Early death is one of the major causes of failure in acute promyelocytic leukemia (APL), it occurs in approximately 5–10% of newly diagnosed cases and is most frequently due to severe intracranial or pulmonary bleeding. In the present single center study, we reviewed the clinical and biological features of patients who developed severe hemorrhagic complications before starting all-trans retinoic acid and chemotherapy, and found that late diagnosis and delayed treatment initiation, in conjunction with elevated WBC counts, are significantly associated with severe bleeding and early death.
Severe bleeding is a well known major complication of APL which leads to early death in approximately 5–10% of patients diagnosed in developed countries and in 20–30% of patients living in less privileged regions. Hemorrhages are commonly attributed in this leukemia to the frequent diffuse activation of coagulation, hyperfibrinolysis, and non-specific proteolytic activity,1–4 and have also been reported to occur before the diagnosis of APL has been made and therapy started.3 Systematic data on patients developing this severe complication before treatment are extremely scarce in the literature and these cases are usually omitted from large clinical trial reports.
We reviewed our database of 105 consecutive patients with newly diagnosed APL observed at a single institution from March 1993 to October 2008. Diagnosis was initially established morphologically and subsequently confirmed in all cases by RT-PCR identification of the specific PML/RARA fusion gene. In all cases, records were available for complete blood counts, peripheral and bone marrow blast count, PML/RARA fusion gene type, coagulation laboratory parameters, time interval between diagnosis and hospitalization in our institution, and time elapsed from APL diagnosis to start of ATRA treatment. Furthermore, the type and amount of supportive treatment (fresh frozen plasma, packed RBCs, platelets) were recorded. Life-threatening hemorrhages were defined as catastrophic bleeding in all sites requiring major non-elective intervention and hemorrhagic vascular event with neurological signs and symptoms in the CNS site, according to NCI common toxicity criteria version 2.0.5 For statistical analysis, the Wilcoxon-Mann-Whitney test was performed for comparison of non-parametric series and Fisher’s exact test was used to compare categories. Values of P<0.05 were considered statistically significant. Five patients (4%) had life-threatening hemorrhages before starting therapy. Their presenting features were compared to those of the remaining 100 patients. As shown in Table 1, median WBC count at presentation was 18×109/L (versus 2.8×109/L, P=0.001) and 3 patients were classified as high-risk according to Sanz’s score.5 All 5 patients who experienced life-threatening hemorrhage had the short form (bcr3) of PML/RARA fusion.
Table 1.
Comparison of clinical and biological features at diagnosis between patients with fatal hemorrhages and the remaining patients.
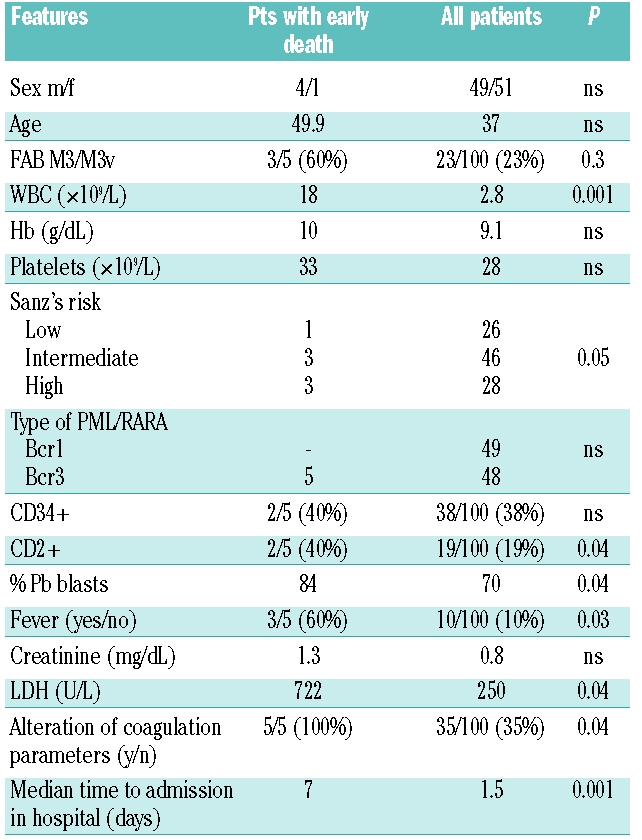
All patients with life-threatening hemorrhages before starting therapy had delayed diagnosis due to initial hospitalization in non-specialized, primary care institutions with no experience in the treatment of acute leukemia. These 5 patients were admitted to our institution after a median time of seven days (range 4–12) from admission to general hospitals as compared to the remaining 100 patients who were admitted and managed with specific therapy in a median time of 1.5 days. All patients developed severe leukocytosis (median WBC count 70×109/L, range 55–250) and acute renal failure (creatinine levels above 1.6 mg/dL). Fresh frozen plasma and platelet transfusions were received by all patients immediately on admission to maintain the fibrinogen concentration and platelet count above 100–150 mg/dL and 30–50×109/L, respectively. All 5 patients died within 24 hours from hospitalization in our institute due to massive cerebral hemorrhage, in spite of the prompt start of ATRA therapy which was initiated upon morphological diagnosis and clinical suspect according to established guidelines,6 and within a median of three hours (range 2–5) from admission.
Although a general consensus exists on the need to confirm the diagnosis of APL at the genetic level, current guideline recommendations establish that the disease should be managed as a medical emergency simply upon morphological and clinical suspicion, with immediate therapeutic actions aimed at counteracting the coagulopathy, including platelet/plasma support and prompt ATRA administration.6
Recently, de la Serna et al.7 reported on causes and prognostic factors predictive of induction remission failure in a large cohort of patients enrolled in the Spanish PETHEMA studies. They reported that central nervous system and lungs were the main sites of fatal bleeding, and 50% of deaths occurred in the first week of treatment despite intensive transfusional support. Fatal hemorrhage was the main cause of death also in patients who died after the first week or who were registered but were considered ineligible for ATRA plus idarubicin treatment. In agreement with data reported by de la Serna et al.,7 also in our experience the presence of coagulopathy, elevated WBC and the percentage of peripheral blast count were predictive factors for early hemorrhagic death. In addition, we found an association with phenotypic expression of CD2 and delayed treatment initiation. CD2 expression in APL has been associated with higher WBC count, bcr3 type of PML/RARA fusion, morphological variant (M3v) form and thrombotic events with a possible role in leukoagglutination, contributing to tissue damage and microvascular occlusion.8
In conclusion, the results of this study confirm that early recognition and treatment of APL is required in specialized institutions to counteract, with prompt and aggressive supportive therapy as well as with specific antileukemic agents (ATRA), the severe hemorrhagic risk associated with this disease, the most curable acute leukemia in adults.
References
- 1.Lo Coco F, Ammatuna E, Montesinos P, Sanz MA. Acute promyelocytic leukemia: recent advances in diagnosis and management. Semin Oncol. 2008;35(4):401–9. doi: 10.1053/j.seminoncol.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Yanada M, Matsushita T, Asou N, Kishimoto Y, Tsuzuki M, Maeda Y, et al. Severe hemorrhagic complications during remission induction therapy for acute promyelocytic leukemia: incidence, risk factors, and influence on outcome. Eur J Haematol. 2007;78(3):213–9. doi: 10.1111/j.1600-0609.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 3.Di Bona E, Avvisati G, Castaman G, Luce Vegna M, De Sanctis V, Rodeghiero F, et al. Early haemorrhagic morbidity and mortality during remission induction with or without all-trans retinoic acid in acute promyelocytic leucemia. Br J Haematol. 2000;108(4):689–95. doi: 10.1046/j.1365-2141.2000.01936.x. [DOI] [PubMed] [Google Scholar]
- 4.Tallman Ms, Altamn JK. Curative strategies in acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008:391–9. doi: 10.1182/asheducation-2008.1.391. [DOI] [PubMed] [Google Scholar]
- 5.NCI Common toxicity criteria version 2.0. link: www.fda.gov/cder/cancer/toxicityframe.
- 6.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;26;113(9):1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 7.de la Serna J, Montesinos P, Vellenga E, Rayón C, Parody R, León A, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukaemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111(7):3395–402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- 8.Breccia M, Latagliata R, Carmosino I, Cannella L, Diverio D, Guarini A, et al. Clinical and biological features of acute promyelocytic leukemia patients developing retinoic acid syndrome during induction treatment with all-trans retinoic acid and idarubicin. Haematologica. 2008;93(12):1918–20. doi: 10.3324/haematol.13510. [DOI] [PubMed] [Google Scholar]


