In a recent issue of this Journal, Andreani et al.1 reported a potential role of iron loading modifier in poly-transfused beta-thalassemic patients for the -582 A>G polymorphic change (rs10421768)2 in the 5’ flanking region of the HAMP (Hepcidin AntiMicrobial Peptide) gene encoding hepcidin. According to these Authors, the presence of the less common “G” at -582 was associated with higher liver iron concentrations (LIC), as determined at liver biopsy, and with higher serum ferritin in patients as compared with the presence of “A” at the same position. This difference was observed only in patients (52 males and 45 females) irregularly treated by iron chelators, since regular iron chelation treatment is likely able to override possible differences. Obviously several determinants may contribute to the clinical condition of iron overload in beta-thalassemia patients, including blood transfusions, chelation regimen and also iron absorption secondary to erythropoietic expansion. However, after considering several variables, the Authors speculate that these patients might theoretically have lower hepcidin levels than the corresponding patients without the polymorphic change.
Although the paper does not report measurements of serum or urinary hepcidin of the patients studied, it indirectly suggests that the presence of the “G” nucleotide substitution makes these patients more prone to iron loading. As the Authors pointed out, this SNP affects a conserved non-coding transcriptional box of the HAMP gene promoter and might change its affinity binding to transcriptional factors.3,4
These results prompted us to evaluate our analysis of the same SNP in a population of 263 healthy blood donors (196 males and 67 females), previously studied for mutations in the HFE gene.5 Informed consent was available for all the studied individuals.
The analysis of the -582A>G polymorphism of HAMP promoter was performed with the NMW 1000 NanoChipTM Molecular Biology Workstation (Nanogen®, Inc. San Diego, CA), previously used in several genetic applications.6 Oligonucleotide sequences of PCR primers, reporter probes, stabilizers and amplification conditions are available on request. Conditions for PCR product purification, addressing the chip, denaturation, hybridization and thermal stringency have been previously described.6 Assay conditions were validated in a blinded fashion on 226 DNA samples who had been found to be either -582G/A heterozygous or homozygous (A/A or G/G) by direct sequencing.7
The allele frequency of -582 A->G change in the cohort of healthy blood donors is similar to that reported in the database (Table 1) and in thalassemic patients.1 Table 2 shows transferrin saturation and serum ferritin levels in the 143/263 blood donors with normal HFE genotype, either carriers or non-carriers of the -582 A->G polymorphism. To avoid the bias of physiologically lower serum ferritin levels in females, statistical calculations on iron parameters were performed considering only male subjects (105/143). A t-test for independent samples after log transformation showed that in the absence of HFE mutations, serum ferritin levels were significantly lower in subjects who carried the “G” allelic variant compared to subjects homozygous for the “A” allelic variant. Conversely, there was no difference in transferrin saturation levels between the two groups.
Table 1.
Frequency of the -582 A->G polymorphism in Italian blood donors compared to Caucasians (CEU) from NCBI database.
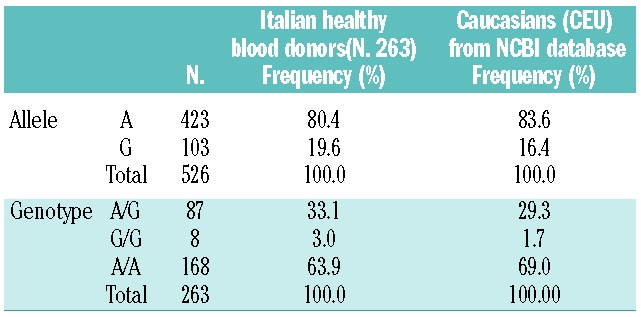
Table 2.
Transferrin saturation and serum ferritin levels according to HAMP promoter variant in 105 male blood donors.
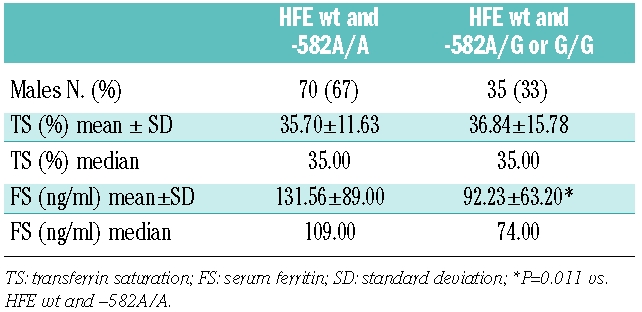
The difference between our results and those by Andreani and co-workers, who found an association between the less common “G” variant in the HAMP promoter gene and higher serum ferritin levels, could be explained mainly on the basis of the different phenotype of the populations evaluated in the two studies: poly-transfused thalassemic patients versus healthy blood donors. Although the presence of “G” at -582 in normal conditions causes even a reduction in serum ferritin levels, when the hepcidin transcription must be reduced, as in thalassemic patients, the “G” variant could favor a stronger hepcidin inhibition, increasing iron absorption and ferritin levels. We can speculate that the mutant promoter could abnormally respond to both activatory and inhibitory signals. This hypothesis should be confirmed by direct hepcidin measurements in groups of individuals/patients with different -582 HAMP genotypes.
References
- 1.Andreani M, Radio FC, Testi M, De Bernardo C, Troiano M, Majore S, et al. Association of hepcidin promoter c.-582 A>G variant and iron overload in thalassemia major. Haematologica. 2009;94(9):1293–6. doi: 10.3324/haematol.2009.006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.http://www.ncbi.nlm.nih.gov/projects/SNP/
- 3.Bayele HK, McArdle H, Srai SK. Cis and transregulation of hepcidin expression by upstream stimulatory factor. Blood. 2006;108(13):4237–45. doi: 10.1182/blood-2005-07-027037. [DOI] [PubMed] [Google Scholar]
- 4.Bayele HK, Srai SK. Genetic variation in hepcidin expression and its implications for phenotypic differences in iron metabolism. Haematologica. 2009;94(9):1185–8. doi: 10.3324/haematol.2009.010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Gobbi M, D’Antico S, Castagno F, Testa D, Merlini R, Bondi A, et al. Screening selected blood donors with biochemical iron overload for hemochromatosis: a regional experience. Haematologica. 2004;89(10):1161–7. [PubMed] [Google Scholar]
- 6.Foglieni B, Cremonesi L, Travi M, Giambona A, Rosatelli MC, Perra C, et al. Beta-thalassemia microelectronic chip: a fast and accurate method for mutation detection. Clin Chem. 2004;50(1):73–9. doi: 10.1373/clinchem.2003.023077. [DOI] [PubMed] [Google Scholar]
- 7.Biasiotto G, Roetto A, Daraio F, Polotti A, Gerardi GM, Girelli D, et al. Identification of new mutations of hepcidin and hemojuvelin in patients with HFE C282Y allele. Blood Cells Mol Dis. 2004;33(3):338–43. doi: 10.1016/j.bcmd.2004.08.002. [DOI] [PubMed] [Google Scholar]


