Abstract
Previously, phenethyl isothiocyanate (PEITC) and dibenzoylmethane (DBM) had been shown to inhibit intestinal carcinogenesis in Apc(Min/+) mice. In this study, we investigated the chemopreventive efficacy of PEITC and DBM in the azoxymethane (AOM)-initiated and dextran sodium sulfate (DSS)-promoted colon cancer mouse model and to compare their potential in vivo mechanisms leading to chemoprevention. The mice were fed with diet supplemented with 0.05% PEITC or 1% DBM before or after AOM initiation. Our results showed that AOM/DSS mice fed with PEITC- or DBM-supplemented diet had lower tumor incidence, lower colon tumor multiplicities and smaller polyps as compared with mice fed with the standard AIN-76A diet. PEITC was effective even after AOM initiation, whereas DBM was not as effective when fed after AOM initiation. Hematoxylin and eosin staining showed that mice fed with PEITC or DBM had attenuated loss of crypt, a marker of inflammation. To examine potential in vivo mechanisms involved in chemoprevention, western blotting was performed and showed that inhibition of growth of adenomas by PEITC was associated with an increase of apoptosis (increased cleaved caspase-3 and-7) and cell cycle arrest (increased p21). In contrast DBM's effect on cell cycle arrest and apoptosis markers was not as substantial as PEITC. Instead, DBM showed increased induction of NF-E2-related factor-2 (Nrf2) transcription factor and phase II detoxifying enzymes, which appears to correlate with in vitro cell lines results that DBM is a more potent Nrf2 activator than PEITC. In summary, our present study shows that PEITC and DBM are potent natural dietary compounds for chemoprevention of colon cancer induced by AOM/DSS and appears to be associated with different in vivo mechanism of actions. PEITC's chemopreventive effect appears to be due to induction of apoptosis and cell cycle arrest, whereas DBM's effect is due to prevention of AOM initiation via induction of Nrf2 and phase II detoxifying enzymes.
Introduction
Colorectal cancer (CRC) is the third most common cancer of men and women and the third leading cause of cancer mortality with an estimated new cases and deaths from colon and rectal cancer in the USA in 2009: new cases—106 100 (colon) and 40 870 (rectal) and deaths—49 920 (colon and rectal combined) (1). The etiology of colon cancer is multifactorial, including familial, environmental, dietary and other unknown factors. Familial adenomatous polyposis is an autosomal dominant inherited condition in humans that is characterized by the progressive development of hundreds to thousands of adenomatous colon polyps. The gene associated with familial adenomatous polyposis coli appears to be an early mutational event found to be responsible in 50–80% of the CRC cases and ApcMin mice have been used extensively for gastrointestinal carcinogenesis research (2). However, a single gene mutation alone such as Apc might not be sufficient to drive colon carcinogenesis. Therefore, chemical-induced carcinogenesis models are also widely utilized. Aberrant crypt foci (ACF) are early morphological changes observed in rodents after administration of colon-specific carcinogen such as azoxymethane (AOM). ACF are considered to be putative preneoplastic lesions (3,4) and are widely used as a surrogate biomarker to rapidly evaluate chemopreventive potential of compounds (5). ACF share many morphological and biochemical characteristics with tumors and ACF found on the surface of cancer-predisposed colons of rodents have been regarded as early appearing preneoplastic lesions. However, it is not clear if such lesions are truly precancerous lesions for CRCs in rodents.
Inflammatory bowel diseases, such as ulcerative colitis (chronic inflammation of colon mucosa) and Crohn's disease (chronic inflammation often involves small intestine along with colon and other organs) have been strongly linked to an increase risk of CRC (6). Indeed, inflammatory bowel disease ranks among the top three high-risk conditions for CRC, together with hereditary syndromes of familial adenomatous polyposis and hereditary non-polyposis CRC (6). In this context, the AOM initiated and promoted by the inflammatory dextran sulfate sodium (DSS) would appear to simulate colon tumor development in human (7,8). AOM causes oxidative and DNA damage, which is then promoted by inflammatory agent DSS. DSS induces colitis and promotes AOM-induced colon cancer in mice (8). Although the AOM-alone model recapitulates many features of human CRC, AOM/DSS appears to represent a potentially more physiologically relevant model since human CRC is often caused by promoting factor (e.g. inflammation) in addition to initiation factors (e.g. somatic mutation caused by carcinogens and or inheritance).
Phenethyl isothiocyanates (PEITC) is a metabolite of glucosinolate that are found abundantly in cruciferous vegetables such as watercress. The chemopreventive efficacy of PEITC has been widely investigated in vitro and in vivo. It has been shown that PEITC induces cell cycle arrest and apoptosis in colon (9), prostate (10) and lung cancer cells (11). Recent studies suggested that other potential mechanism of actions of PEITC include reactive oxygen species-mediated cell killing (12), NF-E2-related factor-2 (Nrf2) induction (13) and protein binding-mediated apoptosis and cell cycle arrest (10,11,14). The efficacy of PEITC as a chemopreventive agent has also been shown in various animal cancer models including rat esophagus (15), prostate (16) and lung (17). However, in terms of PEITC's efficacy in the colon, two previous studies appear to show some contradictory results. One earlier study showed that PEITC was effective in chemoprevention of colonic ACF in Fischer rats (18), whereas a recent report showed that there was no significant difference in the number of ACF found between the PEITC-treated group and the control group (19). Furthermore, as discussed above, ACF lesions might not be true indicators of precancerous lesions for CRC and hence, in this study, we investigated PEITC's efficacy in AOM-induced/DSS-induced mouse CRC model, as well as examined PEITC's potential in vivo mechanisms.
Dibenzoylmethane (DBM) is a beta-diketone structurally related to curcumin, which is a minor constituent of licorice that possesses anti-carcinogenic properties in several animal models (20,21). Early study shows that the efficacy of DBM in the prevention of rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene (20). Our previous study showed the chemopreventive potential of DBM using ApcMin mouse model and that the combination of DBM and another isothiocyanate sulforaphane appears to enhance the effectiveness of inhibition of carcinogenesis in the ApcMin mice (21). The mechanism of actions of DBM remained unclear. In vitro, it was reported that DBM induced cell cycle deregulation in human prostate cancer LNCap cells (22). The anti-inflammatory effect of DBM has been shown to be related to the modulation of arachidonic acid metabolism (23). In vivo, DBM was shown to activate Nrf-2 detoxification pathway and inhibit benzo[a]pyrene-induced DNA adducts in lungs (24). Considering the results of these studies, we thereby investigated the chemopreventive efficacy and the potential in vivo mechanism of actions of DBM in colon cancer using the AOM/DSS model.
Materials and methods
Animals, chemicals and diets
Male C57BL/6 mice were purchased from Jackson. Fifteen to twenty mice were used for each treatment group. AOM was purchased from Sigma–Aldrich (St Louis, MO) and DSS was purchased from MP Biomedicals, LLC (Solon, OH). Both PEITC and DBM were purchased from Sigma and were sent to Dyets (Bethlehem, PA) where they were mixed into 0.05% PEITC and 1% DBM with AIN-76 diet.
Experimental procedure
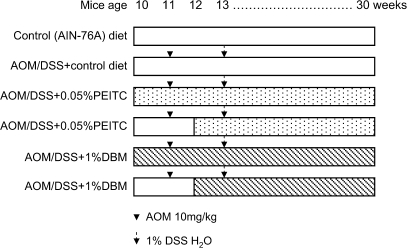
The experimental protocol for the present study is shown in Figure 1. Animals were maintained at 12 h light–12 h dark cycles with free access to water and food (AIN-76A powder diet from Dyets). After 1 week of acclimatization, animals were randomly divided into six groups of between 15–20 animals in each group and fed with pellet diets according to the protocol in Figure 1. After 20 weeks, the mice were killed, and their colons were evaluated for polyps and other markers as described below.
Fig. 1.
Experimental protocol for chemoprevention study with PEITC and DBM in AOM/DSS mice. Ten-week-old mice were used for each group and fed with AIN-76 diet or diet supplemented with 0.05% PEITC or 1% DBM before AOM injection (pre-initiation) or after AOM injection (after initiation). Twenty weeks later, the mice were killed for analysis.
Histopathological analysis
At the end of the experiment, all the mice were killed by CO2. At autopsy, the large bowel was flushed with saline and excised. It was cut open longitudinally along the main axis and washed with saline. The large bowel was then carefully inspected for the presence of polyps, the size and the number of polyps was carefully measured. The polyps and the apparently normal mucosa were excised and fixed in 10% buffered formalin for at least 24 h. Paraffin-embedded sections of the large bowel were then made by routine procedures. Any histopathological alterations in the colon were examined on hematoxylin and eosin-stained sections.
Preparation of tissue homogenates and western blotting
Colonic normal mucosa and polyps were excised from individual animal and pooled together based on the treatment groups. To prepare total cell lysates, about three to five polyps were incubated with 400 μl lysis buffer in an ice-cold French Douncer for 15 min before homogenization of 40 strokes. The homogenates were cleared by centrifugation at 14 000g for 10 min at 4°C. The supernatants were diluted and the protein concentrations were measured by Bicinchoninic acid kit (Pierce, Rockford, IL). A total of 30 μg of proteins were resolved on precasted sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels (Bio-Rad, Hercules, CA) and western blotting was performed. The primary antibodies used include actin, Nrf2, GSTM1, HO-1, NQO-1, p21, p27 (Santa Cruz Biotechnology, Santa Cruz, CA), cleaved caspase-3, cleaved caspase-7 and Bim (Cell Signaling Technology, Danvers, MA).
Statistical analysis
The differences between the groups in terms of the multiplicity and the average size of tumors per mouse were determined by Student's t-test, whereas the difference in the percentage of mice with tumors was tested by Fisher's exact test.
Results
Dietary administration of PEITC or DBM for 20 weeks significantly reduced AOM-induced/DSS-induced polyps without affecting body weight
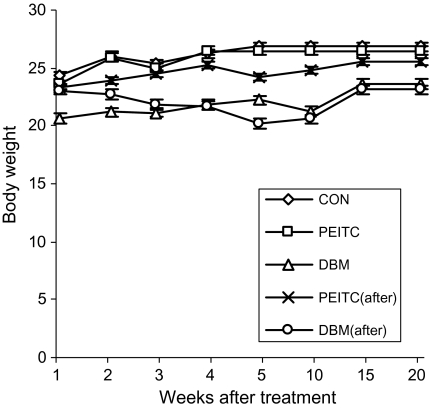
Figure 2 shows that neither the body weight nor any noticeable signs of toxicity were observed in the treatment groups as compared with the control group during the PEITC and DBM treatments. Mice in the control group (AOM/DSS) had a 70% tumor incidence, an average polyp number of 1.6 and an average polyp size of 5 mm (Figure 3). Dietary supplementation of 0.05% PEITC or 1% DBM before initiation (AOM) reduced the polyps incidence to 37.5% (P < 0.05) and 36.4% (P < 0.05) (Figure 3A), average polyps number to 0.375 (P < 0.05) and 1.09 (Figure 3B) and average polyps size to 1.5 mm (P < 0.05) and 3.36 mm (Figure 3C). On the other hand, dietary consumption of 0.05% PEITC after initiation (AOM) reduced the polyps incidence to 40% (P < 0.05) (Figure 3A), average polyps number to 0.6 (P < 0.05) (Figure 3B) and average polyps size to 2.6 mm (P < 0.05) (Figure 3C), whereas 1% DBM after initiation (AOM) showed no apparent inhibitory effect at all (polyps incidence of 77.8%, average polyps number of 1.89 and average polyps size of 5.78 mm) (Figure 3A–C).
Fig. 2.
No observable weight loss of mice. The mice were weighed every week from the start of the treatment for 20 weeks. The administration of compounds did not cause significant weight loss over time.
Fig. 3.
PEITC and DBM decreased tumor incidence, average tumor number and size. (A) 0.05% PEITC and 1% DBM before initiation (AOM) reduced the polyps incidence from 70 to 37.5% and 36.4 and 0.05% PEITC after initiation (AOM) reduced the polyps incidence to 40%. (B) 0.05% PEITC and 1% DBM before initiation (AOM) reduced average polyps number from 1.6 to 0.375 and 1.09 and 0.05% PEITC after initiation (AOM) reduced average polyps number to 0.6. (C) 0.05% PEITC and 1% DBM before initiation (AOM) reduced the average polyps size from 5 to 1.5 mm and 3.36 mm and 0.05% PEITC after initiation (AOM) reduced the average polyps size to 2.6 mm, 1% DBM after initiation (AOM) showed no inhibitory effect at all (polyps incidence of 77.8%, average polyps number of 1.89 and average polyps size of 5.78 mm).
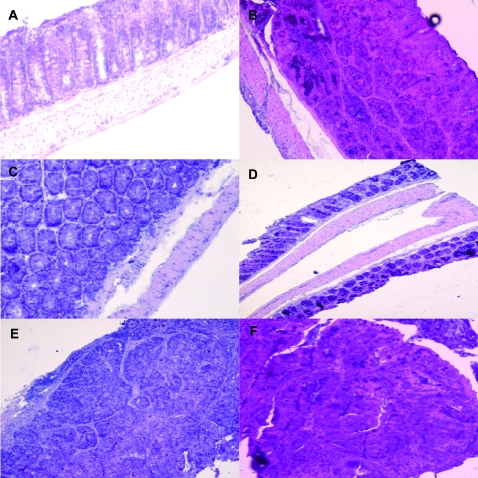
General histological observation of AOM-induced/DSS-induced polyps and normal mucosa in C57/B6 mice treated with PEITC or DBM
AOM-treated/DSS-treated mice resulted in histological alterations of colonic mucosa including infiltration of inflammatory cells into the lamina propria and loss of crypts (Figure 4B). PEITC or DBM treatment decreased the loss of crypts in the colonic mucosa (Figure 4C and D). The polyps developed in AOM-treated/DSS-treated group were mostly adenocarcinomas, treatments with PEITC or DBM did not reverse the severity of the adenocarcinomas developed in the AOM-treated/DSS-treated mice (Figure 4E and F).
Fig. 4.
General histological observation of AOM–DSS polyps and normal mucosa in C57/B6 mice treated with PEITC or DBM. (A) No loss of crypts was observed in mice without AOM/DSS treatment. (B) AOM/DSS treatment resulted in observable crypts loss in normal mucosa. (C) PEITC or (D) DBM treatment decreased loss of crypts in normal mucosa and a better crypt organization was observed. (E) Treatment with PEITC or (F) DBM did not reverse the severity of the adenocarinomas developed in the AOM-treated/DSS-treated mice.
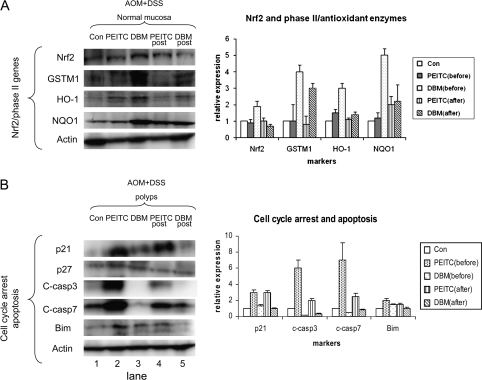
Dietary administration of PEITC or DBM altered proteins involved in cell cycle regulation, apoptosis and Nrf2-regulated detoxification enzymes
DBM greatly induced Nrf2 and its downstream detoxifying enzymes in normal mucosa.
Administration of DBM but not PEITC before AOM initiation induced Nrf2 protein (Figure 5A, lane 3). However, DBM administration after initiation had no effect on Nrf2 induction (Figure 5A, lane 5). The Nrf2-mediated downstream detoxifying/antioxidant enzymes GSTM1, HO-1 and NQO-1 were also greatly induced by DBM treatment before AOM initiation.
Fig. 5.
PEITC or DBM altered proteins involved in cell cycle regulation, apoptosis and Nrf2-regulated detoxification enzymes. (A) DBM greatly induced Nrf2 and its downstream detoxifying enzymes. DBM but not PEITC before AOM initiation induced Nrf2 protein in the normal mucosa. The Nrf2-regulated downstream detoxifying enzymes GSTM1, HO-1 and NQO-1 were also greatly induced by DBM treatment. (B) PEITC greatly induced p21 and cleaved caspase-3 and-7. PEITC administration showed a substantial p21 induction in the polyps, before or after initiation. Induction of another cell cycle arrest marker p27 was less substantial. Cleaved caspase-3 and-7 (C-casp3 and 7) was substantially induced by PEITC administrated both before and after AOM initiation. Induction of pro-apoptotic protein Bim was also higher by PEITC than by DBM.
PEITC induced p21 and cleaved caspase-3 and -7 in the polyps.
The expression level of cell cycle arrest protein p21 was low in polyps (Figure 5B, lane 1). PEITC administration induced p21 in the polyps, before as well as after AOM initiation (Figure 5B, lanes 2 and 4). Administration of PEITC or DBM had no effect on p27 induction. Cleaved caspase-3 and cleaved caspase-7 were more robustly induced by PEITC before AOM than after AOM initiation, and Bim was induced by PEITC and DBM before AOM initiation (Figure 5B, lanes 2 and 4).
Discussion
The purpose of the present study was to investigate the efficacy of PEITC and DBM in the cancer chemoprevention of colon cancer. In addition, we aimed to elucidate the potentially in vivo mechanisms of actions of PEITC and DBM that would contribute to their cancer inhibitory effects. Previously, the use of the AOM–ACF model to determine the chemopreventive efficacy of PEITC was somewhat contradictory (18,19). One paper reported that PEITC inhibited the formation of ACF while another paper did not. The efficacy of DBM has been demonstrated in the lung, in the small intestine but not in the colon. In this study, we showed that both PEITC and DBM are effective chemopreventive compounds in colon carcinogenesis induced by AOM/DSS model (although DBM is only effective before AOM initiation); their in vivo molecular targets and mechanisms appear to be somewhat different.
PEITC appears to be a more potent compound than DBM due to the significantly lower average tumor numbers and sizes. Importantly, if DBM was administrated after AOM initiation, the inhibitory effect of DBM in tumor incidence, number and size was completely attenuated as compared with DBM administered before AOM initiation. Interestingly, the major mechanism of action of DBM in chemoprevention appears to be targeting the molecular events before AOM initiation. This could be through increasing the Nrf2-mediated detoxifying/antioxidant genes for carcinogen detoxification leading to decreased carcinogen-induced oxidative stress, DNA and protein damages. The tumor incidence, number and size remained unchanged for PEITC, whether administrated before or after AOM initiation. These indicate that in contrast to DBM, PEITC's effect may not depend only on the prevention of AOM initiation but also after AOM initiation. Indeed, the inhibitory effect could be due to induction of cell cycle arrest and apoptosis during the progression of carcinogenesis. Interestingly, our recent study using the transgenic adenocarcinoma of the mouse prostate tumor model shows that DBM is more effective when given before the prostatic intraepithelial neoplasia development at 8 weeks old, whereas it is somewhat less effective when DBM is given at 12 weeks when prostatic intraepithelial neoplasia apparently have developed (25). It will be interesting whether PEITC given at a later time point 12 weeks or older transgenic adenocarcinoma of the mouse prostate mice will be as effective as when it was given at 8 weeks old (16).
In this study, we have also examined the normal mucosa and the polyps sections after PEITC and DBM treatments. The AOM/DSS treatment promoted the formation of polyps and all of them were adenomas. The normal mucosa from AOM-treated/DSS-treated group was exemplified with the loss of colonic crypts and the infiltration of inflammatory cells into the lamina propria. The polyps showed no signs of decreased severity for those polyps obtained from mice treated with PEITC or DBM as compared with the controls. However, we did observe less or no crypt loss in the mucosa for the PEITC- and DBM-treated groups. These general observations from the histological analysis suggest that PEITC and DBM have no apparent effect in reversing the tumor grade of already formed malignant polyps.
Comparisons of some of the potential anti-carcinogenesis makers in the normal mucosa versus the polyps between the different groups using western blotting shed some lights on the potentially different in vivo mechanisms elicited by PEITC and DBM. DBM appears to induce a higher Nrf2 expression level, resulting in a more robust induction of phase II detoxifying and antioxidant genes, particularly GSTM1, HO-1 and NQO1, which would play an important role in detoxifying carcinogenic reactive intermediates and reactive oxygen/nitrogen species. Several studies have shown that DBM is effective in reducing DNA-carcinogen adducts in the lungs (24) and mammary glands (26). In this context, the same mechanism could be applied in the colon. Induction of Nrf2 and its downstream target defense enzymes by DBM before AOM initiation would lead to AOM detoxification and potentially less DNA damage. In contrast, when cell cycle and apoptosis markers were compared, PEITC appears to have greater ability to induce cell cycle arrest and apoptosis than DBM, and these results are quite analogous to our previous study with sulforaphane in the ApcMin mouse model (21). PEITC appears to be more potent in inducing p21, Bim and cleaved caspase-3 and caspase-7 than DBM. This might possibly explain why the tumor incidence, number and size were not affected with PEITC was given either before or after AOM initiation. Our result suggests that AOM detoxification might not be the major mechanism for PEITC's chemopreventive effects, rather PEITC's ability to induce apoptosis and cell cycle arrest and possibly keeps the transformed malignant colon cells in check would be the mechanism. However, future studies involving proteomic and or metabolomics would be needed to further elucidate the potential in vivo differential mechanisms.
In summary, using the AOM-induced/DSS-induced colon cancer model, we showed that the major in vivo mechanism for PEITC appears to be induction of cell cycle arrest and apoptosis, whereas DBM's in vivo mechanism of action could be through AOM and or reactive oxygen/nitrogen species detoxification by Nrf2-regulated detoxifying/antioxidant enzymes. This appears to be consistent with our observation that PEITC is effective in reducing tumor incidence, numbers and size, whereas DBM is only effective toward inhibiting tumor incidence when used before AOM initiation. These differential in vivo mechanisms between PEITC and DBM could potentially offer new insights into the in vivo anti-carcinogenesis mechanisms by different dietary cancer chemopreventive compounds and would help to advance future clinical human colorectal chemoprevention trials.
Funding
National Institute of Health (R01 CA-073674-07).
Acknowledgments
We thank all the members in Dr Tony Kong's lab for their helpful discussion and preparation of this manuscript.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ACF
aberrant crypt foci
- AOM
azoxymethane
- CRC
colorectal cancer
- DBM
dibenzoylmethane
- DSS
dextran sodium sulfate
- Nrf2
NF-E2-related factor-2
- PEITC
phenethyl isothiocyanate
References
- 1.Horner MJ, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2006. [Google Scholar]
- 2.Moser AR, et al. ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur. J. Cancer. 1995;31A:1061–1064. doi: 10.1016/0959-8049(95)00181-h. [DOI] [PubMed] [Google Scholar]
- 3.Bird RP, et al. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol. Lett. 2000;15:395–402. doi: 10.1016/s0378-4274(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 4.Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995;93:55–71. doi: 10.1016/0304-3835(95)03788-X. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T, et al. Inhibition of colon carcinogenesis by dietary non-nutritive compounds. J. Toxicol. Pathol. 2007;20:215–235. [Google Scholar]
- 6.Itzkowitz SH, et al. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 7.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 8.Clapper ML, et al. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol. Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheung KL, et al. PEITC induces G1 cell cycle arrest on HT-29 cells through the activation of p38 MAPK signaling pathway. AAPS J. 2008;10:277–281. doi: 10.1208/s12248-008-9032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin P, et al. Phenethyl isothiocyanate induces cell cycle arrest and reduction of alpha- and beta-tubulin isotypes in human prostate cancer cells. Cell Biol. Int. 2009;33:57–64. doi: 10.1016/j.cellbi.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mi L, et al. Binding to protein by isothiocyanates: a potential mechanism for apoptosis induction in human nonsmall lung cancer cells. Nutr. Cancer. 2008;60(suppl 1):12–20. doi: 10.1080/01635580802381287. [DOI] [PubMed] [Google Scholar]
- 12.Trachootham D, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Xu C, et al. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther. 2006;5:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- 14.Mi L, et al. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J. Biol. Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoner GD, et al. Carcinogen-altered genes in rat esophagus positively modulated to normal levels of expression by both black raspberries and phenylethyl isothiocyanate. Cancer Res. 2008;68:6460–6467. doi: 10.1158/0008-5472.CAN-08-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barve A, et al. Murine prostate cancer inhibition by dietary phytochemicals—curcumin and phenyethylisothiocyanate. Pharm. Res. 2008;25:2181–2189. doi: 10.1007/s11095-008-9574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conaway CC, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 18.Chung FL, et al. Chemoprevention of colonic aberrant crypt foci in Fischer rats by SFN and PEITC. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 19.Plate AY, et al. Effects of indole-3-carbinol and phenethyl isothiocyanate on colon carcinogenesis induced by azoxymethane in rats. Carcinogenesis. 2006;27:287–292. doi: 10.1093/carcin/bgi210. [DOI] [PubMed] [Google Scholar]
- 20.Singletary K, et al. Effect of the beta-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1998;19:1039–1043. doi: 10.1093/carcin/19.6.1039. [DOI] [PubMed] [Google Scholar]
- 21.Shen G, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937–9944. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 22.Frazier MC, et al. Proteomic analysis of proteins altered by dibenzoylmethane in human prostatic cancer LNCaP cells. Proteomics. 2004;4:2814–2821. doi: 10.1002/pmic.200400834. [DOI] [PubMed] [Google Scholar]
- 23.Hong J, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 24.Thimmulappa RK, et al. Dibenzoylmethane activates Nrf2-dependent detoxification pathway and inhibits benzo(a)pyrene induced DNA adducts in lungs. Med. Chem. 2008;4:473–481. doi: 10.2174/157340608785700199. [DOI] [PubMed] [Google Scholar]
- 25.Khor TO, et al. Dietary feeding of dibenzoylmethane inhibits prostate cancer in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009;69:7096–7102. doi: 10.1158/0008-5472.CAN-09-0597. [DOI] [PubMed] [Google Scholar]
- 26.Lin CC, et al. Inhibition by dietary dibenzoylmethane of mammary gland proliferation, formation of DMBA-DNA adducts in mammary glands, and mammary tumorigenesis in Sencar mice. Cancer Lett. 2001;168:125–132. doi: 10.1016/s0304-3835(01)00506-7. [DOI] [PubMed] [Google Scholar]