the migratory response to chemokines. Since it was reported that T cells from CLL patients regulate actin polymerization inappropriately when in contact with antigen-presenting cells,29 we examined the ability of CXCL12 to induce changes in the actin cytoskeleton of T cells from healthy donors and ZAP-70+ and ZAP-70− CLL patients by evaluating the expression of filamentous actin (F-actin) in response to CXCL12. As shown in Figure 3C, there were robust, transient increases in F-actin in T cells within 5 sec after exposure to the chemokine, followed by depolymerization in all the samples analyzed. However, CXCL12 induced significantly less F-actin polymerization in T cells from CLL patients than in T cells from healthy donors; there were no differences between the two CLL groups.
The impaired migratory capacity of T cells from ZAP-70−patients was not related to lower expression of ZAP-70, CD3, CD38, CD45 or CXCR7 on these cells
It was previously reported that ZAP-70, a key element in T-cell receptor (TCR) signaling, is required for CXCR4 signal transduction.30,31 However, when we compared the expression of ZAP-70 within T cells from ZAP-70− and ZAP-70+ CLL samples we found no differences between groups (Online Supplementary Figure S5A) indicating that a dissimilar expression of ZAP-70 in T cells could not explain the different migratory capacity towards CXCL12 found in ZAP-70− and ZAP-70+ samples.
Since it was reported that CXCL12 stimulates the physical association of CXCR4 and TCR/CD3 and utilizes the ZAP-70 binding ITAM domains of the CD3 for signal transduction,32 we evaluated CD3 expression in PBMC from both groups of CLL patients without finding any significant difference in its expression (Online Supplementary Figure S5B). In addition, when we evaluated the expression of two other molecules which could influence T-cell chemotaxis towards CXCL12, the ectoenzyme CD38 and the tyrosine phosphatase CD45 (Online Supplementary Figure S5 C and D)33,34 we found comparable levels of expression in T cells from ZAP-70− and ZAP-70+ CLL samples. Finally, we evaluated the expression of CXCR7, a recently identified receptor for the chemokine CXCL12 which might influence CXCR4-mediated T-cell chemotaxis35 and found no differences in surface or intracellular CXCR7 expression between groups (Online Supplementary Figure S5E). Collectively, these results indicate that the lower migratory response towards CXCL12 found in T cells from ZAP-70− samples could not be ascribed to lesser expression of ZAP-70, CD3, CD45, CD38 or CXCR7 molecules on these cells.
Leukemic cells from ZAP-70− patients, but not from ZAP-70+ samples, reduce T-cell migration towards CXCL12
Since it was previously reported that the presence of leukemic CLL cells induces specific changes in T cells resulting in functional impairment,22,29 we evaluated whether CLL cells from ZAP-70− and ZAP-70+ samples can modulate autologous T-cell responses towards CXCL12. To this aim, purified T cells were cultured alone or with autologous CLL cells (ratio T cells: CLL cells = 1:4). T-cell chemotaxis towards CXCL12 was evaluated with freshly purified cells and cells cultured for 48 h. As shown in Figure 4A, comparable migratory capacity towards CXCL12 was found between freshly purified T cells alone (pT) or with autologous CLL cells (pT+pCLL) in both ZAP-70− and ZAP-70+ samples. Similar results were obtained with T cells from ZAP-70+ patients after 48 h of culture (Figure 4B). By contrast, in all of the ZAP-70− patients evaluated, purified T cells cultured for 48 h alone (pT) showed greater migratory capacity towards CXCL12 compared to purified T cells cultured with autologous CLL cells (pT+pCLL) (Figure 4B), although CXCR4 surface expression was similar in both groups (Online Supplementary Figure S6).
Figure 4.
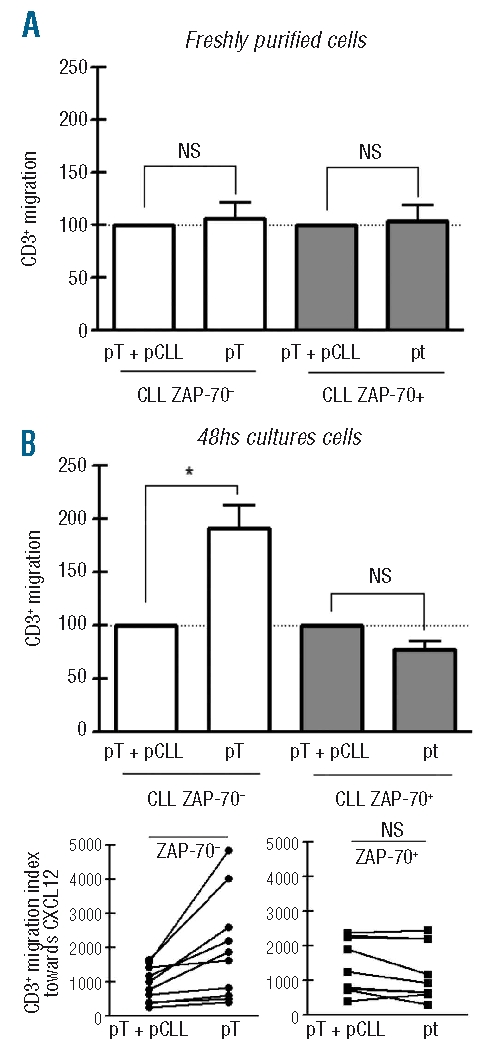
CLL cells from ZAP-70− patients reduce T-cell migration towards CXCL12. Purified T cells from ZAP-70− (n= 10) and ZAP-70+ CLL patients (n=9) were cultured in complete medium alone (pT cultures) or at a 1:4 ratio with autologous purified CLL cells (pT+pCLL cultures). Chemotaxis assays towards CXCL12 (1 μg/mL) were performed with freshly purified cells (A) and 48 h cultured cells (B). The migration of CD3+ cells was calculated by taking the migration index of T cells in pT+pCLL cultures as 100%. Bars represent the mean values±SEM for the CD3+ migration. NS: not significant, *P=0.001, Wilcoxon’s signed rank test. The lower panels show the migration indices of pT+pCLL and pT from 48 h cultured cells of each patient, *P<0.01, Wilcoxon’s signed rank test.
Discussion
During the past few years, it has increasingly been recognized that signals from the microenvironment make pivotal contributions to the progression of CLL. T lymphocytes and stromal cells appear to be essential players,36 interacting with leukemic cells in anatomical structures called proliferation centers. Since there was no information about the ability of T cells from CLL patients to respond to lymphoid organ chemokines, we evaluated the migratory potential of T cells from high risk and low risk CLL patients by performing chemotaxis assays towards CXCL12, CCL21 and CCL19 in CLL samples and in healthy donors.
When CLL patients as a whole group were compared with healthy donors, we found that T cells from the CLL patients responded to CXCL12, CCL21 and CCL19, albeit less well than T cells from their healthy counterparts. Since CCR7 and its ligands are essentially involved in T-cell and dendritic-cell homing to the lymph nodes, where T cells establish close physical contacts with dendritic cells to allow their antigen-specific activation, the lower CCR7-induced chemotaxis of T cells from CLL patients might contribute to their impaired cell-mediated immunity.37
We found similar CXCR4 and CCR7 expression in T cells from CLL patients and healthy donors, confirming a previous report of comparable CCR7 expression in T cells from normal subjects and CLL patients.38 Regarding CXCR4 expression, our results differ from those of Kratchard et al. who reported a higher expression in T cells from CLL patients.39 This discrepancy may be due to different experimental conditions used, since we directly stained whole blood samples while Kratchard et al. evaluated CXCR4 expression after isolation of mononuclear leukocytes from peripheral blood by Ficoll-Hypaque centrifugation. Despite having similar CXCR4 and CCR7 expression, T cells from CLL patients consistently showed a lower migratory capacity towards their ligands compared to healthy T cells. Other studies have already shown that chemokine responsiveness does not correlate with chemokine receptor expression levels. Concerning CXCR4, it was reported that its expression in bone marrow B cells and the migratory response towards CXCL12 was not associated at all.40 In addition, it was observed that B cells become highly responsive to the chemokine CCL20 after cellular activation without changes in the expression of its receptor41 and also that mature dendritic cells express the homing receptor CCR7 but migrate poorly in response to CCL19 and CCL21 without prior exposure to prostaglandin E2.42
When CLL patients were divided according to ZAP-70 expression, we found, surprisingly, that the lower migration towards CXCL12 is a distinctive feature of T cells from ZAP-70− CLL patients. The combination of ZAP-70 and CD38 appears to be more useful than either ZAP-70 or CD38 alone in identifying patients with a worse (ZAP-70+CD38+) or better (ZAP-70−CD38−) prognosis.1,2,4 In our samples, when ZAP-70− and ZAP-70+ CLL patients were further separated according to CD38 expression, we found that only T cells from ZAP-70−CD38− CLL samples had a significantly reduced migratory capacity towards CXCL12 compared to healthy T cells, although more CLL samples must be analyzed to allow firm conclusions to be drawn. Interestingly, Roth et al. recently reported that the average telomere loss was higher for T cells in ZAP-70+CD38+ CLL patients than in ZAP-70−CD38− ones indicating an extensive expansion within the T-cell compartment in the former group of CLL patients.43 Altogether, our results and the observations made by Roth indicate that accessory T cells from ZAP-70+ and ZAP-70− patients are different.
Given that CXCL12-induced migration seems to be regulated by intricate mechanisms besides CXCR4 expression, we further investigated other molecules and mechanisms capable of regulating CXCL12 responsiveness of T cells from CLL patients. Since it was previously reported that ZAP-70, CD38 and CD45 expression was associated with an enhanced response through CXCR4,33,34 we evaluated their expression in T cells from CLL patients without finding any significant difference between ZAP-70+ and ZAP-70− groups. Likewise, expression of CXCR7, a recently identified receptor for the chemokine CXCL12, was comparable in T cells from both groups. The lower migratory response of T cells from ZAP-70− CLL patients could not be ascribed to a deficiency in receptor endocytosis upon CXCL12 treatment, since T cells from both groups of CLL patients and healthy donors comparably down-regulated CXCR4 in a time- and dose-dependent fashion. Finally, as previously described by Gribben et al. for synapse formation29 we observed an F-actin polymerization defect upon CXCL12 stimulation in T cells from CLL patients compared to normal T cells, but no significant differences between the two groups of CLL patients, indicating that actin polymerization could not explain the different migratory capacity observed between T cells from ZAP-70− and ZAP-70+ CLL samples.
A number of reports suggested that CLL cells are not passive players, but that they actively modify their own microenvironment: for example, they have the capacity to attract accessory cells within lymphatic tissues,13,44,45 and can induce specific changes in T cells22 which can alter T-cell immunological recognition and function.29 We, therefore, investigated whether the leukemic clone itself was involved in the reduced migratory capacity towards CXCL12 displayed by T cells from the ZAP-70− group. We found that purified T cells from ZAP-70−, and not from ZAP-70+ patients, cultured alone for 48 h showed a significantly greater capacity to migrate towards CXCL12 compared to T cells cultured with autologous CLL cells, suggesting that T-cell migratory responses to CXCL12 are strongly affected by leukemic cells in ZAP-70− patients but not in ZAP-70+ samples. It remains to be determined which processes involved in CXCL12-induced migration of T cells are affected by ZAP-70− CLL cells.
Since T cells in proliferation centers may help CLL cells to survive and proliferate, the low migratory response towards CXCL12 in T cells from ZAP-70− CLL patients might favor the indolent clinical course of the disease in these patients. Experiments evaluating the quantity and functional quality of the T-cell compartment from ZAP-70+ and ZAP-70− CLL lymphoid organs are warranted.
Acknowledgments
the authors would like to thank Beatriz Loria and Edit Mabel Horvat for technical assistance. We also thank Olga Ramos for providing samples from healthy adults and Jorge Geffner for critical reading of the article.
Footnotes
Funding: this work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica, CONICET, Fundación Roemmers and Fundación Florencio Fiorini.
The online version of the article has a supplementary appendix
Authorship and Disclosures
MB designed and carried out the experiments and created the figures; PRN, JGG and PEM contributed to the analysis and interpretation of the data; RFB and JSA provided patients’ samples and advice; MG participated in the project conception and critically reviewed the manuscript; RG designed and supervised the study and wrote the manuscript.
The authors reported no potential conflicts of interest.
References
- 1.D’Arena G, Tarnani M, Rumi C, Vaisitti T, Aydin S, De Filippi R, et al. Prognostic significance of combined analysis of ZAP-70 and CD38 in chronic lymphocytic leukemia. Am J Hematol. 2007;82(9):787–91. doi: 10.1002/ajh.20936. [DOI] [PubMed] [Google Scholar]
- 2.Del Giudice I, Morilla A, Osuji N, Matutes E, Morilla R, Burford A, et al. Zeta-chain associated protein 70 and CD38 combined predict the time to first treatment in patients with chronic lymphocytic leukemia. Cancer. 2005;104(10):2124–32. doi: 10.1002/cncr.21437. [DOI] [PubMed] [Google Scholar]
- 3.Schroers R, Griesinger F, Trumper L, Haase D, Kulle B, Klein-Hitpass L, et al. Combined analysis of ZAP-70 and CD38 expression as a predictor of disease progression in B-cell chronic lymphocytic leukemia. Leukemia. 2005;19(5):750–8. doi: 10.1038/sj.leu.2403707. [DOI] [PubMed] [Google Scholar]
- 4.Hus I, Podhorecka M, Bojarska-Junak A, Rolinski J, Schmitt M, Sieklucka M, et al. The clinical significance of ZAP-70 and CD38 expression in B-cell chronic lymphocytic leukaemia. Ann Oncol. 2006;17(4):683–90. doi: 10.1093/annonc/mdj120. [DOI] [PubMed] [Google Scholar]
- 5.Dighiero G, Binet JL. When and how to treat chronic lymphocytic leukemia. N Engl J Med. 2000;343(24):1799–801. doi: 10.1056/NEJM200012143432410. [DOI] [PubMed] [Google Scholar]
- 6.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115(3):755–64. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosati S, Kluin PM. Chronic lymphocytic leukaemia: a review of the immuno-architecture. Curr Top Microbiol Immunol. 2005;294:91–107. [PubMed] [Google Scholar]
- 8.Soma LA, Craig FE, Swerdlow SH. The proliferation center microenvironment and prognostic markers in chronic lymphocytic leukemia/small lymphocytic lymphoma. Hum Pathol. 2006;37(2):152–9. doi: 10.1016/j.humpath.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Ghia P, Circosta P, Scielzo C, Vallario A, Camporeale A, Granziero L, et al. Differential effects on CLL cell survival exerted by different microenvironmental elements. Curr Top Microbiol Immunol. 2005;294:135–45. doi: 10.1007/3-540-29933-5_8. [DOI] [PubMed] [Google Scholar]
- 10.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol. 2003;123(3):380–8. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 11.Fluckiger AC, Rossi JF, Bussel A, Bryon P, Banchereau J, Defrance T. Responsiveness of chronic lymphocytic leukemia B cells activated via surface Igs or CD40 to B-cell tropic factors. Blood. 1992;80(12):3173–81. [PubMed] [Google Scholar]
- 12.Buske C, Gogowski G, Schreiber K, Rave-Frank M, Hiddemann W, Wormann B. Stimulation of B-chronic lymphocytic leukemia cells by murine fibroblasts, IL-4, anti-CD40 antibodies, and the soluble CD40 ligand. Exp Hematol. 1997;25(4):329–37. [PubMed] [Google Scholar]
- 13.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113(13):3050–8. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Giral S, Quintana NE, Cabrerizo M, Alfonso-Perez M, Sala-Valdes M, De Soria VG, et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol. 2004;76(2):462–71. doi: 10.1189/jlb.1203652. [DOI] [PubMed] [Google Scholar]
- 15.Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110(9):3316–25. doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]
- 16.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94(11):3658–67. [PubMed] [Google Scholar]
- 17.Richardson SJ, Matthews C, Catherwood MA, Alexander HD, Carey BS, Farrugia J, et al. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107(9):3584–92. doi: 10.1182/blood-2005-04-1718. [DOI] [PubMed] [Google Scholar]
- 18.Deaglio S, Aydin S, Vaisitti T, Bergui L, Omede P, Bonello L, et al. CD38 and ZAP-70 regulate CLL cells chemotaxis Leuk Lymphoma 200748suppl 110917325854 [Google Scholar]
- 19.Shanafelt T, Kay N. T-cell abnormalities in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47(7):1197–8. doi: 10.1080/10428190600687976. [DOI] [PubMed] [Google Scholar]
- 20.Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma. 2003;44(3):383–9. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- 21.Ravandi F, O’Brien S. Immune defects in patients with chronic lymphocytic leukemia. Cancer Immunol Immunother. 2006;55(2):197–209. doi: 10.1007/s00262-005-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115(7):1797–805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42(7):799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 24.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, et al. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002;196(1):65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips R, Ager A. Activation of pertussis toxin-sensitive CXCL12 (SDF-1) receptors mediates transendothelial migration of T lymphocytes across lymph node high endothelial cells. Eur J Immunol. 2002;32(3):837–47. doi: 10.1002/1521-4141(200203)32:3<837::AID-IMMU837>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Durig J, Naschar M, Schmucker U, Renzing-Kohler K, Holter T, Huttmann A, et al. CD38 expression is an important prognostic marker in chronic lymphocytic leukaemia. Leukemia. 2002;16(1):30–5. doi: 10.1038/sj.leu.2402339. [DOI] [PubMed] [Google Scholar]
- 27.Gentile M, Mauro FR, Calabrese E, De Propris MS, Giammartini E, Mancini F, et al. The prognostic value of CD38 expression in chronic lymphocytic leukaemia patients studied prospectively at diagnosis: a single institute experience. Br J Haematol. 2005;130(4):549–57. doi: 10.1111/j.1365-2141.2005.05659.x. [DOI] [PubMed] [Google Scholar]
- 28.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7. [PubMed] [Google Scholar]
- 29.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427–37. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ticchioni M, Charvet C, Noraz N, Lamy L, Steinberg M, Bernard A, et al. Signaling through ZAP-70 is required for CXCL12-mediated T-cell transendothelial migration. Blood. 2002;99(9):3111–8. doi: 10.1182/blood.v99.9.3111. [DOI] [PubMed] [Google Scholar]
- 31.Ottoson NC, Pribila JT, Chan AS, Shimizu Y. Cutting edge: T cell migration regulated by CXCR4 chemokine receptor signaling to ZAP-70 tyrosine kinase. J Immunol. 2001;167(4):1857–61. doi: 10.4049/jimmunol.167.4.1857. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, et al. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25(2):213–24. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Fernandis AZ, Cherla RP, Ganju RK. Differential regulation of CXCR4-mediated T-cell chemotaxis and mitogen-activated protein kinase activation by the membrane tyrosine phosphatase, CD45. J Biol Chem. 2003;278(11):9536–43. doi: 10.1074/jbc.M211803200. [DOI] [PubMed] [Google Scholar]
- 34.Deaglio S, Vaisitti T, Aydin S, Ferrero E, Malavasi F. In-tandem insight from basic science combined with clinical research: CD38 as both marker and key component of the pathogenetic network underlying chronic lymphocytic leukemia. Blood. 2006;108(4):1135–44. doi: 10.1182/blood-2006-01-013003. [DOI] [PubMed] [Google Scholar]
- 35.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–6. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 36.Caligaris-Cappio F, Ghia P. Novel insights in chronic lymphocytic leukemia: are we getting closer to understanding the pathogenesis of the disease? J Clin Oncol. 2008;26(27):4497–503. doi: 10.1200/JCO.2007.15.4393. [DOI] [PubMed] [Google Scholar]
- 37.Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33(2):240–9. doi: 10.1053/j.seminoncol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Alfonso-Perez M, Lopez-Giral S, Quintana NE, Loscertales J, Martin-Jimenez P, Munoz C. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol. 2006;79(6):1157–65. doi: 10.1189/jlb.1105623. [DOI] [PubMed] [Google Scholar]
- 39.Krackhardt AM, Harig S, Witzens M, Broderick R, Barrett P, Gribben JG. T-cell responses against chronic lymphocytic leukemia cells: implications for immunotherapy. Blood. 2002;100(1):167–73. doi: 10.1182/blood.v100.1.167. [DOI] [PubMed] [Google Scholar]
- 40.Honczarenko M, Douglas RS, Mathias C, Lee B, Ratajczak MZ, Silberstein LE. SDF-1 responsiveness does not correlate with CXCR4 expression levels of developing human bone marrow B cells. Blood. 1999;94(9):2990–8. [PubMed] [Google Scholar]
- 41.Liao F, Shirakawa AK, Foley JF, Rabin RL, Farber JM. Human B cells become highly responsive to macrophage-inflammatory protein-3 alpha/CC chemokine ligand-20 after cellular activation without changes in CCR6 expression or ligand binding. J Immunol. 2002;168(10):4871–80. doi: 10.4049/jimmunol.168.10.4871. [DOI] [PubMed] [Google Scholar]
- 42.Scandella E, Men Y, Legler DF, Gillessen S, Prikler L, Ludewig B, et al. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103(5):1595–601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 43.Roth A, de Beer D, Nuckel H, Sellmann L, Duhrsen U, Durig J, et al. Significantly shorter telomeres in T-cells of patients with ZAP-70+/CD38+ chronic lymphocytic leukaemia. Br J Haematol. 2008;143(3):383–6. doi: 10.1111/j.1365-2141.2008.07363.x. [DOI] [PubMed] [Google Scholar]
- 44.Ghia P, Strola G, Granziero L, Geuna M, Guida G, Sallusto F, et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32(5):1403–13. doi: 10.1002/1521-4141(200205)32:5<1403::AID-IMMU1403>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Zucchetto A, Benedetti D, Tripodo C, Bomben R, Dal Bo M, Marconi D, et al. CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Res. 2009;69(9):4001–9. doi: 10.1158/0008-5472.CAN-08-4173. [DOI] [PubMed] [Google Scholar]