Abstract
Background
Disease stage is the most important prognostic parameter in allogeneic hematopoietic cell transplantation (HCT) for acute lymphoblastic leukemia, but other factors such as donor/host histocompatibility and gender combination, recipient age, performance status and comorbidities need to be considered. Several scoring systems are available to predict outcome in HCT recipients; however, their prognostic relevance in acute lymphoblastic leukemia is not well defined.
Design and Methods
In the present study we evaluated a modified EBMT risk score (mEBMT) and the HCT-specific comorbidity index (HCT-CI) in 151 adult acute lymphoblastic leukemia patients who received allogeneic HCT from 1995 until 2007 at our center.
Results
Disease status was first complete remission (CR1) (47%), CR>1 (21%) or no CR (32%). Overall survival (OS) at one, two and five years was 62%, 51% and 40% and non-relapse mortality (NRM) was 21%, 24% and 32%. Median mEBMT was 3 (0–6). Higher mEBMT was associated with inferior OS (hazard ratio per score unit (HR): 1.50, P<0.001), higher NRM (HR: 1.36, P=0.042) and higher relapse mortality (HR: 1.68, P<0.001). Disease stage was the predominant prognostic factor in this score. Comorbidities were present in 71% of patients with mild hepatic disease (29%), moderate pulmonary disease (28%) and infections (23%) being the most common. Median HCT-CI was 1 (0–9). In univariate analysis a trend for inferior OS (HR: 1.08, P=0.20) and higher NRM (HR: 1.14, P=0.11) with increasing HCT-CI was observed but the level of significance was not reached. In additional analyses we found that reduced Karnofsky Performance Status (KPS) was associated with inferior OS (HR: 1.34, P=0.023) and higher relapse mortality (HR: 1.71, P=0.001) when analyzed univariately. However, KPS was associated with disease stage and significance was lost in multivariate analysis.
Conclusions
The mEBMT was prognostic in our patient cohort with predominant influence of disease stage, whereas a trend but no significant prognostic value was observed for the HCT-CI.
Keywords: hematopoietic stem cell transplantation, acute lymphoblastic leukemia, HCT recipients
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an integral part of post-remission therapy in adult patients with acute lymphoblastic leukemia (ALL) who present with high risk features such as high initial leukocyte counts, unfavorable cytogenetics or delayed response to induction chemotherapy.1 HCT is also indicated in cases of primary refractory ALL or after relapse has occurred. Although HCT has considerable curative potential in these situations, its application is limited by transplant-related complications such as infections and graft-versus-host disease (GVHD) which can lead to mortality rates of up to 50% in older or less fit patients. Therefore, a careful assessment of risk and benefits prior to transplantation is mandatory.
Disease stage appears as the most prominent prognostic parameter in allogeneic HCT for ALL, but other factors such as cytomegalovirus status, donor/host histocompatibility, donor/host gender combination, general performance status and recipient age need to be considered.2–4 In addition, comorbid diseases may also have an important influence on transplant outcome in ALL patients, as already shown for other hematologic malignancies.5 Several composite risk scores have been introduced to allow an integral assessment of the aforementioned parameters. However, none has yet been established as a standard of clinical care for ALL patients.6–11 Possibly, an assessment of specific patient-, disease- and transplant-related factors as made with the European Group for Blood and Marrow Transplantation (EBMT) risk score9, 10 in combination with an extensive assessment of comorbidities as made with the HCT-specific comorbidity index (HCT-CI)11 may be most potent.
The EBMT risk score is based on an analysis of registry data of 3,142 patients transplanted between 1989 and 1997 for CML in Europe.9 The score accounts for recipient age, disease stage, donor/host histocompatibility, interval from diagnosis to transplantation and donor/host gender combination and is highly predictive for leukemia-free survival (LFS), overall survival (OS) and non-relapse mortality (NRM) in CML patients. The HCT-CI accounts for seventeen comorbidities which were found to influence the 2-year NRM in a cohort of 1,055 patients who received allogeneic HCT at the Fred Hutchinson Cancer Research Center (FHCRC) between 1997 and 2003.11 Main diagnoses in this study were acute myeloid leukemia (AML) (27%), chronic myeloid leukemia (CML) (20%) and myelodysplastic syndrome (MDS) (19%) but some patients with ALL (10%) were also included.
In the present study, we analyzed whether a modified version of the EBMT risk score, where the definition of disease stage was adapted for ALL and where the parameter “time from diagnosis to transplantation” was omitted due to multiple sources of bias (mEBMT), and the HCT-CI were able to predict OS, NRM and relapse mortality in 151 adult ALL patients who received allogeneic HCT at our center from 1995 until 2007. We also included the Karnofsky Performance Status Scale (KPS) in our analysis, as this parameter had been previously established as a prognostic factor in ALL patients.2
Design and Methods
Patients
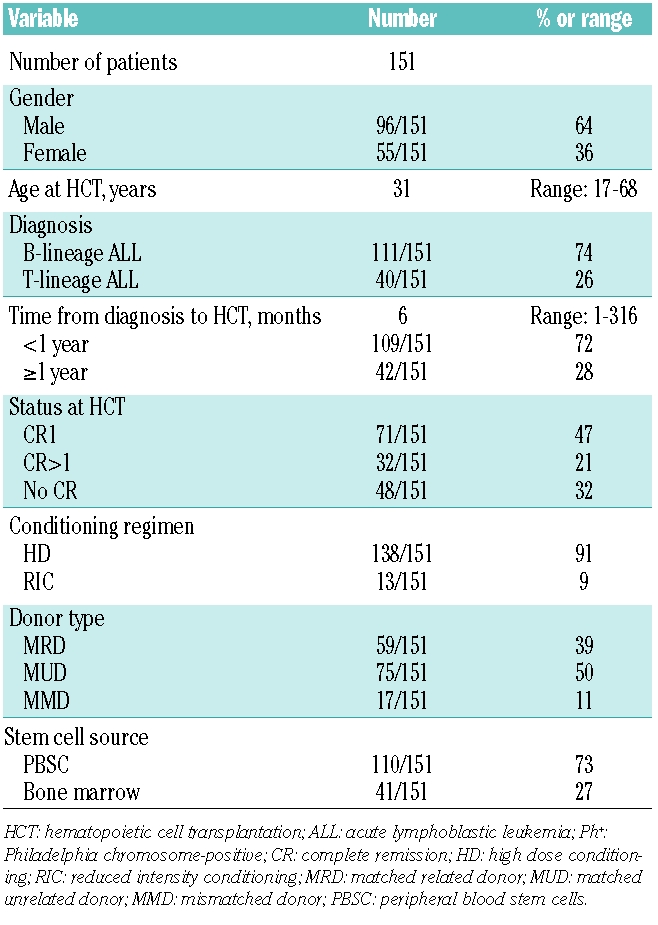
One hundred and fifty-one consecutive adult ALL patients who were transplanted between 1995 and 2007 were included in this retrospective analysis. Median age was 31 years (range: 17–68). All patients had received initial treatment according to the German Multicenter Study Group for Adult ALL protocols (GMALL).12 Status at the time of HCT was first complete remission (CR1) and presence of high risk or very high risk disease according to GMALL criteria in 48% of patients, second or higher CR (CR>1) in 21% of patients, and no CR in 32% of patients (Table 1).
Table 1.
Patients’ and treatment characteristics.
Risk assessment
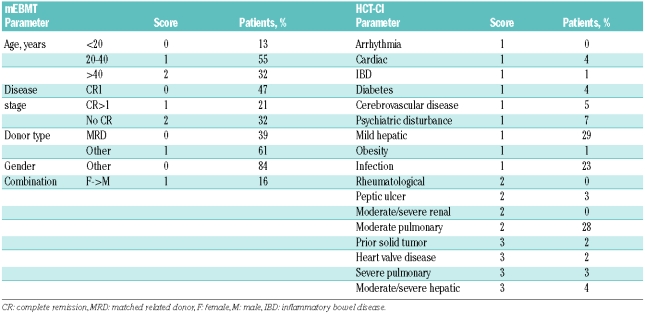
The HCT-CI was calculated as described in the original publication.11 The calculation of the EBMT risk score, which had been initially developed for CML,9 was modified to account for disease-specific differences (mEBMT). For the parameter “disease stage”, scores of 0, 1 or 2 were assigned to patients transplanted in CR1, higher CR and active disease (Table 2) according to published survival rates in ALL patients.2,3 The parameter “time from diagnosis to transplantation” was omitted from the score calculation, as it strongly correlated with the parameter “disease stage” and was, therefore, not an independent prognosticator (Spearman’s rank correlation: r=0.413, P<0.0001). In addition, various sources of bias exist for this parameter which could influence OS, NRM and relapse mortality in one direction or the other. For example, a long interval may indicate the occurrence of prior complications which would be associated with increased NRM, but it may also indicate an adequate recovery time from chemotherapy leading to less NRM. Also, an important selection bias occurs for patients in CR because patients with a long interval have not experienced early relapse and may, therefore, have a better prognosis. Finally, the time interval depends on unspecific factors such as donor availability and availability of a transplant bed.
Table 2.
Parameters of analyzed risk assessment scores.
Required parameters for the calculation of the scores were extracted from patient files, discharge letters and computer databases. All patients were scored by two independent investigators and in case of diverging results the final score was specified by review of original data from the patient files and subsequent consensus discussion. Laboratory studies, pulmonary function tests and echocardiograms had been performed in all patients at admission for allogeneic HCT. Retrospective data collection was complete (Table 2). Karnofsky performance status had been prospectively assessed at time of admission in 130/151 patients. For the remaining patients, we retrospectively assigned scores according to the general state of health at the time of admission as documented in the discharge letters. For analysis of the HCT-CI, patients were assigned to risk groups as described in the original publication (HCT-CI score 0: low risk, score 1–2: intermediate risk, score > 2: high risk). For analysis of the mEBMT, patients with scores 0 and 1 and with scores 5 and 6 were analyzed together to increase group sizes.
HCT
One hundred and thirty-eight patients (91%) received standard high-dose conditioning (HD) whereas 13 patients (9%) received reduced intensity conditioning (RIC) (Table 1). HD consisted of 6 × 2 Gy total body irradiation (TBI) in combination with either 2 × 60 mg/kg cyclophosphamide (n=78, 52%), or 60 mg/kg etoposide (n=30, 20%) or a combination of 2 × 50 mg/kg cyclophosphamide and 50 mg/kg etoposide (n=28, 19%). Two patients received non-TBI based HD with 4 × 4 mg/kg busulfan and 2 × 60 mg cyclophosphamide. RIC consisted of 6 × 30 mg/m2 fludarabine, 2 × 4 mg/kg busulfan and 4 × 10 mg/kg rabbit antithymocyte globulin (ATG, Fresenius, Germany).
Transplants were from HLA-matched related donors (n=59, 39%), HLA-matched unrelated (n=75, 50%) donors or HLA-mismatched donors (n=17, 11%) according to serological typing (before 1997) or high-resolution genotyping (from 1997). As stem cell source, either peripheral blood stem cells (PBSC) (n=110, 73%) or bone marrow (n=41, 27%) were used. Prophylaxis of GVHD and grading was performed as previously described.13
Statistics
Data were analyzed as of September 10, 2008. Statistical analysis was performed using SPSS 15.0 (SPSS, Chicago, USA), GraphPad 4.0 (GraphPad Software Inc., San Diego, USA) and the cmprsk package in R statistical software (The R Foundation for Statistical Computing, Vienna, Austria). The level of significance was 0.05 (two-sided).
Patients’ and treatment characteristics are reported with medians and range where applicable. OS was measured from the date of HCT to the date of death from any cause. LFS was defined as survival from the date of HCT while in continuous CR. In the analysis of time to relapse patients dying from reasons not related to the disease were censored. For surviving patients, data were censored at the date of last follow-up. Overall survival data were analyzed according to Kaplan-Meier and tested univariately using the log rank test. Graphs were truncated at ten years of follow-up because no further events occurred beyond that point. Univariate and multivariate analysis were performed using Cox’s proportional hazard regression model. Prognostic variables examined were gender, year of HCT, conditioning regimen, stem cell source, time from diagnosis to transplantation, patient age, disease stage, donor type, donor/recipient gender combination, mEBMT, HCT-CI and KPS. First, for all potential predictors, a univariate model was calculated. Then, forward and backward variable selection (inclusion P=0.05, exclusion P=0.10) was applied for those variables which were significant in univariate analysis. In the analysis of the mEBMT, variables already included in the score were not included in the multivariate model. For each analysis, hazard ratios (HR) and 95% confidence intervals (95%CI) are given together with P values for comparisons with the reference category and when applicable for the overall test. Cumulative incidence curves for non-relapse mortality and relapse mortality were calculated in a competing-risks setting according to published methods and were compared using Gray’s test.14 Correlations were evaluated by Spearman’s rank correlation.
Results
Overview
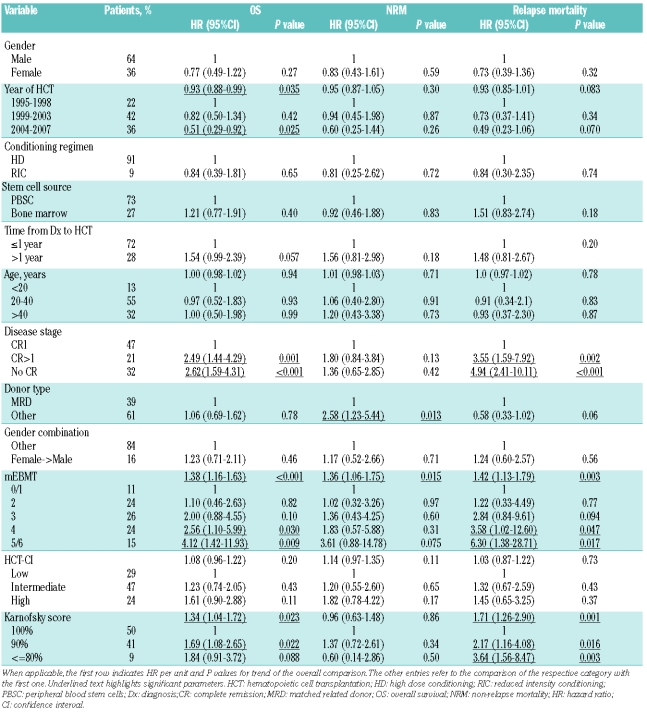
At time of analysis, after a median follow-up of the survivors of 48 months (range: 5–144) and after a median follow-up of all patients of 19 months (range: 1–144), 64 patients (42%) remained alive. Projected OS of the whole cohort at one, two and five years was 62%, 51% and 40%, respectively, with a median OS of 26 months (95%CI: 12–41, range: 1–144) (Figure 1A). LFS at one, two and five years was 57%, 48% and 38%, respectively (Figure 1A). Causes of death were relapse in 31% of patients, acute or chronic GVHD in 10% of patients, infections in 11% of patients and toxicity in 5% of patients. Median time to relapse was five months (range: 1–49) and median time to death from relapse was ten months (range: 3–75). NRM at one, two and five years was 21%, 24% and 32%, respectively. Median time to death from NRM was four months (range: 1–92) (Figure 1B). Cox’s regression analysis showed that OS improved and NRM and relapse mortality decreased over time (Tables 3 and 4). Acute GVHD grade I–II occurred in 42% of patients, acute GVHD grade III–IV occurred in 21% of patients and chronic GVHD occurred in 59% of patients who survived more than 100 days after HCT.
Figure 1.
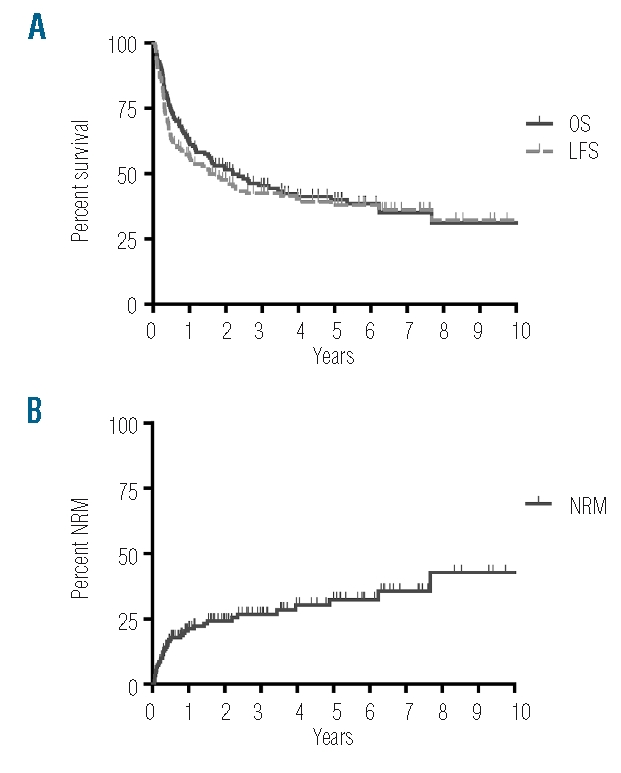
OS, LFS and NRM of 151 patients receiving HCT for ALL: 151 adult ALL patients received allogeneic HCT between 1995 and 2007 at our center. Shown are A) OS and LFS and B) NRM.
Table 3.
Pre-transplant risk assessment in adult acute lymphoblastic leukemia: univariate analysis of OS, NRM and relapse mortality.
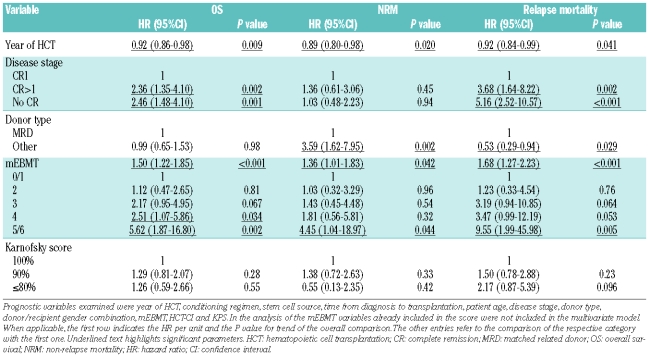
Table 4.
Pre-transplant risk assessment in adult acute lymphoblastic leukemia: multivariate analysis showing results for significant variables from univariate analysis.
mEBMT
As detailed above, a modified version of the EBMT risk score was determined, where the definition of disease stage was adapted for ALL and where the parameter “time from diagnosis to transplantation” was omitted due to multiple sources of bias. All patients were scored independently by two investigators with divergent results in 4/151 cases (3%). The cause of disagreement in all 4 cases was incongruence between information sources and the final score was then determined by review of original data from the patient files.
The prevalence of individual risk factors according to the definitions of the mEBMT is shown in Table 2. Median mEBMT was 3 (range: 0–6) with 11%, 24%, 26%, 24% and 15% of patients scoring 0 or 1, 2, 3, 4 and 5 or 6, respectively (Table 3). In univariate Kaplan-Meier analysis the mEBMT proved to be prognostic for OS with projected 2-year OS rates of 65%, 65%, 52%, 34% and 22% for scores 0/1, 2, 3, 4 and 5/6, respectively and 5-year OS rates ranging from 58% for score 0/1 to 24% for score 4 (P<0.001) (Figure 2A). In Cox’s regression analyses hazard ratios for OS increased with each additional score point, which was highly significant in univariate and multivariate comparisons (multivariate HR per score unit: 1.50 (95%CI: 1.16–1.63), P<0.001) (Tables 3 and 4). The mEBMT was also prognostic for NRM (multivariate HR per score unit: 1.36 (95%CI: 1.01–1.83), P=0.042) and relapse mortality (multivariate HR per score unit: 1.68 (95%CI: 1.27–2.23), P<0.001) (Figure 2B, Table 4). When NRM and relapse mortality were analyzed in a competing risk setting similar trends were observed. However the level of significance was not reached (data not shown). In an analysis of single mEBMT parameters, HCT performed in CR>1 or no CR was associated with inferior OS (P=0.002 and P=0.001, respectively) which was due to higher relapse mortality (P=0.002 and P<0.001, respectively) (Table 4). Patients with an HLA-identical family donor had less NRM (P=0.002). However, due to higher relapse mortality (P=0.029) survival was not improved. Age group and donor/recipient gender combination had no significant prognostic value in our patients (Tables 3 and 4).
Figure 2.
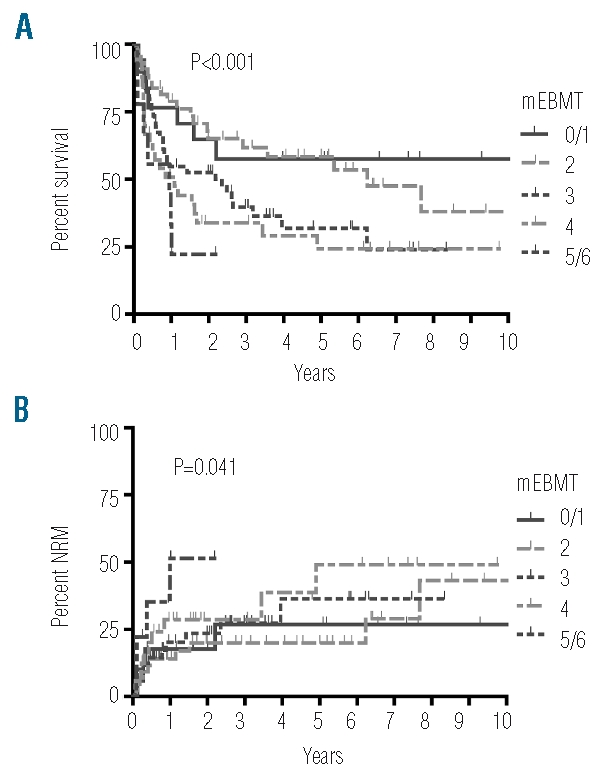
The mEBMT is predictive for OS and NRM. A) OS and B) NRM are shown for the respective mEBMT scores.
HCT-CI
The HCT-CI was scored by two independent investigators with divergent results in 34 score items affecting 31/151 patients (21%). Causes of disagreement were different understanding of the HCT-CI comorbidity definitions among scorers (n=19), obvious scoring errors (n=7), incongruence between information sources (n=4) and different time points used for analysis (n=4). In affected patients, the final HCT-CI was then specified by review of original data from the patient files and subsequent consensus discussion.
Comorbidities as defined by the HCT-CI criteria were identified in 71% of all patients with 40% of patients presenting with one comorbid condition and 21%, 8% and 2% presenting with two, three and more than three comorbid conditions, respectively. Main comorbidities in our cohort were mild hepatic disease, moderate pulmonary disease and infections with a prevalence of 29%, 28% and 23%, respectively. All other comorbidities had a prevalence of 7% or less (Table 2). The most frequent infectious foci observed in our patients were persistent fungal pneumonia (9%), fever of unknown origin (3%) and urinary tract infection (2%). Median HCT-CI score was 1 (range: 0–9). There was no significant association of the HCT-CI with age (r=0.074, P=0.37) or disease risk (r=0.144, P=0.078) as assessed by Spearman’s rank correlation whereas a weak association of higher HCT-CI with lower KPS was observed (r=−0.179, P=0.028). According to the HCT-CI risk classification 29% of patients belonged to the low risk group, 47% belonged to the intermediate risk group and 24% belonged to the high risk group (Table 3). When OS was compared for these three risk groups we observed a trend for a decrease in overall survival in patients with higher HCT-CI. However, this difference was not statistically significant (2-year OS of 58%, 50% and 46% and 5-year OS of 44%, 42% and 32% for HCT-CI low, intermediate and high risk patients, P=0.11) (Figure 3A, Table 3). The HR per score unit was 1.08 (95%CI: 0.96–1.22, P=0.2) with an HR of 1.23 (95%CI: 0.74–2.05) for intermediate risk patients and an HR of 1.61 (95%CI: 0.90–2.88) for high risk patients. Also, in patients with higher HCT-CI, NRM was increased (HR per score unit: 1.14 (95%CI: 0.97–1.35), P=0.11), but again the level of significance was not reached (Figure 3B, Table 3). There was no considerable change in these results in competing risk analysis and when other cut-off points for definition of risk groups were used or when we analyzed subgroups stratified by disease stage, conditioning regimen or age group (data not shown). In further univariate analysis of single HCT-CI comorbidities, cardiac disease had a significant influence on NRM (HR: 3.88 (95%CI: 1.51–9.97), P=0.005), whereas all other comorbidities had no relevant effect on OS or NRM (data not shown).
Figure 3.
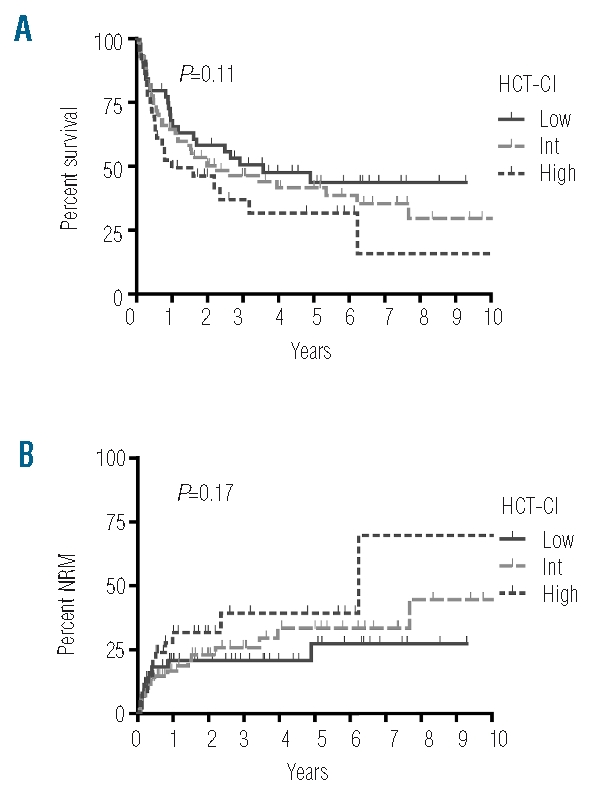
A prognostic trend for the HCT-CI is observed but differences do not reach the level of significance. A) OS and B) NRM are shown for the respective HCT-CI risk categories (HCT-CI score 0: low risk, score 1–2: intermediate risk, score > 2: high risk).
Karnofsky Performance Status Scale
Most patients had good or very good general performance on admission for HCT with a KPS of 100% in 50% of patients and a KPS of 90% in 41% of patients. A KPS of 80% or less was observed in only 9% of patients (Table 3). According to Spearman’s rank correlation there was no association of KPS with age (r=−0.009, P=0.917). In univariate analysis a significant association of lower KPS with a decrease in OS was observed (HR per score unit: 1.34 (95%CI: 1.04–1.72), P=0.023) (Figure 4A, Table 3). However, KPS was not an independent prognostic variable as significance was lost in multivariate analysis and as we observed a significant correlation of KPS with disease stage (r=−0.327, P<0.0001) and mEBMT (r=−0.258, P=0.001). In accordance with these results, the difference in OS seen in univariate analysis was not due to a difference in NRM (HR per unit: 0.96 (95%CI: 0.63–1.48), P=0.86) (Figure 4B, Table 3) but rather due to higher relapse mortality in the groups with lower KPS (HR per unit: 1.71 (95%CI: 1.26–2.90), P=0.001) (Table 3).
Figure 4.
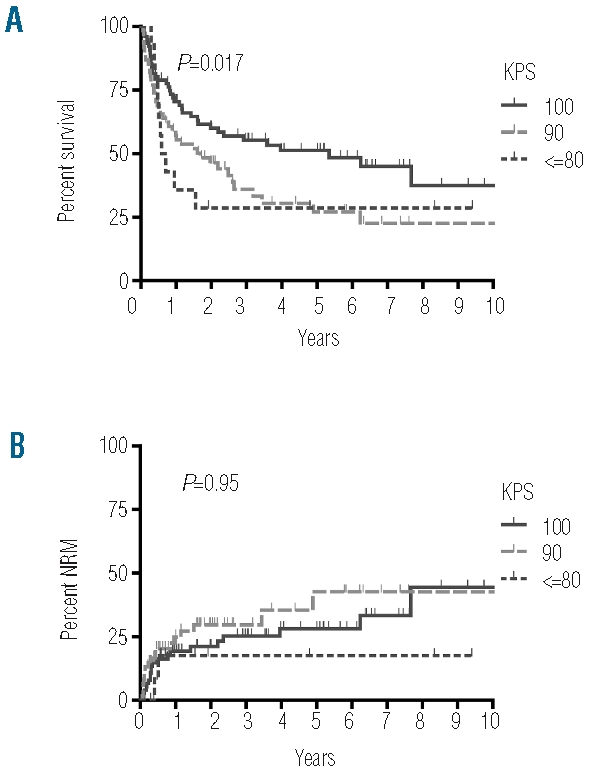
The Karnofsky Performance Status Scale is predictive for OS but not for NRM. A) OS and B) NRM are shown for the respective KPS.
Discussion
This study was intended to determine whether an assessment of patient-, disease- and transplant-related variables by calculation of a modified EBMT risk score and an assessment of pre-transplant comorbidities by calculation of the HCT-CI allowed prognostication of OS, NRM and relapse mortality in a cohort of 151 adult ALL patients who were treated at our center between 1995 and 2007. These scores had not so far been analyzed in a large homogeneously treated group of ALL patients. Especially the role of pre-transplant comorbidities needed to be more clearly defined in this patient population.
Our analysis showed that the HCT-CI was able to identify comorbid conditions in more than two-thirds of our patients. Hepatic disease, pulmonary disease and infections had the highest prevalence while all other comorbidities were only rarely seen in our study. Among the comorbidities included in the HCT-CI only cardiac disease was predictive for increased NRM whereas all other comorbidities had no significant prognostic value. In the analysis of the composite score we observed a trend for inferior OS and higher NRM with higher HCT-CI. However, the level of prognostic significance was not reached. Subgroup analyses with stratification by disease stage, conditioning regimen or age group and competing risk analysis did not change these results.
Various studies had validated the HCT-CI in patients with lymphoma and chronic lymphocytic leukemia (CLL),15–17 AML and MDS18–21 and in other more heterogeneous patient cohorts.22–25 No significant prognostic role of the HCT-CI was seen in a study from Canada26 and in a study from France (which determined a reduced score without results of pulmonary function tests).27 In addition to these negative studies, one report found that a significant correlation of the HCT-CI with treatment outcome was lost in subgroup analysis of different donor sources and conditioning regimens.28 Moreover, in a two-center study, the HCT-CI was able to predict OS and NRM at one center but failed to do so in the other center in multivariate analysis.29 Also, two studies described a correlation of the HCT-CI with OS30 and LFS,31 but not with NRM. Three smaller studies,32–34 including one on the use of RIC in 22 ALL patients,34 had negative results. However, these studies were possibly not powered to detect differences. The reasons behind these conflicting results are not totally clear. However, the predictive value of the HCT-CI may depend on specific patient characteristics and treatment protocols. In this respect, it is important to note that median patient age in our study (31 years) and the larger negative studies (39 and 31 years)26, 27 was considerably lower compared with the original report and most confirmative studies (43–60 years)11,17–25. It is possible that younger patients with comorbidities have a lower risk for treatment-related deaths than older patients with the same comorbidities. Another important confounder may be the distribution of HCT-CI risk groups. The negative studies reported HCT-CI intermediate and high risk in 94% of patients,27 82% of patients26 and 71% of patients (our study), whereas the original report and most confirmative studies had considerably lower frequencies of intermediate and high risk patients (33–62%)11,17,19,20,23,29. Discriminative properties of the HCT-CI might have been diminished in the negative studies as a result of very high sensitivity. Furthermore, influence of comorbidities on overall treatment outcome might depend on center-specific factors as well as on the analyzed disease with a possible loss of predictive value in situations with many high relapse risk patients. We observed a comparatively high interscorer variability for the HCT-CI with divergent scores in 31 of 151 patients. The variability was mainly due to a different interpretation of comorbidity definitions among scorers and it, therefore, appears that the single HCT-CI comorbidities need to be defined in more detail.
The EBMT risk score was initially established for CML patients based on registry data9 and was recently validated in a second registry analysis for other hematologic malignancies including ALL.10 The original score accounts for recipient age, disease stage, donor/host histocompatibility, time from diagnosis to HCT and donor/recipient sex combination, all of which have been validated as important prognosticators in ALL patients.2–4 However, for reasons discussed above we have omitted the parameter “time from diagnosis to HCT” in our score calculation. The modified EBMT risk score had a good prognostic value for OS with increasing hazard ratio for each additional score point in univariate and multivariate analysis. The score was also predictive for relapse mortality and NRM. However, significance was not observed in competing risk analysis. In an analysis of single risk score parameters, advanced disease stage had a negative impact on OS and led to increased relapse mortality, whereas transplants from unrelated donors or mismatched sibling donors increased NRM but reduced relapse mortality. Although disease stage was the single factor with significant influence on OS in our study, we have found that the remaining EBMT risk score parameters also had considerable prognostic relevance. When analyzed together a trend towards reduced OS for patients with higher scores and a clear trend towards increased NRM was seen (data not shown). Also, our multivariate analysis showed that scoring the mEBMT allowed better discrimination of groups with distinct hazard ratios than only scoring for disease stage.
In an attempt to determine whether a combined assessment of conventional prognostic parameters and comorbidities would have superior prognostic strength we also tested the Pretransplantation Assessment of Mortality score (PAM) which accounts for patient age, disease risk, donor type, type of conditioning regimen, pulmonary function tests and laboratory values for serum creatinine and serum alanine aminotransferase levels.7 However, the missing discrimination between matched and mismatched unrelated donors, the definition of age groups (<50 years, 50–60 years, >60 years) and the assignment of both CR1 and CR2 patients to the intermediate disease risk group was not ideal for our patient population and no relevant discriminative properties of this score were found in our patient population (data not shown). Also no relevant prognostic effect was seen when only PAM score comorbidities were analyzed (data not shown).
In the final analysis, we found that a simple assessment of overall health of transplant recipients based on Karnofsky performance status, allowed a relatively good estimation of overall survival when analyzed univariately. However, prognostic significance was lost in the multivariate analysis showing that KPS was not an independent prognostic parameter in our patients. Lower KPS scores correlated with advanced disease stage which explains our observation that the reduced OS rate was due to an increase in relapse mortality but not due to increased NRM. In contrast to our data, a previous study in ALL patients found that KPS was an independent prognostic variable for NRM.2 Reports in other disease entities also described that KPS or other indicators of general clinical performance such as the Eastern Cooperative Oncology Group (ECOG) are important independent prognostic variables for OS and NRM.22,25,26,35
An important limitation of our study is the single-institution design with retrospective collection of risk score parameters. Also, the limited patient numbers might have prevented detection of relevant differences between the groups. Another confounder is the long accrual period of 13 years during which physician experience, supportive care and patient selection have changed. Additionally, selection of patients for RIC or HD conditioning and the inclusion of multiple donor types may have influenced our results.
Conclusions
The modified EBMT risk score was prognostic for OS, NRM and relapse mortality with predominant effect of disease stage. For the HCT-CI, a non-significant prognostic trend for OS and NRM was observed. The KPS allowed relatively good prognostication of OS and relapse mortality in univariate analysis, but it was not an independent parameter. Although our data show that prognostic categories for allogeneic HCT in ALL patients can be defined, larger multi-center studies, ideally in a prospective setting, need to be performed. Further specific adaptations of the tested scores for ALL patients will be required to give optimal prognostic strength. For now, it appears that clinical judgment by the treating physician incorporating factors such as disease stage, HLA-match, patient age, performance status and presence of significant comorbidities remains the most important tool for predicting transplant outcome and for allocating patients to a specific transplant regimen. However, composite scores such as the EBMT risk score and the HCT-CI may already be useful to compare patient populations and trial results among different studies and institutions.
Acknowledgments
the authors would like to thank the nursing staff for excellent patient care, Sabine Diehl for management of the patient database and Ramona Scheufele for providing assistance with the statistical analysis.
Footnotes
Authorship and Disclosures
TT designed the study, recruited patients, analyzed the data and wrote the manuscript. PGH and GM recruited patients and revised the manuscript. PM and ED were responsible for the statistical analyses. LGV analyzed data. BD revised the manuscript. RA coordinated the study, recruited patients and revised the manuscript. All authors discussed and agreed on the final version of the paper. The authors reported no potential conflicts of interest.
References
- 1.Terwey TH, Kim TD, Arnold R. Allogeneic hematopoietic stem cell transplantation for adult acute lymphocytic leukemia. Curr Hematol Malig Rep. 2009;4:139–47. doi: 10.1007/s11899-009-0020-7. [DOI] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, Carston M, Kollman C, King R, Dekker AW, Lowenberg B, et al. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood. 2001;97(6):1572–7. doi: 10.1182/blood.v97.6.1572. [DOI] [PubMed] [Google Scholar]
- 3.Doney K, Hagglund H, Leisenring W, Chauncey T, Appelbaum FR, Storb R. Predictive factors for outcome of allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2003;9(7):472–81. doi: 10.1016/s1083-8791(03)00149-6. [DOI] [PubMed] [Google Scholar]
- 4.Frassoni F, Labopin M, Gluckman E, Prentice HG, Vernant JP, Zwaan F, et al. Results of allogeneic bone marrow transplantation for acute leukemia have improved in Europe with time--a report of the acute leukemia working party of the European group for blood and marrow transplantation (EBMT) Bone Marrow Transplant. 1996;17(1):13–8. [PubMed] [Google Scholar]
- 5.Sorror M. Impacts of pretransplant comorbidities on allogeneic hematopoietic cell transplantation (HCT) outcomes. Biol Blood Marrow Transplant. 2008;15:149–53. doi: 10.1016/j.bbmt.2008.12.498. [DOI] [PubMed] [Google Scholar]
- 6.Gorin NC, Labopin M, Polge E, Cordonnier C, Jouet JP, Michallet M, et al. Risk assessment in adult acute lymphoblastic leukaemia before early haemopoietic stem cell transplantation with a geno-identical donor: an easy clinical prognostic score to identify patients who benefit most from allogeneic haemopoietic stem cell transplantation. Leukemia. 2003;17(8):1596–9. doi: 10.1038/sj.leu.2403030. [DOI] [PubMed] [Google Scholar]
- 7.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144(6):407–14. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 8.Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Ritz J, et al. A prognostic score for patients with acute leukemia or myelodysplastic syndromes undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(1):28–35. doi: 10.1016/j.bbmt.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352(9134):1087–92. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 10.Gratwohl A, Stern M, Apperley J, De Witte T, Passweg J, Rocha V, et al. The EBMT risk score predicts outcome after allogeneic HSCT in all haematological disease categories and is independent of stem cell source or conditioning intensity Bone Marrow Transplant 200841S318545240 [Google Scholar]
- 11.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gokbuget N, Hoelzer D, Arnold R, Bohme A, Bartram CR, Freund M, et al. Treatment of Adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14(6):1307–25. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 13.Lutz C, Massenkeil G, Nagy M, Neuburger S, Tamm I, Rosen O, et al. A pilot study of prophylactic donor lymphocyte infusions to prevent relapse in adult acute lymphoblastic leukemias after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(9):805–12. doi: 10.1038/sj.bmt.1705981. [DOI] [PubMed] [Google Scholar]
- 14.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381–7. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 15.Pollack SM, Steinberg SM, Odom J, Dean RM, Fowler DH, Bishop MR. Assessment of the hematopoietic cell transplantation comorbidity index in non-Hodgkin lymphoma patients receiving reduced-intensity allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(2):223–30. doi: 10.1016/j.bbmt.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111(1):446–52. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26(30):4912–20. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246–54. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 19.Lim ZY, Ho AY, Ingram W, Kenyon M, Pearce L, Czepulkowski B, et al. Outcomes of alemtuzumab-based reduced intensity conditioning stem cell transplantation using unrelated donors for myelodysplastic syndromes. Br J Haematol. 2006;135(2):201–9. doi: 10.1111/j.1365-2141.2006.06272.x. [DOI] [PubMed] [Google Scholar]
- 20.Boehm A, Sperr WR, Leitner G, Worel N, Oehler L, Jaeger E, et al. Comorbidity predicts survival in myelodysplastic syndromes or secondary acute myeloid leukaemia after allogeneic stem cell transplantation. Eur J Clin Invest. 2008;38(12):945–52. doi: 10.1111/j.1365-2362.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 21.Platzbecker U, Bornhauser M, Germing U, Stumpf J, Scott BL, Kroger N, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS) Biol Blood Marrow Transplant. 2008;14(11):1217–25. doi: 10.1016/j.bbmt.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror M, Storer B, Sandmaier BM, Maloney DG, Chauncey TR, Langston A, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112(9):1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama D, Fukuda T, Kato R, Yamasaki S, Usui E, Morita-Hoshi Y, et al. Comparable antileukemia/lymphoma effects in nonremission patients undergoing allogeneic hematopoietic cell transplantation with a conventional cytoreductive or reduced-intensity regimen. Biol Blood Marrow Transplant. 2007;13(8):932–41. doi: 10.1016/j.bbmt.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Mielcarek M, Storer BE, Sandmaier BM, Sorror ML, Maloney DG, Petersdorf E, et al. Comparable outcomes after nonmyeloablative hematopoietic cell transplantation with unrelated and related donors. Biology of Blood and Marrow Transplant. 2007;13(12):1499–507. doi: 10.1016/j.bbmt.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farina L, Bruno B, Patriarca F, Spina F, Sorasio R, Morelli M, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009;23(6):1131–8. doi: 10.1038/leu.2009.1. [DOI] [PubMed] [Google Scholar]
- 26.Guilfoyle R, Demers A, Bredeson C, Richardson E, Rubinger M, Szwajcer D, et al. Performance status, but not the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), predicts mortality at a Canadian transplant center. Bone Marrow Transplant. 2009;43(2):133–9. doi: 10.1038/bmt.2008.300. [DOI] [PubMed] [Google Scholar]
- 27.Xhaard A, Porcher R, Chien JW, de Latour RP, Robin M, Ribaud P, et al. Impact of comorbidity indexes on non-relapse mortality. Leukemia. 2008;22(11):2062–9. doi: 10.1038/leu.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majhail NS, Brunstein CG, McAvoy S, DeFor TE, Al-Hazzouri A, Setubal D, et al. Does the hematopoietic cell transplantation specific comorbidity index predict transplant outcomes¿ A validation study in a large cohort of umbilical cord blood and matched related donor transplants. Biol Blood Marrow Transplant. 2008;14(9):985–92. doi: 10.1016/j.bbmt.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606–13. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron F, Storb R, Storer BE, Maris MB, Niederwieser D, Shizuru JA, et al. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. J Clin Oncol. 2006;24(25):4150–7. doi: 10.1200/JCO.2006.06.9914. [DOI] [PubMed] [Google Scholar]
- 31.Bornhauser M, Illmer T, Oelschlaegel U, Schetelig J, Ordemann R, Schaich M, et al. Gemtuzumab ozogamicin as part of reduced-intensity conditioning for allogeneic hematopoietic cell transplantation in patients with relapsed acute myeloid leukemia. Clin Cancer Res. 2008;14(17):5585–93. doi: 10.1158/1078-0432.CCR-08-0894. [DOI] [PubMed] [Google Scholar]
- 32.Rezvani AR, Norasetthada L, Gooley T, Sorror M, Bouvier ME, Sahebi F, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol. 2008;143(3):395–403. doi: 10.1111/j.1365-2141.2008.07365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid C, Schleuning M, Hentrich M, Markl GE, Gerbitz A, Tischer J, et al. High antileukemic efficacy of an intermediate intensity conditioning regimen for allogeneic stem cell transplantation in patients with high-risk acute myeloid leukemia in first complete remission. Bone Marrow Transplantation. 2008;41(8):721–7. doi: 10.1038/sj.bmt.1705965. [DOI] [PubMed] [Google Scholar]
- 34.Bachanova V, Verneris MR, DeFor T, Brunstein CG, Weisdorf DJ. Prolonged survival in adults with acute lymphoblastic leukemia after reduced-intensity conditioning with cord blood or sibling donor transplantation. Blood. 2009;113(13):2902–5. doi: 10.1182/blood-2008-10-184093. [DOI] [PubMed] [Google Scholar]
- 35.Artz AS, Pollyea DA, Kocherginsky M, Stock W, Rich E, Odenike O, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12(9):954–64. doi: 10.1016/j.bbmt.2006.05.015. [DOI] [PubMed] [Google Scholar]