We read with interest the article by Lucas et al. in this Journal focussing on the impact of the BCR-ABL transcript type (e13a2 versus e14a2) in newly diagnosed patients with chronic myeloid leukemia (CML).1 In a series of 71 adults treated with imatinib 400mg, they demonstrated that those 39 patients expressing the e14a2 transcript type had a higher and more rapid complete cytogenetic response than the 32 patients exhibiting the e13a2 transcript. Thus, they concluded that the transcript type yields additional prognostic information to predict the long-term response to imatinib.
CML in pediatrics is rare and constitutes only approximately 2% of all childhood leukemias. Imatinib has been licensed since the year 2003 for use in children; however, its efficacy has so far been evaluated only in prospective trials with small patient numbers. Long-term experience in pediatric patients of all age groups is still very limited and the durability of responses, as well as long-term side effects of this drug, must still be determined.2–4
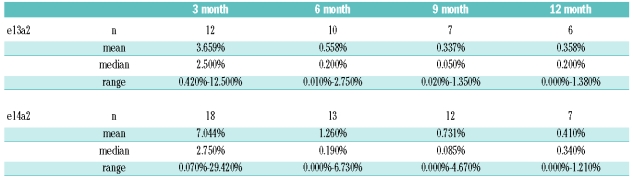
Stimulated by the article by Lucas et al., we analyzed our data from children with Philadelphia-positive CML enrolled in trial CML-paed-II and questioned whether assessment of the BCR-ABL/ABL ratio by quantitative RT-PCR would also show a difference in molecular response with respect to the underlying transcript type.5 Thirty pediatric patients (19 males, 11 females; age: 1 – 17 yrs, mean age: 10.5 yrs) diagnosed with CML in chronic phase were treated with imatinib at a dose of 250 mg/m2 (equivalent to 400 mg in adults; maximum absolute dose 400 mg). Mean duration of treatment was eight months (range 1 – 15 months). Twelve patients (8 males) exhibited transcript type e13a2 and 18 pts (11 males) type e14a2. From month three to month nine after the start of the treatment the mean ratio of the BCR-ABL/ABL transcripts in patients exhibiting the e13a2 type was approximately twice as high as mean ratio of patients with the e14a2 type, respectively (Table 1). No difference was observed when the median values were considered. A graphical plot of all data applying the best fit mathematical model of a logarithmic decline over time showed that children exhibiting the e14a2 transcript type experienced a more rapid decline of BCR/ABL transcripts than children with an e13a2 transcript type (Figure 1). However, due to the small number of patients under analysis, differences of the curves cannot be considered to be significant (P≥0.05) since confidence limits overlay (SPSS statistical package, Version 14, Chicago, USA).
Table 1.
Absolute values of the BCR-ABL/ABL transcript ratio (mean, range, median) over time (three month intervals) under imatinib treatment in pediatric patients depending on the underlying transcript type e13a2 or e14a2, respectively.
Figure 1.
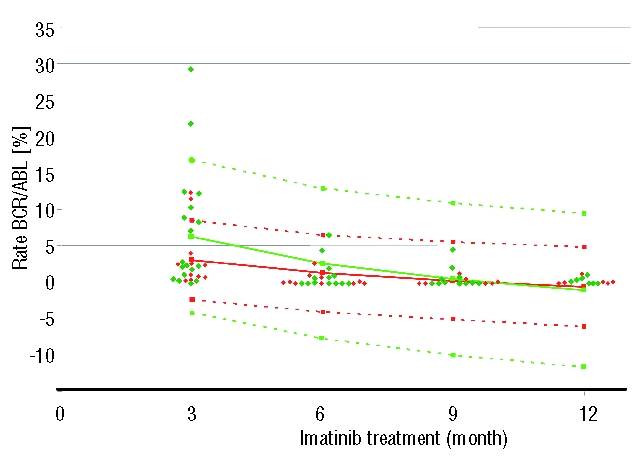
Time course of the transcript ratio BCR-ABL/ABL in individual patients exhibiting either the e13a2 rearrangement type (red dots) or the e14a2 type (green dots) in pediatric patients with chronic myeloid leukemia under ongoing imatinib treatment. Full lines denote the best fitted curves adapted by logarithmic function for decline over time while dotted lines show the upper and lower limits of confidence (red lines: e13a2; green lines e14a2).
The molecular data accumulated in our small pediatric cohort thus confirm the findings reported by Lucas et al. showing that patients exhibiting the e14a2 transcript type respond better to a fixed dose of imatinib. These patients not only have a lower mean BCR/ABL transcript ratio as early as after three months of treatment but also achieved low transcript levels faster than patients with e13a2 transcript types. As published earlier by our group in more than 140 children, and confirmed in this small series of 31 individuals, the e14a2 transcript type is over-represented in children, a finding which has been also described in the majority but not in all adult cohorts.6 This skewed distribution may weaken any statistical analysis especially in pediatrics with small cohorts due to the rarity of the diseases in this age group. Of note, Lucas et al. describe an almost balanced distribution of the e13a2 (n=32) and the e14a2 (n=39) transcript.
The authors could also show an increased pCrKL/CrKL ratio as a surrogate marker for the bcr-abl tyrosine kinase (TK) activity in patients exhibiting the e13a2 transcript type. It is tempting to speculate that patients with higher TK-activity should also be treated by either higher doses of imatinib or more potent second generation TK-inhibitors. Whether this approach would also augment recently reported side effects of imatinib on bone metabolism is of special concern in a pediatric cohort which is still in a period of growth.7,8 We, therefore, fully agree with the suggestion by Lucas et al. that the analysis of the transcript type in patients with CML should be included in the analysis of response data from trials with TK inhibitors.
Footnotes
Response to the Original Article by: Claire M. Lucas, Robert J. Harris, Athina Giannoudis, Andrea Davies, Katy Knight, Sarah J. Watmough, Lihui Wang and Richard Clark. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica 2009, 94:1362–7.
References
- 1.Lucas CM, Harris RJ, Giannoudis A, Davies A, Knight K, Watmough SJ, et al. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica. 2009;94(10):1362–7. doi: 10.3324/haematol.2009.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champagne MA, Capdeville R, Krailo M, Qu W, Peng B, Rosamilia M, et al. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: results from a Children's Oncology Group phase 1 study. Blood. 2004;104(9):2655–60. doi: 10.1182/blood-2003-09-3032. [DOI] [PubMed] [Google Scholar]
- 3.Millot F, Guilhot J, Nelken B, Leblanc T, De Bont ES, Bekassy AN, et al. Imatinib mesylate is effective in children with chronic myelogenous leukemia in late chronic and advanced phase and in relapse after stem cell transplantation. Leukemia. 2006;20(2):187–92. doi: 10.1038/sj.leu.2404051. [DOI] [PubMed] [Google Scholar]
- 4.Suttorp M. Innovative approaches of targeted therapy for chronic myeloid leukaemia of childhood in combination with paediatric haematopoietic stem cell transplantation (Review) Bone Marrow Transplant. 2008;42(Suppl 2):S40–S46. doi: 10.1038/bmt.2008.282. [DOI] [PubMed] [Google Scholar]
- 5.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors - review and recommendations for “harmonizing” current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler R, Viehmann S, Kuhlisch E, Martiniak Y, Röttgers S, Harbott J, et al. Correlation of BCR/ABL Transcript Variants with Patients’ Characteristics in Childhood Chronic Myeloid Leukaemia. Eur J Haematol. 2008;82(2):112–8. doi: 10.1111/j.1600-0609.2008.01170.x. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson S, Olsson B, Ohlsson C, Lorentzon M, Mellstrom D, Wadenvik H. Increased cortical bone mineralization in imatinib treated patients with chronic myelogenous leukemia. Haematologica. 2008;93(7):1101–3. doi: 10.3324/haematol.12373. [DOI] [PubMed] [Google Scholar]
- 8.Schmid H, Jaeger BAS, Lohse J, Suttorp M. Longitudinal growth retardation in a prepupertal girl with chronic myeloid leukemia on long-term imatinib treatment. Haematologica. 2009;94(8):1177–9. doi: 10.3324/haematol.2009.008359. [DOI] [PMC free article] [PubMed] [Google Scholar]