Summary
Background
The global burden of disease attributable to respiratory syncytial virus (RSV) remains unknown. We aimed to estimate the global incidence of and mortality from episodes of acute lower respiratory infection (ALRI) due to RSV in children younger than 5 years in 2005.
Methods
We estimated the incidence of RSV-associated ALRI in children younger than 5 years, stratified by age, using data from a systematic review of studies published between January, 1995, and June, 2009, and ten unpublished population-based studies. We estimated possible boundaries for RSV-associated ALRI mortality by combining case fatality ratios with incidence estimates from hospital-based reports from published and unpublished studies and identifying studies with population-based data for RSV seasonality and monthly ALRI mortality.
Findings
In 2005, an estimated 33·8 (95% CI 19·3–46·2) million new episodes of RSV-associated ALRI occurred worldwide in children younger than 5 years (22% of ALRI episodes), with at least 3·4 (2·8–4·3) million episodes representing severe RSV-associated ALRI necessitating hospital admission. We estimated that 66 000–199 000 children younger than 5 years died from RSV-associated ALRI in 2005, with 99% of these deaths occurring in developing countries. Incidence and mortality can vary substantially from year to year in any one setting.
Interpretation
Globally, RSV is the most common cause of childhood ALRI and a major cause of admission to hospital as a result of severe ALRI. Mortality data suggest that RSV is an important cause of death in childhood from ALRI, after pneumococcal pneumonia and Haemophilus influenzae type b. The development of novel prevention and treatment strategies should be accelerated as a priority.
Funding
WHO; Bill & Melinda Gates Foundation.
Introduction
Acute lower respiratory infection (ALRI) is the leading cause of global child mortality.1,2 Respiratory syncytial virus (RSV) is believed to be the most important viral pathogen causing ALRI in young children, although its contribution to ALRI deaths is uncertain.3–5 Many data for incidence of and mortality from RSV-associated ALRI in developing countries remain unpublished. Therefore, we formed an RSV study group to supplement a systematic literature review with unpublished data. No global or regional estimates have been made previously of the burden of RSV-associated ALRI in children. Previous reviews have focused either on developing countries4,6 or industrialised countries.7 We aimed to estimate the burden of disease worldwide due to RSV-associated ALRI in children younger than 5 years for 2005. We estimated only the ALRI component of the burden due to RSV infection because the main costs of RSV disease relate to health-service use for ALRI, and first-generation RSV vaccines are likely to protect against ALRI.3 This report is especially timely in view of the development of candidate vaccines that are sufficiently attenuated yet immunogenic in infants,8–10 and the increased funding for development and implementation of novel vaccines in developing countries that is available through the Global Alliance for Vaccines and Immunisation. We also aimed to emphasise important gaps in knowledge and to provide information about potential sites for vaccine trials.
Methods
Search strategy and selection criteria
We did a systematic literature review using a combination of search terms (webappendix pp 3–4), hand searching of online journals, and scanning of reference lists of identified citations. The search was limited to Medline (Ovid), Embase, CINAHL, Global Health, Web of Science, WHOLIS, LILACS, IndMed, and the grey literature (SIGLE) databases and to studies published between January, 1995, and June, 2009. Panel 1 shows eligibility criteria. No language or publication restrictions were applied. We invited the participation of researchers who had done similar studies resulting in unpublished data or supplementary data from published work.
Panel 1. Eligibility criteria.
Inclusion criteria
-
•
Reported data for respiratory syncytial virus (RSV)-infected children with acute lower respiratory infection (eg, pneumonia, bronchiolitis) or acute respiratory infection necessitating hospital admission
-
•
Undertook surveillance within a defined population for a minimum of 1 year (apart from studies reporting case fatality ratios)
-
•
Provided data specific to children younger than 5 years
-
•
Reported RSV incidence or mortality for at least the first year of life
Exclusion criteria
-
•
Reported data for children with acute upper respiratory infections not necessitating hospital admission
-
•
Investigated RSV as a co-infection rather than the primary outcome
-
•
Used a case definition of influenza or influenza-like illness
-
•
Used a case definition that was not clearly defined or was not consistently applied
Definitions
Most investigators used the clinical pneumonia and severe pneumonia syndromic case definitions that were established by WHO.11 We chose to use ALRI and severe ALRI, including bronchiolitis and pneumonia, to recognise the important contribution of bronchiolitis in RSV infections. ALRI has been defined as equivalent to clinical pneumonia, which is characterised by acute-onset cough or difficulty in breathing with fast breathing for age. Acute cough or difficulty in breathing with indrawing of the lower chest wall indrawing (with or without fast breathing for age) necessitating hospital admission has been defined as severe ALRI, and is equivalent to severe clinical pneumonia.
We used the American Association for Respiratory Care definition of hypoxaemia and regarded oxygen saturation (at sea level in room air) lower than 90% (measured by pulse oximetry) in children older than 1 month and lower than 88% in neonates as hypoxaemic,12 unless indicated otherwise. We used a modification of the definition for RSV season that was provided by Mullins.13 Any month of a year with RSV detected in at least four (or 5%) of the submitted specimens were deemed to be within the RSV season. We regarded countries as industrialised if they fell within: high-income Asia Pacific, high-income North America, western Europe, and Australasia. Remaining countries were classified as developing. Population estimates by region for 2005 are as in The State of the World's Children 2007.14
Adjustment for differences across studies
Few studies adopted the WHO acute respiratory infection case definition or reported RSV data for the full age range (0 to <5 years).3 Where necessary, data imputation was used on the basis of the approach adopted by Rudan and colleagues.15 Relative to an incidence of 1·0 for RSV-associated severe ALRI in the younger than 1 year age group, we calculated an incidence rate ratio of 0·58 for children younger than 2 years and 0·30 for those younger than 5 years by taking the median of the incidence rate ratios of studies reporting incidence for the full age range. For RSV-associated ALRI, an incidence rate ratio of 1·3 was calculated for children younger than 2 years, whereas for those younger than 5 years the ratio was 0·81. Too few studies reported neonatal RSV-associated ALRI for us to report this finding reliably as a separate group. We assumed that cases defined as hospitalised acute respiratory infection or hospitalised lower or acute lower respiratory infection represented severe ALRI (webappendix p 5). Figure 1 summarises our overall approach and associated rationale for decisions adopted.
Figure 1.
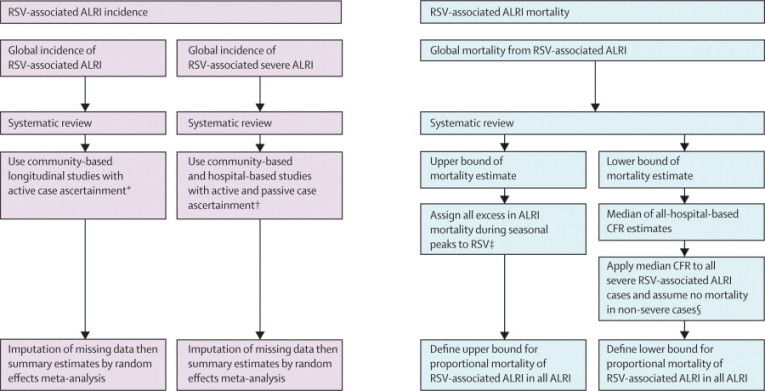
Approaches for estimation of global RSV incidence and mortality in children aged 0–5 years
RSV=respiratory syncytial virus. ALRI=acute lower respiratory infection. CFR=case fatality ratio. *Approach justified by large difference in reported incidence between studies using active and passive case ascertainment; studies with passive ascertainment reported much lower estimates than did those with active ascertainment. †Approach justified by the decision that hospital-based data would be most useful for population-based projections, since all severe episodes are likely to need hospital treatment; also, we noted no difference in reported incidence of RSV-associated severe ALRI between studies with active and passive case ascertainment. †Approach based on assumptions that: i) baseline proportional mortality of RSV-associated ALRI in all ALRI would be similar to proportional incidence of severe ALRI in all severe ALRI, and ii) there is no overall effect from seasonality of other respiratory pathogens; then, if all excess ALRI mortality during RSV seasonal peaks is assigned to RSV as the only cause (in a setting with many seasonal peaks) and this mortality is added to baseline mortality estimates, this approach is likely to overestimate the contribution of RSV to mortality from all ALRI. §Approach deemed to yield a lower bound for RSV-associated ALRI mortality because patients with severe RSV-associated ALRI treated in hospital might have higher CFRs than do all severe cases of RSV (treated and untreated), but a substantial (but unknown) proportion of severe cases will not present to health services for treatment, thereby increasing overall CFR.
Statistical analysis
Using Stata (version 10.1), we did a meta-analysis of incidence data and reported pooled estimates and 95% CIs using the random effects model (DerSimonian-Laird method) since data were heterogeneous (p<0·0001). Studies of Indigenous populations in industrialised countries were excluded from this analysis. We estimated incidence for industrialised and developing countries for 2005, and summed these estimates to yield the global incidence estimate for that year.
Since data were scarce, we did not model a point estimate for RSV-associated ALRI mortality. Instead, we assessed the possible boundaries of mortality that could plausibly be attributed to RSV using three approaches. First, the median of all RSV-associated ALRI case fatality ratio data from hospital-based reports was combined with hospital-based inpatient data for RSV-associated severe ALRI incidence. Since access to hospital treatment in most developing countries is typically limited, we judged this result to represent a lower bound for mortality. The second approach was similar to the first, but severity was defined on the basis of presence of hypoxaemia (an established marker of RSV-associated severe ALRI). We estimated the proportion of children with severe disease and hypoxaemia at presentation and applied a 14% case fatality ratio on the basis of published data from developing country studies reporting outcomes in hypoxaemic children with ALRI.16,17 This approach adjusted for the fact that no RSV test was done in 4–28% of children admitted with ALRI in the studies we included (webappendix p 6).
A third approach assumed that all excess mortality due to ALRI in children younger than 5 years in the RSV season was due to RSV, and that non-RSV mortality is equal within and between RSV epidemic periods. Since approach three represents an extreme scenario, we assumed that this method yielded an upper bound for RSV-associated ALRI mortality. We defined the duration (in months) of the RSV season for each calendar year of the study (MonRSV). For each year, we calculated the average number of total ALRI deaths (in and out of hospital) that occurred per month during (AvgRSV) and outside (AvgOTHER) the RSV season, as well as the total number of deaths (TOTAL) during the year. The proportion of yearly deaths due to RSV was then calculated as:
Application of this approach to the estimated mortality of children younger than 5 years due to ALRI in Indonesia (with 2005 population data14 and WHO estimates1 of mortality attributable to ALRI in this age group) would provide an estimate of all deaths attributable to RSV if community-based case ascertainment was used. This approach needed population-based data for RSV seasonality and monthly death records (with cause of death attribution) from the same population, and this information was only available from one developing country site (with data for a 3-year period). The proportion of excess deaths computed with this method for Indonesia was extrapolated to estimate the global mortality of children younger than 5 years attributable to RSV-associated ALRI.
Role of the funding source
The funding sources supported the organisation of a meeting of the RSV study group in Edinburgh on Jan 29 and 30, 2008. The study sponsors had no role in the writing of the report or the decision to submit for publication. HC had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
We identified 36 studies with suitable data (figure 2): 19 published population-based studies reporting incidence of RSV-associated severe or non-severe ALRI in populations under surveillance; seven published studies estimating incidence on the basis of hospital discharge records or laboratory diagnosis reports and a census-based denominator of children at risk; and unpublished data from ten population-based studies, again reporting a clear denominator of children at risk (figure 3, table 1).18–42
Figure 2.
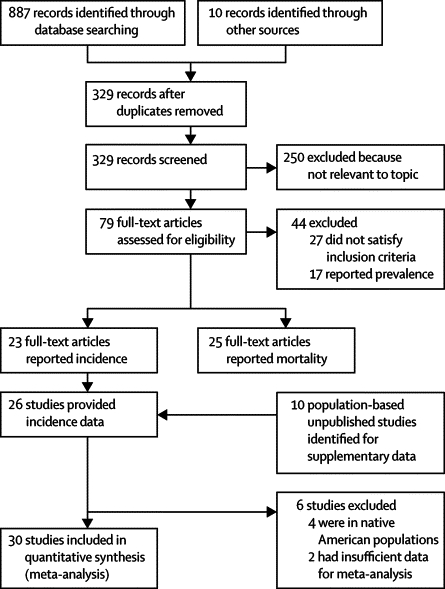
Flow diagram for selection of studies
Figure 3.

Location of the 36 studies by Global Burden of Diseases, Injuries and Risk Factors regions
Table 1.
Incidence estimates of RSV-associated ALRI and severe ALRI in children younger than 5 years from published and unpublished studies
| Case ascertainment | Study denominator | Diagnostic tests |
Incidence of RSV-associated ALRI (per 1000 children per year)* |
Incidence of RSV-associated severe ALRI (per 1000 children per year)* |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Children aged <1 year | Children aged <2 years | Children aged <5 years | Children aged <1 year | Children aged <2 years | Children aged <5 years | ||||
| Europe, western | |||||||||
| Gipuzoka, Spain18 | Passive, hospital (IP) | Defined population base | ELISA | NA | NA | NA | 26 | 15 | 6 |
| Kiel, Germany19 | Passive, hospital (IP) | Defined population base | RT-PCR | NA | NA | NA | 16 | 9 | (5) |
| Multicentric, Germany23 | Passive, hospital (IP) | Defined population base | PCR using test kit | NA | NA | NA | (28) | (16) | (8) |
| Shropshire, UK24 | Passive, hospital (IP) | Defined population base | IF | NA | NA | NA | (28) | 16 | (8) |
| Northern Stockholm, Sweden25 | Passive, hospital (IP) | Defined population base | DFA | NA | NA | NA | 14 | (8) | (4) |
| Southern Austria, Austria26 | Passive, hospital (IP) | Defined population base | ELISA | NA | NA | NA | (12) | 7 | (4) |
| UK27 | Passive, hospital (IP)† | Census-derived estimate | Not available | NA | NA | NA | 28 | (16) | (8) |
| Netherlands28 | Passive, hospital (OP/IP)†‡ | Census-derived estimate | Not available | NA | NA | NA | 10 | (6) | (3) |
| Sub-Saharan Africa, east | |||||||||
| Kilifi birth cohort, Kenya (Nokes et al)§ | Active, community based | Defined population base | IFA | 104 | (135) | (84) | 13 | (8) | (4) |
| Kilifi hospital study, Kenya (Nokes et al)§ | Passive, hospital (IP) | Defined population base | IFA | NA | NA | NA | 11 | (6) | 3 |
| Manhiça district, Mozambique (Roca et al) | Passive, hospital (OP) | Defined population base | ELISA | 44 | (57) | (36) | 16 | (9) | 4 |
| Sub-Saharan Africa, west | |||||||||
| Ibadan, Nigeria22 | Active, community-based | Defined population base | ELISA | 116 | (151) | 94 | NA | NA | NA |
| Western region, The Gambia29 | Passive, hospital (IP) | Defined population base | IF | NA | NA | NA | 18¶ | (10) | (5) |
| Sub-Saharan Africa, southern | |||||||||
| Soweto, South Africa (Madhi et al)‖ | Passive, hospital (IP) | Defined population base | DFA | NA | NA | NA | 10 | (6) | 2 |
| Agincourt, South Africa22 | Passive, hospital (IP) | Defined population base | ELISA | NA | NA | NA | 15 | (9) | 9 |
| Asia, south | |||||||||
| Mirzapur, Bangladesh30 | Active, community-based | Defined population base | ELISA | (32) | 42 | (22) | NA | NA | NA |
| Ballabgarh, India31 | Active, community-based | Defined population base | IF (DFA, IFA) | 33 | 43 | (27) | 14 | 11 | (4) |
| Asia, southeast | |||||||||
| Bandung, Indonesia (Simões et al) | Active, community-based | Defined population base | PCR** | 53 | 60 | 48 | 17 | 14 | 10 |
| Takhli district, Thailand32 | Passive, hospital (IP) | Defined population base | IF | NA | NA | NA | (30) | (17) | 9 |
| Lombok, Indonesia (Gessner et al) | Passive, hospital (IP) | Defined population base | ELISA | NA | NA | NA | 13 | 8 | 4 |
| Asia, east | |||||||||
| Eastern and Northern New Territories, Hong Kong, China33 | Passive, hospital (IP) | Defined population base | DFA, virus isolation | NA | NA | NA | (10) | (6) | 3 |
| Australasia | |||||||||
| Townsville (Queensland), Australia34 | Passive, hospital (IP) | Defined population base | DFA, Culture | NA | NA | NA | 18 | (10) | 5 |
| North America, high income | |||||||||
| Monroe and Davidson County, USA20 | Passive, hospital (IP) | Defined population base | RT-PCR and culture | NA | NA | NA | 13 | (8) | 4 |
| YK Delta, Alaska, USA35††‡‡ | Passive, hospital (IP) | Defined population base | ELISA | NA | NA | NA | 166 | (96) | 50 |
| Milwaukee, USA36 | Passive, hospital (IP) | Defined population base | MPCR, virus culture, and ELISA | NA | NA | NA | (20) | (12) | 6 |
| YK Delta, Alaska, USA (Singleton et al)††§§ | Passive, hospital (IP) | Defined population base | ELISA | NA | NA | NA | 113 | 65 | (34) |
| Navajo and White Mountain Apache reservations, USA (Epi study)†† | Passive, hospital (IP) | Defined population base | ELISA | NA | NA | NA | 91 | 64 | (27) |
| Nashville, Tennessee, USA (Wright et al)¶¶ | Passive, hospital (OP) | Defined population base | Culture | 46 | (60) | 24 | 10 | (6) | 3 |
| Tennessee, USA37 | Passive, hospital (IP)† | Census-derived estimate | Not available | NA | NA | NA | 63 | 41 | (19) |
| USA38 | Passive, hospital (IP)† | Census-derived estimate | Not available | NA | NA | NA | 23 | (13) | (7) |
| American Indian and Alaska Native, USA39†† | Passive, hospital (IP)† | Census-derived estimate | Not available | NA | NA | NA | 34 | (20) | (10) |
| USA39 | Passive, hospital (IP)† | Census-derived estimate | Not available | NA | NA | NA | 27 | (16) | (8) |
| Hawaii, USA21 | Passive, hospital (IP)† | Census-derived estimate | Not available | NA | NA | NA | 10 | (6) | 3 |
| Nashville, Rochester and Cincinnati, USA40 | Passive, hospital (IP) | Defined population base | RT-PCR and tissue culture | NA | NA | NA | 11 | 3 | 3 |
| Latin America, central | |||||||||
| San Marcos, Guatemala (Bruce et al)‖‖ | Active, community-based | Defined population base | ELISA | 158 | (205) | (128) | 60 | (35) | (18) |
| Latin America, tropical | |||||||||
| Rio de Janeiro, Brazil41 | Passive, hospital (IP) | Defined population base | IF and culture | NA | NA | NA | (47) | (27) | 14 |
RSV=respiratory syncytial virus. ALRI=acute lower respiratory infections. IP=inpatient. NA=not applicable. RT-PCR=reverse transcriptase PCR. IF=immunofluorescence. DFA=direct fluorescent antibody test. OP=outpatient. IFA=indirect immunofluorescent antibody test. MPCR=multiplex reverse transcription PCR.
Data in parentheses are computed incidence estimates.
Incidence estimated with hospital discharge records.
Incidence estimated with weekly lab reports from 17 virological laboratories.
RSV incidence estimates are scaled by fraction of lower respiratory infection samples tested; proportion of RSV-positive patients in the tested group is thus assumed to be the same as that in the eligible but untested group.
Incidence in rural areas; in urban areas incidence is 8·7 per 1000 children per year.
Included children aged 6 weeks up to 5 years.
ELISA was used initially as diagnostic assay for published results22 and 120 patients with ALRI tested positive for RSV; all samples were later reanalysed with PCR and an additional 43 cases tested positive.
Between July, 1994, and June, 2001.
Although these areas have low incomes and have factors favouring RSV transmission similar to those of developing countries, they are included in North America (high income) by virtue of being part of the USA.
Between July, 2001, and June, 2004.
Excluded children with known risk factors such as prematurity, bronchopulmonary dysplasia, and congenital heart disease.
RESPIRE trial; refer to reference 42 for methods.
We estimated incidence in children younger than 5 years for all studies within Global Burden of Disease regions (table 1). Ten studies,18–22 six published and four unpublished (Madhi et al, Wright et al, Roca et al, Simões et al), reported incidence for the full age range. All studies adopted one or a combination of three methods of case ascertainment: active community-based (ALRI cases sought by health workers by regular house-to-house visits); or passive hospital-based or clinic-based (children with ALRI who have sought treatment in hospital or clinic are enrolled), which was further stratified as outpatient or inpatient. Most studies were passive hospital-based (inpatient), with only six studies using active community-based case ascertainment and two having a passive hospital-based (outpatient) approach. In active community-based studies, investigators reported a raised incidence of RSV-associated ALRI compared with passive studies. Incidence varied widely across age groups (webappendix pp 15–16). Although incidence was usually the highest in the 0–5 month age group, some investigators reported a higher incidence in children aged 6–11 months.
All studies (apart from one) from developing countries estimating incidence for RSV-associated ALRI used active case ascertainment. Investigators using passive case ascertainment reported substantially lower incidence than did those using an active approach, as would be expected in developing country settings in which access to health services is limited (table 2; webappendix pp 7–11, 18–29). Hence, we based our incidence estimate on a summary of data from developing country studies with active case ascertainment only (table 2), as the most accurate reflection of the true value. We estimated that 33·8 (95% CI 19·3–46·2) million new cases of non-severe RSV-associated infection occurred globally in children younger than 5 years (including neonates) in 2005 (table 2), of which 96% of episodes were in developing countries (where 90% of the world's population aged younger than 5 years reside).
Table 2.
Estimates of incidence and number of new cases of RSV-associated ALRI and severe ALRI in children younger than 5 years from studies§ with active and passive case ascertainment, by GBD region
|
RSV-associated ALRI |
RSV-associated severe ALRI |
||||
|---|---|---|---|---|---|
| Incidence in children aged <1 year (per 1000 per year) | Number of new cases in children aged <5 years in 2005 (×103) | Incidence in children aged <1 year (per 1000 per year) | Number of new cases in children aged <5 years in 2005 (×103) | ||
| Developing countries | |||||
| Active | |||||
| Number of studies | 6 (1) | 6 (4) | 4 (0) | 4 (3) | |
| Median estimate | 78·5 (33–116) | 66 (27–94) | 15·5 (13·5–38·6) | 7 (4–14) | |
| Meta-estimate | 74·2 (50·2–109·7) | 59·1 (40–87·5) | 22·3 (9·4–52·9) | 8·3 (4·4–15·6) | |
| Passive | |||||
| Number of studies | 1 | 1 | 10 (4) | 10 (3) | |
| Median estimate | NA | NA | 15·4 (11–19) | 4·7 (3–9) | |
| Meta-estimate | NA | NA | 16·4 (13·2–20·5) | 5 (3·7–6·7) | |
| Active and passive | |||||
| Number of studies | 7 (1) | 7 (5) | 14 (4) | 14 (6) | |
| Median estimate | 53 (33–116) | 48 (27–94) | 15·4 (13–19) | 4·7 (4–10) | |
| Meta-estimate | 68·4 (46·5–100·5) | 54·6 (37·1–80·4) | 17·9 (14·5–22·2) | 5·6 (4·3–7·4) | |
| Industrialised countries | |||||
| Active | |||||
| Number of studies | 0 | 0 | 0 | 0 | |
| Passive | |||||
| Number of studies | 1 | 1 | 15 (4) | 15 (10) | |
| Median estimate | NA | NA | 18 (12–28) | 4·7 (3–9) | |
| Meta-estimate | NA | NA | 19 (14·6–24·7) | 5·5 (4·2–7·2) | |
| Global | |||||
| Developing* | 59·1 (40–87·5) | 32512 | 5·6 (4·3–7·4) | 3080·7 | |
| Industrialised† | 24 (19·8–30) | 1301·7 | 5·5 (4·2–7·2) | 298·3 | |
| Total‡ | 48·5 (31·4–74·9) | 33813·7 | 5·6 (4·5–7) | 3379 | |
Data are number of studies (imputed number), median estimate (IQR), or meta-estimate (95% CI). References 35 and 36, Singleton et al, and the Epi study were excluded because these studies were of Indigenous populations in industrialised countries; references 19 and 38 excluded because of insufficient data. RSV=respiratory syncytial virus. ALRI=acute lower respiratory infection. GBD=global burden of disease.
Incidence estimates for RSV-associated ALRI based on the meta-estimate for active studies only and for severe ALRI on the meta-estimate for both active and passive studies.
Incidence for RSV-associated ALRI based on the estimate from one passive study only and incidence for severe ALRI on the meta-estimate from passive studies.
Total incidence for RSV-associated ALRI was calculated on the basis of the meta-estimate from active studies in developing countries plus the estimate from passive study in industrialised countries; total incidence for RSV-associated severe ALRI was on the basis of the meta-estimate from active and passive studies in developing countries plus the meta-estimate from passive studies in industrialised countries.
Reported incidences of RSV-associated severe ALRI were similar in studies with active and passive case ascertainment, which is consistent with increased health-service use for children with severe disease. We thus based the estimate of incidence of severe infection on data from studies with both active and passive case ascertainment. The estimated global incidence of severe disease in children younger than 5 years was roughly 3·4 million cases, of which 91% occurred in developing countries (table 2).
We identified 25 published and six unpublished studies providing case fatality ratio data for deaths due to RSV-associated severe ALRI in children admitted to hospital (webappendix pp 7–8). We considered separately the case fatality ratios for severe disease from hospital-based studies in developing and industrialised countries. Not all studies that were included in the younger than 5 years age group provided case fatality ratios for the full age range. Age-stratified mortality data from unpublished studies (Madhi et al, Nokes et al) show that mortality due to RSV-associated severe ALRI is very low beyond age 2 years, and the number of cases of severe infection also decreases sharply. Hence, we assumed that all deaths in severe disease occurred in the first 2 years of life. Table 3 shows median case fatality ratio estimates for developing and industrialised countries. In industrialised countries, almost all deaths due to RSV-associated severe ALRI occurred in infants, whereas in developing countries deaths also occurred in the second year of life.
Table 3.
Estimated case fatality ratio in children younger than 5 years admitted to hospital for RSV-associated severe ALRI in industrialised and developing countries
|
Number of studies included* |
RSV-associated ALRI case fatality ratio (%) |
|||
|---|---|---|---|---|
| Children aged <1 year | Children aged <5 years | Children aged <1 year | Children aged <5 years | |
| Industrialised countries | 5 | 7 | 0·7% (0·3–4·8) | 0·3% (0·2–0·4) |
| Developing countries | 3 | 12 | 2·1% (1·6–2·2) | 2·1% (1·3–3·4) |
Data are number, or median (IQR). RSV=respiratory syncytial virus. ALRI=acute lower respiratory infections.
Seven studies reporting zero case fatality ratios were excluded from final analysis.
Approach 1 was based on the estimated yearly number of new cases of RSV-associated severe ALRI from hospital-based studies (table 2) and the case fatality ratios from children admitted with severe disease reported in hospital-based studies (table 3) separately for developing and industrialised countries. This approach showed that roughly 66 000 children younger than 5 years died in 2005 because of RSV-associated severe ALRI (panel 2). Approach 2 considered the median proportion of children admitted to hospital with severe disease who had hypoxaemia (0·36 [IQR 0·17–0·59]; webappendix p 14) and the related case fatality ratios reported for this group of children in resource-poor settings (28% and 14% in children admitted to hospital with a mixture of bacterial and viral pneumonias who had hypoxaemia at presentation).16,17 Adoption of the most conservative estimate of 14% leads to a crude estimate of roughly 155 000 deaths due to severe disease in children younger than 5 years in 2005 (panel 2). Data were insufficient to allow calculation of precise age-specific case fatality ratio estimates in children admitted to hospital, which would have yielded an improved estimate. Since this estimate includes only children admitted to hospital (a large underascertainment of all severe cases), we judged it to represent a plausible lower bound of RSV-associated severe ALRI mortality.
Panel 2. Estimated mortality due to respiratory syncytial virus (RSV)-associated acute lower respiratory infection (ALRI) in children younger than 5 years.
Approach 1: Case fatality ratios and incidence rate
-
•
Estimated new cases (in children younger than 5 years) of RSV-associated severe ALRI yearly in industrialised countries, a=0·29 million
-
•
Estimated case fatality ratio (CFR; for children younger than 5 years) due to RSV-associated severe ALRI in industrialised countries, b=0·3%
-
•
Estimated mortality due to RSV-associated severe ALRI in children (younger than 5 years) in industrialised countries, c=a×b=895
-
•
Estimated new cases (in children younger than 5 years) of RSV-associated severe ALRI yearly in developing countries, d=3·08 million
-
•
Estimated CFR (in children younger than 5 years) due to RSV-associated severe ALRI in developing countries, e=2·1%
-
•
Estimated mortality due to RSV-associated severe ALRI in children (younger than 5 years) in developing countries, f=d×e=64 695
-
•
Estimated global mortality due to RSV-associated severe ALRI in children (younger than 5 years), g=c+f=65 590
Approach 2: Hypoxaemia in RSV-associated severe ALRI cases admitted to hospital
-
•
Estimated proportion of hypoxaemia in children admitted to hospital with RSV-associated severe ALRI, h=0·36
-
•
Yearly estimated new cases (in children younger than 5 years) of RSV-associated severe ALRI in developing countries, i=3·08 million
-
•
Assumed mortality rate in untreated severe hypoxaemia, j=14%
-
•
Estimated mortality due to RSV-associated severe ALRI in children younger than 5 years in developing countries, h×i×j=155 232
Approach 3: ALRI mortality during RSV season (based on study in Lombok, Indonesia)
-
•
Proportion of ALRI mortality due to RSV during 3 RSV seasons=8%, 12%, 27%
-
•
Estimated mortality due to ALRI in Indonesian children younger than 5 years=35 640 (22% of mortality in children younger than 5 years1)
-
•
Estimated mortality due to RSV-associated ALRI in children younger than 5 years, k=5596 (mean of the three yearly estimates)
-
•
Estimated mortality due to RSV-associated ALRI in Indonesian children (from approach 1, with incidence rates from table 1 and CFR for developing countries), l=1812
-
•
Proportion of mortality from this approach compared with approach 1, k/l=2·67
-
•
Estimated global mortality due to RSV-associated ALRI (by extrapolating Indonesian model), 2·67×f=199 260
Only one study, in Lombok, Indonesia, reported cause of death in children not admitted to hospital, who were assigned by verbal autopsy and RSV isolations in the same population (mid-study analysis, reference 16; unpublished data, Gessner et al). Data were reported continuously during a 3-year period (figure 4, table 4). We estimated that the number of deaths calculated with approach 3 was 2·67 times higher than was estimated with approach 1. If we assume that these data are broadly representative of Indonesia, then 16% of all ALRI deaths are associated with RSV. If extrapolated to other developing country settings, this approach yields a crude estimate (for developing countries) of roughly 199 000 deaths attributable to with RSV-associated ALRI in young children for 2005 (panel 2). We think that this method is likely to overestimate the number of deaths because it assumes that all excess ALRI mortality during the RSV season is due to RSV, an assumption that is unlikely to be true, in view of the seasonality of other respiratory pathogens and the likelihood that RSV deaths occur outside the defined RSV season. Though data from Lombok were for children younger than 2 years only, this limitation is not likely to be important since most pneumonia deaths in children occur during the first 2 years of life.
Figure 4.

ALRI-associated mortality pattern in children younger than 2 years in Lombok, Indonesia
RSV=respiratory syncytial virus. ALRI=acute lower respiratory infection.
Table 4.
Estimated RSV-associated ALRI deaths in Indonesia based on total ALRI deaths occurring in and out of hospital in children younger than 2 years in Lombok, Indonesia
| Months of RSV season (a) | Average total ALRI deaths per month during RSV season (b) | Average total ALRI deaths per month outside RSV season (c) | Total ALRI deaths per year (d) | Proportion of ALRI deaths due to RSV (e)* | Indonesia RSV deaths†‡ | |
|---|---|---|---|---|---|---|
| 2000 | 6 | 73 | 62 | 814 | 0·081 | 2887 |
| 2001 | 6 | 85 | 49 | 802 | 0·27 | 9623 |
| 2002 | 7 | 45 | 37 | 499 | 0·12 | 4277 |
| Summary | .. | .. | .. | .. | .. | 5596§ |
RSV=respiratory syncytial virus. ALRI=acute lower respiratory infections.
e=[(b−c)a]/d.
35 640e.
Calculated with the WHO proportion estimate of 22% of all deaths in children younger than 5 years (19% of all deaths in children aged 1–59 months and a third of the 26% of all neonatal deaths) in Indonesia in 2005.1
Calculated as the mean of individual year values.
In summary, insufficient data are available from which to make valid estimates of global mortality from RSV-associated ALRI. We have adopted three independent approaches with differing assumptions and limitations to obtain a rough data-derived estimate of the plausible lower and upper bounds for RSV-associated ALRI mortality. Our estimates of mortality are consistent with RSV being associated with roughly 3–9% of deaths from ALRI in young children. Data for Indonesia show substantial yearly variation in magnitude of RSV epidemic activity and associated ALRI deaths. This finding suggests that national, regional, and global RSV mortality also varies widely from year to year.
Discussion
We estimated that in 2005, at least 33·8 (95% CI 19·3–46·2) million episodes of RSV-associated ALRI occurred worldwide in children younger than 5 years, with incidence in developing countries more than twice that of industrialised countries. This estimate represents roughly 22% of all episodes of ALRI in young children. By comparison, 13·8 (10·8–17·2) million episodes of pneumococcal pneumonia and 7·9 (7·2–12·9) million episodes of Haemophilus influenzae type b pneumonia occurred in the same age group.43,44 However, this comparison should be interpreted with caution since the estimates for pneumococcal pneumonia and Haemophilus influenzae type b were calculated by extrapolation from data derived from vaccine probe studies. A substantial proportion of RSV-associated morbidity occurs in the first year of life, with incidence in infants that is twice or three times greater than is reported for children younger than 5 years overall. We also estimated that in 2005, 3·4 (2·8–4·3) million young children worldwide developed RSV-associated severe ALRI necessitating hospital admission, and 66 000–199 000 children younger than 5 years died from RSV-associated ALRI, with 99% of these deaths occurring in developing countries.
Estimates of RSV-associated ALRI incidence are highly variable within countries or regions and between regions. We cannot deduce how much of this variation is due to methodological differences and how much is due to variation in RSV epidemiology between study populations. Thus, the true uncertainty is wider than that expressed in a standard 95% CI. Three methodological factors affect estimates: method of case ascertainment; precise case definition for non-severe and severe episodes; and differences in sensitivity and specificity of diagnostic assays to identify RSV infection.
Hospital-based passive case ascertainment is likely to yield falsely low estimates of RSV-associated ALRI incidence, especially in developing countries.45 For example, Nokes and colleagues46 reported that only 20–25% of cases of RSV-associated severe infections that were referred from the community could be identified as hospital admissions, mainly showing low health-service use. Additionally, in some studies a substantial proportion of all children with ALRI were not tested for RSV for various reasons, resulting in falsely low estimates. In hospital-based passive ascertainment studies, distance from hospital is an important factor. Results of a study in the western region of The Gambia29 showed that the rate of hospital admission for RSV-associated severe ALRI was inversely proportional to the cost of getting to the health centre. Similarly, in the Kilifi hospital study,46 though overall incidence for RSV-associated ALRI was 11 per 1000 infants, incidence was 21 per 1000 infants in sublocations closest to hospitals. In one study,47 investigators attempted to keep this effect to a minimum by reimbursing patients for travel costs, but still showed that roughly 25% of children referred with RSV-associated severe ALRI did not attend hospital. This finding supports our decision to base the RSV-associated ALRI incidence estimate in developing countries on data from studies with active case ascertainment.
Case definitions varied from the WHO definition to physician-diagnosed ALRI (with or without chest radiographic confirmation) and unspecified acute respiratory infection that was severe enough to necessitate hospital admission. We noted that investigators using the WHO definition reported the highest incidences. For example, Nokes and colleagues reported in the Kilifi birth cohort study (webappendix p 17) that 75% of WHO-defined severe ALRI cases did not warrant admission to hospital, according to study clinical officers. The studies we included also differed in method of nasal sampling and in the specific diagnostic assays used, which will also have contributed to some of the variation in reported incidence estimates.
The RSV-associated ALRI incidence estimates are more likely to underestimate than to overestimate true incidence in developing countries. However, this conclusion is uncertain because of various factors affecting the estimate. First, estimation depends on the relative sensitivity and specificity of the WHO case definition for true ALRI—both are reported to be fairly high (median specificity of 86% for infants and 93% for children aged 1–4 years).11 Second, almost all studies identified RSV by ELISA or immunofluorescence assays, which have a 12–50% lower sensitivity than does PCR-based diagnosis.48 However, the overall effect of these factors on estimates depends also on relative test specificities, which are unknown for most studies. Finally, although we based our estimate on data from community-based studies with active case ascertainment and facilitated referral of patients to hospital, they could have still missed an unknown proportion of cases.
Substantial uncertainty surrounds case fatality ratio estimates from developing countries. First, 4–28% of children admitted to hospital with ALRI were not tested for RSV for various reasons (such as the child being critically ill, sampling not done on weekends or for night-time admissions, death before sampling, or refusal to get the test done; webappendix p 6). The absence of sampling in these children introduces a bias towards falsely low reported estimates because mortality tends to be higher in these groups. Second, we have estimated case fatality ratios separately for broad developing and industrialised country categories and the degree to which included studies are representative of these broad categories is unknown. We omitted three developing country studies reporting data from paediatric intensive care units and reduced bias in combination of incidence and case fatality ratio data by using only data for the incidence and case fatality ratios from cases admitted to hospital. More generally, these studies might be from settings with above-average resources, and thus report an underestimate of the true case fatality ratio in hospital settings in developing countries.
We based our estimate of the lower bound on reported incidence of RSV-associated severe ALRI necessitating hospital admission and on reported case fatality ratios in developing-country hospitals. However, not all cases of severe disease are admitted to hospital. Furthermore, hospital-based case fatality ratios from the studies we included cannot be regarded as representative of whole population groups. In the most resource-poor settings, the case fatality ratio for RSV-associated ALRI could be higher than these reported estimates and closer to that of bacterial pneumonias, especially since RSV-associated ALRI might predispose to bacterial infection. Results of studies from developing countries have shown variable rates of bacterial co-infection in RSV-associated ALRI, ranging from 3·5% in The Gambia49 to 31% in a study from Pakistan.4 In Lombok, Indonesia, peaks in ALRI case fatality ratios occurred after peaks in RSV case fatality ratios, potentially implicating bacterial co-infections (figure 4). During strategic planning to reduce childhood pneumonia deaths, co-attribution of mortality in patients with RSV and bacterial co-infection to RSV in addition to bacteria might be worthwhile.
Our estimate of the upper bound was based on only one study and so replication in other settings is needed. Additionally, although we attributed all excess ALRI mortality during the RSV season to RSV, several other viral and bacterial pathogens causing ALRI have seasonal patterns. We have thus not considered the context of multiplicity of other respiratory viruses (eg, human metapneumovirus or influenza A) that co-circulate during the RSV season and in some areas account for as much as 10–20% of ALRI hospital admissions and probably have a similar, if not higher, case fatality ratio to RSV.50–53
The plausibility of our global mortality boundary estimates are supported by the internal consistency of the RSV-associated severe ALRI incidence, case fatality ratios, and RSV-associated ALRI mortality estimates. However, evidence to support valid and precise estimates of global RSV-associated ALRI mortality is of low quality. Research investment to gather further data is clearly needed—eg, by gathering RSV isolation data from ALRI patients in sites where demographic surveillance records community-based pneumonia mortality. Further large-scale unselected case series reporting age-specific case fatality ratios from many well described clinical settings in developing countries and large-scale post-mortem studies of ALRI that include investigation of possible RSV causes would also substantially improve the evidence base for this estimate. Until the widespread delivery of an effective RSV vaccine, measures such as promotion of health-service use, provision of regular oxygen supplies at health centres54 and hospitals, and immunoprophylaxis with monoclonal antibodies (when appropriate and affordable) can be expected to substantially reduce mortality associated with this disease.55,56
Acknowledgments
Acknowledgments
This work was done as part of the wider programme of the Child Epidemiology Reference Group (CHERG) to establish the major causes of global childhood disease burden. We thank Emelda A Okiro, Ann Bett, John Abwao (KEMRI-Wellcome Trust Research Programme, Kenya); Dana Bruden (Arctic Investigations Program, National Center for Preparedness, Detection and Control of Infectious Disease, CDC, Anchorage, AK, USA); Byron Arana (Center for Health Studies, Universidad del Valle de Guatemala, Guatemala City, Guatemala); Keith P Klugman (University of the Witwatersrand/Medical Research Council: Respiratory and Meningeal Pathogens Research Unit and Hubert Department of Global Health, Rollins School of Public Health and Division of Infectious Diseases, School of Medicine, Emory University, Atlanta, GA, USA); Pedro Alonso (Barcelona Centre for International Health Research (CRESIB), Hospital Clínic/IDIBAPS, Universitat de Barcelona, Spain and Centro de Investigação em Saúde da Manhiça (CISM), Ministerio de Saúde, Mozambique); Llorenç Quintó (Barcelona Centre for International Health Research (CRESIB), Hospital Clínic/IDIBAPS, Universitat de Barcelona, Spain); Kuswandewi Mutyara (Medical Faculty, Padjadjaran University, Hasan Sadikin General Hospital, Bandung, Indonesia); Lesley C McGoohan (Centre for Population Studies, Global Health Academy, The University of Edinburgh) assistance with some of the illustrations in this report; and Douglas Holtzman (Bill & Melinda Gates Foundation, Seattle, WA, USA) for participating in the expert group meeting in Edinburgh and reviewing the report. Financial support for this work was provided by WHO CAH (grant number WHO OD/AP-07-04680) and the Bill & Melinda Gates Foundation (R41202). Studies from Kilifi, Kenya received Wellcome Trust funding (061584, 076278). AR was supported by a grant from the Spanish Ministry of Education and Science (Ramón y Cajal: RYC-2008-02777). MWW is a WHO staff member; he is responsible for the views expressed in this publication and they do not necessarily represent the decisions or the policies of WHO.
Contributors
HN participated in study design, literature review, data analysis, data interpretation and report writing. DJN, BDG, SAM, RJS, KLO'B, AR, PFW, MD, NB, and ES participated in study design, data collection, data interpretation and report writing. AC, AS, ERS, MN, and PKM participated in data collection and analysis. ET participated in data analysis, data interpretation, and report writing. IR and HC participated in study design, literature search, data interpretation, and report writing. MWW participated in design and review of the report. CK participated in data collection, data analysis, and review of the report.
Conflicts of interest
BDG has received honoraria from GlaxoSmithKline, but has not received any funding for work on respiratory syncytial virus. RJS has received grant funding from MedImmune and Wyeth. KLO'B has received grant funding from MedImmune. PFW has received grant funding and honoraria from Sanofi-Aventis, Wyeth, MedImmune, and Merck; however no grants or honoraria were received for the work included in this study. AC has received grant support from Wyeth and MedImmune. CK has received grant funding from Wyeth and Abbott; however no funding was received for work included in this study. EAFS has received research grants and honoraria from Medimmune, research grants from Abbott International, and honoraria from GlaxoSmithKline and Sanofi Pasteur; however no grants or honoraria were received for work included in this study. HN, DJN, MD, SAM, AR, NB, ET, AS, ERS, MN, PKM, IR, MWW, and HC declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE, the WHO Child Health Epidemiology Reference Group WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland EK, Campbell H. Epidemiology and etiology of clinical pneumonia. Bull World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright PF, Cutts FT. Generic protocol to examine the incidence of lower respiratory infection due to respiratory syncytial virus in children less than 5 years of age. World Health Organization; Geneva: 2000. p. 34. [Google Scholar]
- 4.Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 5.Simoes EAF. Respiratory syncytial virus infection. Lancet. 1999;354:847–852. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- 6.Nokes DJ. In: Respiratory syncytial virus disease burden in the developing world. Cane P, editor. Elsevier; Amsterdam: 2007. [Google Scholar]
- 7.Law BJ, Carbonell-Estrany X, Simoes EAF. An update on respiratory syncytial virus epidemiology: a developed country perspective. Respir Med. 2002;96(suppl B):S1–S7. [PubMed] [Google Scholar]
- 8.Karron RA, Wright PF, Belshe RB. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 9.Schickli JH, Dubovsky F, Tang RS. Challenges in developing a pediatric RSV vaccine. Hum Vaccin. 2009;5:582–591. doi: 10.4161/hv.9131. [DOI] [PubMed] [Google Scholar]
- 10.Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. Phase-I study Medi-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr Infect Dis J. 2009;28:655–658. doi: 10.1097/INF.0b013e318199c3b1. [DOI] [PubMed] [Google Scholar]
- 11.WHO . Technical bases for the WHO recommendations on the management of pneumonia in children at first-level health facilities. World Health Organization; Geneva: 1991. [Google Scholar]
- 12.Burton GG, Hodgkin JE, Ward JJ. Respiratory care. A guide to clinical practice. 4th edn. Lipincott; Philadelphia: 1997. [Google Scholar]
- 13.Mullins JA, Lamonte AC, Bresee JS, Anderson LJ. Substantial variability in community respiratory syncytial virus season timing. Pediatr Infect Dis J. 2003;22:857–862. doi: 10.1097/01.inf.0000090921.21313.d3. [DOI] [PubMed] [Google Scholar]
- 14.UNICEF . State of the World's Children 2007. United Nation's Children's Fund; New York: 2007. http://www.unicef.org/sowc07/docs/sowc07.pdf (accessed July 5, 2008). [Google Scholar]
- 15.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of clinical pneumonia among children under 5 years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 16.Djelantik IG, Gessner BD, Sutanto A. Case fatality proportions and predictive factors for mortality among children hospitalised with severe pneumonia in a rural developing country setting. J Trop Pediatr. 2003;49:327–332. doi: 10.1093/tropej/49.6.327. [DOI] [PubMed] [Google Scholar]
- 17.Onyango FE, Steinhoff M, Wafula EM, Wariua S, Musia J, Kitonyi J. Hypoxaemia in young Kenyan children with acute lower respiratory infection. BMJ. 1993;306:612–615. doi: 10.1136/bmj.306.6878.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicente D, Montes M, Cilla G, Perez-Yarza EG, Perez-Trallero E. Hospitalization for respiratory syncytial virus in the paediatric population in Spain. Epidemiol Infect. 2003;131:867–872. doi: 10.1017/s0950268803008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigl JA, Puppe W, Rockahr S, Schmitt HJ. Burden of disease in hospitalized RSV-positive children in Germany. Klin Padiatr. 2002;214:334–342. doi: 10.1055/s-2002-35365. [DOI] [PubMed] [Google Scholar]
- 20.Iwane MK, Edwards KM, Szilagyi PG. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113(6 part 1):1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 21.Yorita KL, Holman RC, Steiner CA. Severe bronchiolitis and respiratory syncytial virus among young children in Hawaii. Pediatr Infect Dis J. 2007;26:1081–1088. doi: 10.1097/INF.0b013e31812e62c2. [DOI] [PubMed] [Google Scholar]
- 22.Robertson SE, Roca A, Alonso P. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ. 2004;82:914–922. [PMC free article] [PubMed] [Google Scholar]
- 23.Forster J, Ihorsst G, Rieger CHL. Prospective population-based study of viral lower respiratory tract infections in children under 3 years of age (the PRI.DE study) Eur J Pediatr. 2004;163:709–716. doi: 10.1007/s00431-004-1523-9. [DOI] [PubMed] [Google Scholar]
- 24.Deshpande SA, Northern V. The clinical and health economic burden of respiratory syncytial virus disease among children under 2 years of age in a defined geographical area. Arch Dis Child. 2003;88:1065–1069. doi: 10.1136/adc.88.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriksson M, Bennet R, Rotzen-Ostlund M, Sydow von M, Wirgart BZ. Population-based rates of severe respiratory syncytial virus infection in children with and without risk factors, and outcome in a tertiary care setting. Acta Paediatr. 2002;91:593–598. doi: 10.1080/080352502753711740. [DOI] [PubMed] [Google Scholar]
- 26.Resch B, Gusenleitner W, Muller W. The impact of respiratory syncytial virus infection: a prospective study in hospitalised infants younger than 2 years. Infection. 2002;4:193–197. doi: 10.1007/s15010-002-2122-1. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Pebody B, Edmunds WJ, Zambon MC, Gay NJ, Crowcroft NS. Contribution of RSV to bronchiolitis and pneumonia-associated hospitalisations in English children, April 1995–March 1998. Epidemiol Infect. 2002;129:99–106. doi: 10.1017/s095026880200729x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen AG, Sanders EA, Hoes AW, van Loon AM, Hak E. Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur Respir J. 2007;30:1158–1166. doi: 10.1183/09031936.00034407. [DOI] [PubMed] [Google Scholar]
- 29.Weber MW, Milligan P, Sanneh M. An epidemiological study of RSV infection in the Gambia. Bull World Health Organ. 2002;80:562–568. [PMC free article] [PubMed] [Google Scholar]
- 30.Hasan K, Jolly P, Marquis G. Viral etiology of pneumonia in a cohort of newborns till 24 months of age in Rural Mirzapur, Bangladesh. Scand J Infect Dis. 2006;38:690–695. doi: 10.1080/00365540600606473. [DOI] [PubMed] [Google Scholar]
- 31.Broor S, Parveen S, Bharaj P. A prospective three year cohort study of the epidemiology and virology of acute respiratory infections in rural India. PLoS One. 2007;2:e491. doi: 10.1371/journal.pone.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suwanjutha S, Sunakorn P, Chantarojanasiri T. Respiratory syncytial virus-associated lower respiratory tract infection in under-5-year old children in a rural community of central Thailand, a population-based study. J Med Assoc Thai. 2002;85(suppl 4):S1111–S1119. [PubMed] [Google Scholar]
- 33.Chan PKS, Sung RYT, Fung KSC. Epidemiology of respiratory syncytial virus infection among pediatric patients in Hong Kong: seasonality and disease impact. Epidemiol Infect. 1999;123:257–262. doi: 10.1017/s0950268899002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitehall JS, Boilsetty S, Whitehall P, Francis F, Norton R, Patole SK. High rate of indigenous bronchiolitis and palivuzumab. J Paediatr Child Health. 2001;37:416–417. doi: 10.1046/j.1440-1754.2001.0720f.x. [DOI] [PubMed] [Google Scholar]
- 35.Singleton RJ, Bruden D, Bulkow L. Respiratory syncytial virus season and hospitalizations in Alaskan Yukon-Kuskokwim delta. Pediatr Infect Dis J. 2007;26:S1–S6. doi: 10.1097/INF.0b013e318157da9b. [DOI] [PubMed] [Google Scholar]
- 36.Henrickson KJ, Hoover S, Kehl KS, Hua W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect Dis J. 2004;23(suppl 1):S11–S18. doi: 10.1097/01.inf.0000108188.37237.48. [DOI] [PubMed] [Google Scholar]
- 37.Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 38.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 suppl):S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 39.Holman RC, Curns AT, Cheek JE. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114:437–444. doi: 10.1542/peds.2004-0049. [DOI] [PubMed] [Google Scholar]
- 40.Hall CB, Weinberg GA, Iwane MK. The burden of respiratory virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutmoller F, Ferro ZPA, Asensi MD, Ferreira V, Mazzei IS, Cunha BL. Etiology of acute respiratory tract infections among children in a combined community and hospital study in Rio de Janeiro. Clin Infect Dis. 1995;20:854–860. doi: 10.1093/clinids/20.4.854. [DOI] [PubMed] [Google Scholar]
- 42.Bruce N, Weber M, Arana B. Pneumonia case-finding in the RESPIRE Guatemala indoor air pollution trial: standardizing methods for resource-poor settings. Bull World Health Organ. 2007;85:535–544. doi: 10.2471/BLT.06.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Brien KL, Wolfson LJ, Watt JP, for the Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 44.Watt JP, Wolfson LJ, O'Brien KL. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–911. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 45.Sutanto A, Gessner BD, Djelantik GG. Acute respiratory illness incidence and death among children under two years of age on Lombok Island, Indonesia. Am J Trop Med Hyg. 2002;66:175–179. doi: 10.4269/ajtmh.2002.66.175. [DOI] [PubMed] [Google Scholar]
- 46.Nokes DJ, Ngama MJ, Bett A. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49:1341–1349. doi: 10.1086/606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nokes DJ, Okiro EA, Ngama M. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi district, Kenya. Clin Infect Dis. 2008;46:50–57. doi: 10.1086/524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henrickson KJ, Hall CB. Diagnostic assays for respiratory syncytial virus disease. Pediatr Infect Dis J. 2007;26:S36–S40. doi: 10.1097/INF.0b013e318157da6f. [DOI] [PubMed] [Google Scholar]
- 49.Weber M, Dackour R, Usen S. The clinical spectrum of respiratory syncytial virus disease in the Gambia. Pediatr Infect Dis J. 1998;17:224–230. doi: 10.1097/00006454-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Madhi SA, Ludewick H, Kuwanda L, van Niekerk N, Cutland C, Klugman KP. Seasonality, incidence, and repeat human metapneumovirus lower respiratory tract infections in an area with a high prevalence of human immunodeficiency virus type-1 infection. Pediatr Infect Dis J. 2007;26:693–699. doi: 10.1097/INF.0b013e3180621192. [DOI] [PubMed] [Google Scholar]
- 51.Hamelin M-E, Abed Y, Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38:983–990. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolf DG, Greenberg D, Kalkstein D. Comparison of human metapneumovirus, respiratory syncytial virus and influenza-A virus lower respiratory tract infection in hospitalised young children. Pediatr Infect Dis J. 2006;25:320–324. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 53.Foulongne V, Guyon G, Rodiere M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:354–359. doi: 10.1097/01.inf.0000207480.55201.f6. [DOI] [PubMed] [Google Scholar]
- 54.Duke T, Wandi F, Jonathan M. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372:1328–1333. doi: 10.1016/S0140-6736(08)61164-2. [DOI] [PubMed] [Google Scholar]
- 55.Simoes EAF, Groothuis JR. Respiratory syncytial virus prophylaxis—the story so far. Respir Med. 2002;96(suppl. B):S15–S24. doi: 10.1016/s0954-6111(02)90066-1. [DOI] [PubMed] [Google Scholar]
- 56.Strutton DR, Stang PE. Prophylaxis against respiratory syncytial virus (RSV), varicella, and pneumococcal infections: economic-based decision-making. J Pediatr. 2003;143:S157–S162. doi: 10.1067/s0022-3476(03)00512-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


