Abstract
Autoantibodies to nucleolar components are a common serological feature of patients suffering from scleroderma, a collagen vascular autoimmune disease. While animal models, which spontaneously develop abundant anti-nucleolar antibodies, have not yet been described, high titers of such antibodies may be induced by treating susceptible strains of mice with mercuric chloride. We have identified the nucleolar autoantigen against which the HgCl2-induced IgG autoantibodies from mice of strain B10.S are directed. It is a protein with an apparent molecular mass of 36 kDa and a pI value of approximately 8.6, which is associated with the nucleolar small nuclear RNA U3, and by these criteria must be identical with a polypeptide called fibrillarin. It is striking that scleroderma patients spontaneously produce autoantibodies against the same U3 ribonucleoprotein (RNP). The HgCl2-induced murine and the scleroderma-specific human anti-U3 RNP autoantibodies were indistinguishable in their reactivities toward fibrillarin. They further resemble each other insofar as both recognize epitopes on the 36-kDa protein, which have been highly conserved throughout evolution. Our results provide a basis to investigate at the molecular level whether similar immunoregulatory dysfunctions may lead to the preferential anti-U3 RNP autoantibody production in the animal model and in scleroderma patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein R. M., Steigerwald J. C., Tan E. M. Association of antinuclear and antinucleolar antibodies in progressive systemic sclerosis. Clin Exp Immunol. 1982 Apr;48(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- Black C., Pereira S., McWhirter A., Welsh K., Laurent R. Genetic susceptibility to scleroderma-like syndrome in symptomatic and asymptomatic workers exposed to vinyl chloride. J Rheumatol. 1986 Dec;13(6):1059–1062. [PubMed] [Google Scholar]
- Bringmann P., Rinke J., Appel B., Reuter R., Lührmann R. Purification of snRNPs U1, U2, U4, U5 and U6 with 2,2,7-trimethylguanosine-specific antibody and definition of their constituent proteins reacting with anti-Sm and anti-(U1)RNP antisera. EMBO J. 1983;2(7):1129–1135. doi: 10.1002/j.1460-2075.1983.tb01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druet E., Fournie G., Mandet C., Sapin C., Günther E., Druet P. Genetic control of susceptibility to immune complex type nephritis induced by HgCl2 in rats. Transplant Proc. 1979 Sep;11(3):1600–1603. [PubMed] [Google Scholar]
- Druet P., Druet E., Potdevin F., Sapin C. Immune type glomerulonephritis induced by HgCl2 in the Brown Norway rat. Ann Immunol (Paris) 1978 Oct-Dec;129 100(6):777–792. [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Habets W. J., de Rooij D. J., Salden M. H., Verhagen A. P., van Eekelen C. A., van de Putte L. B., van Venrooij W. J. Antibodies against distinct nuclear matrix proteins are characteristic for mixed connective tissue disease. Clin Exp Immunol. 1983 Oct;54(1):265–276. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. Sequential association of nucleolar 7-2 RNA with two different autoantigens. J Biol Chem. 1983 Feb 10;258(3):1379–1382. [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Konings D. A., Cesareni G. The yeast homologue of U3 snRNA. EMBO J. 1987 Jul;6(7):2145–2155. doi: 10.1002/j.1460-2075.1987.tb02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman P., Eneström S. Mercury induced antinuclear antibodies in mice: characterization and correlation with renal immune complex deposits. Clin Exp Immunol. 1988 Feb;71(2):269–274. [PMC free article] [PubMed] [Google Scholar]
- Kuppers R. C., Suiter T., Gleichmann E., Rose N. R. The induction of organ-specific antibodies during the graft-vs.-host reaction. Eur J Immunol. 1988 Jan;18(1):161–166. doi: 10.1002/eji.1830180124. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Matter L., Schopfer K., Wilhelm J. A., Nyffenegger T., Parisot R. F., De Robertis E. M. Molecular characterization of ribonucleoprotein antigens bound by antinuclear antibodies. A diagnostic evaluation. Arthritis Rheum. 1982 Nov;25(11):1278–1283. doi: 10.1002/art.1780251102. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Parker K. A., Steitz J. A. Structural analysis of the human U3 ribonucleoprotein particle reveal a conserved sequence available for base pairing with pre-rRNA. Mol Cell Biol. 1987 Aug;7(8):2899–2913. doi: 10.1128/mcb.7.8.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Hirsch F., Sapin C., Druet P. Autoreactive T cells in mercury-induced autoimmune disease: in vitro demonstration. J Immunol. 1986 Oct 15;137(8):2548–2554. [PubMed] [Google Scholar]
- Pinnas J. L., Northway J. D., Tan E. M. Antinucleolar antibodies in human sera. J Immunol. 1973 Oct;111(4):996–1004. [PubMed] [Google Scholar]
- Polák L., Barnes J. M., Turk J. L. The genetic control of contact sensitization to inorganic metal compounds in guinea-pigs. Immunology. 1968 May;14(5):707–711. [PMC free article] [PubMed] [Google Scholar]
- Reardon C. L., Lucas D. O. Heavy-metal mitogenesis: Zn++ and Hg++ induce cellular cytotoxicity and interferon production in murine T lymphocytes. Immunobiology. 1987 Nov;175(5):455–469. doi: 10.1016/S0171-2985(87)80073-6. [DOI] [PubMed] [Google Scholar]
- Reddy R., Tan E. M., Henning D., Nohga K., Busch H. Detection of a nucleolar 7-2 ribonucleoprotein and a cytoplasmic 8-2 ribonucleoprotein with autoantibodies from patients with scleroderma. J Biol Chem. 1983 Feb 10;258(3):1383–1386. [PubMed] [Google Scholar]
- Reimer G., Pollard K. M., Penning C. A., Ochs R. L., Lischwe M. A., Busch H., Tan E. M. Monoclonal autoantibody from a (New Zealand black x New Zealand white)F1 mouse and some human scleroderma sera target an Mr 34,000 nucleolar protein of the U3 RNP particle. Arthritis Rheum. 1987 Jul;30(7):793–800. doi: 10.1002/art.1780300709. [DOI] [PubMed] [Google Scholar]
- Reimer G., Rose K. M., Scheer U., Tan E. M. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest. 1987 Jan;79(1):65–72. doi: 10.1172/JCI112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer G., Scheer U., Peters J. M., Tan E. M. Immunolocalization and partial characterization of a nucleolar autoantigen (PM-Scl) associated with polymyositis/scleroderma overlap syndromes. J Immunol. 1986 Dec 15;137(12):3802–3808. [PubMed] [Google Scholar]
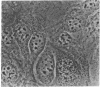
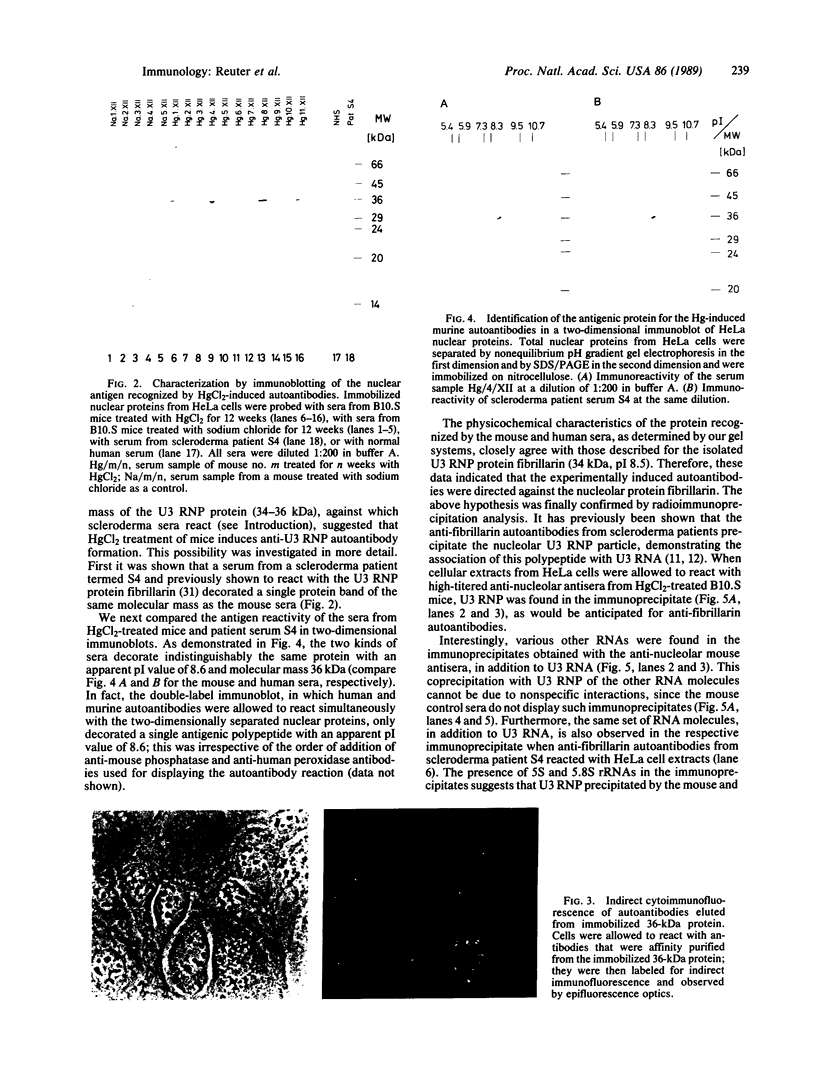
- Reuter R., Appel B., Rinke J., Lührmann R. Localization and structure of snRNPs during mitosis. Immunofluorescent and biochemical studies. Exp Cell Res. 1985 Jul;159(1):63–79. doi: 10.1016/s0014-4827(85)80038-0. [DOI] [PubMed] [Google Scholar]
- Ritchie R. F. Antinucleolar antibodies. Their frequency and diagnostic association. N Engl J Med. 1970 May 21;282(21):1174–1178. doi: 10.1056/NEJM197005212822104. [DOI] [PubMed] [Google Scholar]
- Robinson C. J., Abraham A. A., Balazs T. Induction of anti-nuclear antibodies by mercuric chloride in mice. Clin Exp Immunol. 1984 Nov;58(2):300–306. [PMC free article] [PubMed] [Google Scholar]
- Robinson C. J., Balazs T., Egorov I. K. Mercuric chloride-, gold sodium thiomalate-, and D-penicillamine-induced antinuclear antibodies in mice. Toxicol Appl Pharmacol. 1986 Nov;86(2):159–169. doi: 10.1016/0041-008x(86)90046-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez J. L., Gelpi C., Juarez C., Hardin J. A. Anti-NOR 90. A new autoantibody in scleroderma that recognizes a 90-kDa component of the nucleolus-organizing region of chromatin. J Immunol. 1987 Oct 15;139(8):2579–2584. [PubMed] [Google Scholar]
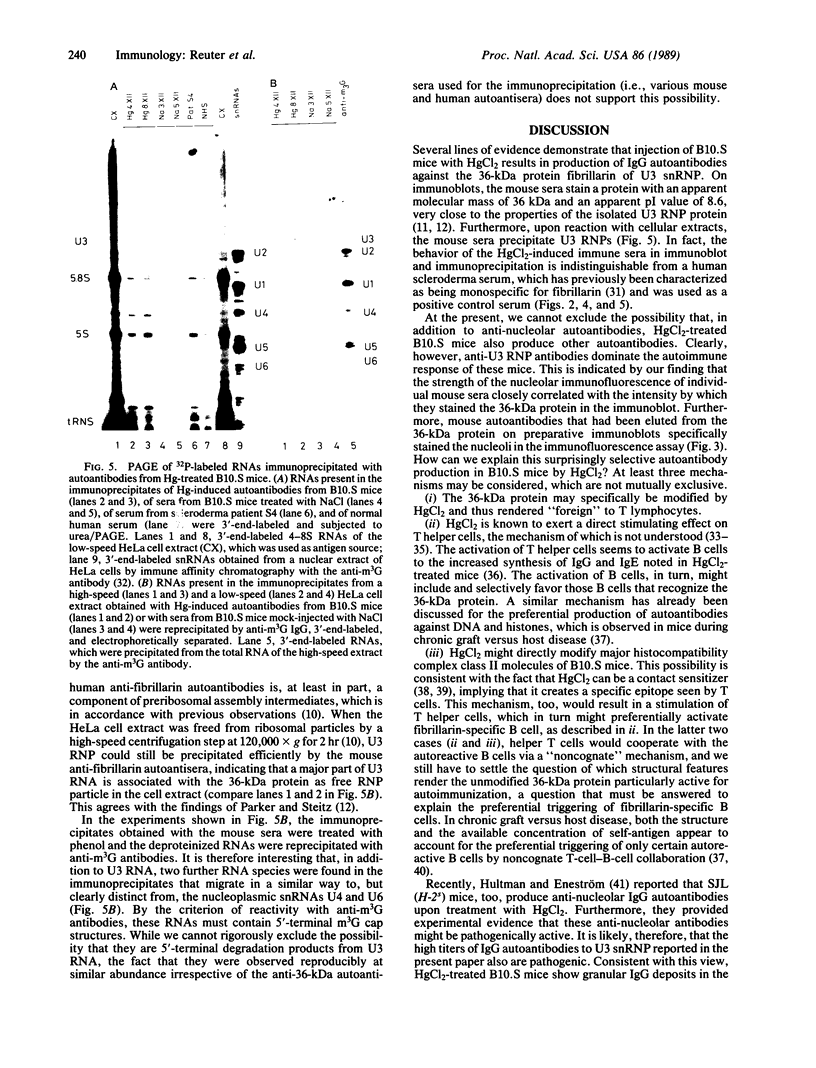
- Sapin C., Druet E., Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977 Apr;28(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler D. A., Rose K. M., Wenger M. E., Berlin C. M., Jacob S. T. Antibodies to distinct polypeptides of RNA polymerase I in sera from patients with rheumatic autoimmune disease. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7499–7503. doi: 10.1073/pnas.79.23.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targoff I. N., Reichlin M. Nucleolar localization of the PM-Scl antigen. Arthritis Rheum. 1985 Feb;28(2):226–230. doi: 10.1002/art.1780280221. [DOI] [PubMed] [Google Scholar]
- Taugner M., Schütz R. Beitrag zur Quecksilber-Allergie. Dermatologica. 1966;133(4):245–261. [PubMed] [Google Scholar]
- Tubbs R. R., Gephardt G. N., McMahon J. T., Pohl M. C., Vidt D. G., Barenberg S. A., Valenzuela R. Membranous glomerulonephritis associated with industrial mercury exposure. Study of pathogenetic mechanisms. Am J Clin Pathol. 1982 Apr;77(4):409–413. doi: 10.1093/ajcp/77.4.409. [DOI] [PubMed] [Google Scholar]
- Vaughan M. A. An autoimmune antibody from scleroderma patients recognizes a component of the plant cell nucleolus. Histochemistry. 1987;86(5):533–535. doi: 10.1007/BF00500629. [DOI] [PubMed] [Google Scholar]
- Weening J. J., Fleuren G. J., Hoedemaeker P. J. Demonstration of antinuclear antibodies in mercuric chloride-induced glomerulopathy in the rat. Lab Invest. 1978 Oct;39(4):405–411. [PubMed] [Google Scholar]
- Weening J. J., Grond J., van der Top D., Hoedemaeker P. J. Identification of the nuclear antigen involved in mercury-induced glomerulopathy in the rat. Invest Cell Pathol. 1980 Apr-Jun;3(2):129–134. [PubMed] [Google Scholar]