Abstract
Polo-like kinase 1 (Plk1) is a key regulator of mitosis. Aberrant Plk1 activity is found in tumors, but little is known regarding its role in the DNA damage response of normal cells and its potential contribution to the early stages of carcinogenesis. Inappropriate survival signaling after DNA damage may facilitate clonal expansion of genetically compromised cells, and it is known that protein tyrosine phosphatase (PTP) inhibitors activate key survival pathways. In this study, we employed hexavalent chromium [Cr(VI)], a well-documented genotoxicant, to investigate the mechanism by which survival pathway activation could lead to loss of checkpoint control via a mechanism involving Plk1. We recently reported that PTP inhibition enhances clonogenic survival and mutagenesis after Cr(VI) exposure by overriding Cr-induced growth arrest. Here, we report that checkpoint bypass, facilitated by PTP inhibition, was associated with decreased Cdk1 Tyr15 phosphorylation, as well as increased Plk1 activity and nuclear localization. Plk1 was necessary for increased survival after PTP inhibition and Cr(VI) exposure in normal human fibroblasts via enhanced mitotic progression. In addition, pharmacological inhibition of Plk1 abolished the PTP inhibitor-induced bypass of the G2/M checkpoint. Notably, Plk1 overexpression increased survival and mutagenesis after Cr(VI) exposure in wild-type Saccharomyces cerevisiae. Taken together, our data indicate that Plk1 activation and nuclear localization are necessary for PTP-regulated mitotic progression after DNA damage. Our studies highlight a role for Plk1 in the loss of checkpoint control, increased survival and mutagenesis after genotoxic exposure in normal cells, which in turn may lead to genomic instability and carcinogenesis.
Introduction
Dysregulated cell proliferation after genotoxic injury is a hallmark of the early stages of neoplastic transformation. Maintenance of cell cycle checkpoints in response to genotoxic stress is critical for the preservation of genomic integrity (1). It is thought that cell cycle checkpoint arrest allows sufficient time for surveillance of damaged DNA and either subsequent repair or activation of terminal growth arrest and/or apoptotic pathways. Specifically, the G2/M checkpoint prevents cells with unrepaired DNA damage from entering mitosis and therefore preserves the genomic integrity of the cell (1,2).
Protein tyrosine phosphorylation plays a central role in maintaining proliferative signals, particularly during neoplastic progression (3). Protein tyrosine kinases promote cell survival, whereas the overall effect of protein tyrosine phosphatases (PTPs) is to decrease cell survival (3,4). Therefore, PTP inhibition promotes activation of key survival pathways (5). We have reported that maintenance of protein tyrosine phosphorylation through PTP inhibition was associated with increased proliferation, clonogenic survival and mutagenesis in normal diploid cells after a single exposure to hexavalent chromium [Cr(VI)], a well-documented human genotoxicant (6). These findings suggest that regulators of tyrosine phosphorylation may induce cell cycle checkpoint bypass as an initial event after genotoxic insult.
Multiple signaling pathways coordinate cell cycle progression and influence cell survival after DNA damage. Recently, it has been postulated that Polo-like kinase 1 (Plk1) functions downstream of receptor tyrosine kinases (7,8). Furthermore, Plk1 activity has been suggested to be necessary for mitotic progression in cancer cells after recovery from DNA damage (9,10). Plk1 appears to be a key regulator of the G2/M checkpoint (9–11), although DNA damage has been associated with inhibition of Plk1 activity in conjunction with G2/M arrest (11,12). Similarly, the yeast homolog of Plk1, Cdc5, is necessary for adaptation in Saccharomyces cerevisiae (13). Likewise, it has been proposed that adaptation in osteosarcoma cells after infrared radiation is partially dependent upon Plk1 (14). Although Plk1 dysregulation in tumor cells is now well established, the role of Plk1 in the DNA damage response after genotoxic exposure in normal cells and its potential contribution to early-stage carcinogenesis remains relatively unclear.
In light of our recent report that PTP inhibition enhances clonogenic survival after Cr(VI) exposure (6), we postulated that Plk1 is involved in the override of genotoxic stress-induced cell cycle arrest that we observed after PTP inhibition. Studies in our laboratory have shown that exposure of normal human cells to Cr(VI) was associated with a prolonged G1/S and G2/M arrest (15). We further identified Akt1 as a key determinant of G1/S checkpoint bypass (16). However, Akt1 had no effect on either clonogenic survival or G2/M checkpoint bypass, and consequent mitotic progression, after Cr(VI) exposure (6). The objective of the present study was to ascertain the role of Plk1 in mitotic progression induced by PTP inhibition after Cr(VI) exposure in normal human lung fibroblasts (HLFs). Moreover, we determined the necessity of Plk1 for cell survival after genotoxic stress and PTP inhibition. Our data suggest that Plk1 mediates cell cycle checkpoint bypass, mitotic progression and enhanced survival induced by PTP inhibition after Cr(VI) exposure. This PTP inhibitor-mediated checkpoint bypass is associated with Plk1 activation as well as with modulation of expression and/or localization of Plk1 and phospho-Tyr15 Cdk1. Furthermore, Plk1 overexpression in wild-type (wt) S.cerevisiae enhanced clonogenic survival and mutagenesis after Cr(VI) exposure. We propose that (i) Plk1 is necessary to bypass the G2/M checkpoint after DNA damage concurrent with upregulation of survival signaling through maintenance of tyrosine phosphorylation and (ii) Plk1 is a key determinant in the bypass of the G2/M checkpoint after genotoxic stress in normal cells, which can foster neoplastic progression.
Materials and methods
Cell culture and experimental treatment of cells
HLFs (American Type Culture Collection, Manassas, VA) were maintained and treated with sodium chromate (Na2CrO4·4H2O) (J.T. Baker, Phillipsburg, NJ) in the absence or presence of the PTP inhibitor, sodium orthovanadate (SOV, Na3VO4) (Sigma, St. Louis, MO) as we have described previously (6). GW843682X [Plk1 inhibitor: 5-(5,6-dimethoxy-1H-benzimidazol-1-yl)-3-[2-(trifluoromethyl)-benzyl]oxythiophene-2-carboxamide] was a kind gift from GlaxoSmithKline R&D (Research Triangle Park, NC) (17). Treatment with GW843682X was for 30 min prior to any other treatment at a final dose of 0.25 μM. Other chemicals were from Fisher Scientific (Pittsburgh, PA) and/or Sigma, unless indicated otherwise. For all experiments, cells were incubated at 37°C for 24 h prior to treatment.
Clonogenic survival
Cells were seeded at 105 per 60 mm dish. Following treatment, cells were collected by trypsinization, washed and reseeded at 2 × 102 per 60 mm dish and colonies were stained as described previously (6).
Mitotic index
Mitotic index was determined as described previously (18). Briefly, HLFs were seeded at 2.5 × 105 per 100 mm dishes, treated with the respective agents, washed and fixed in 70% ethanol. The cells were then incubated with an anti-phospho-Ser 10 histone H3 polyclonal antibody (Upstate, Billerica, MA) and followed by an Alexa 488-conjugated secondary antibody (Invitrogen, Carlsbad, CA). Cells were costained with propidium iodide and analyzed with a FACSort flow cytometer (Becton Dickinson, Franklin Lakes, NJ). The percentage of cells in the G0/G1, S and G2/M regions as well as those containing phosphorylated histone H3, i.e. undergoing mitosis, was determined with 10 000 cells.
Immunoblotting
Total protein lysates were extracted and immunoblotting was performed as we have described previously (16). Antibodies used were as follows: monoclonal Plk1 antibody (Upstate), polyclonal phospho-Cdk1 (Tyr15) antibody (Cell Signaling, Danvers, MA) and β-actin (Sigma).
Determination of Plk1 tyrosine phosphorylation
Immunoprecipitation for pan-phosphotyrosine was carried out as described previously (16), using Catch and Release Phosphotyrosine, clone 4G10 Immunoprecipitation Kit (Millipore, Billerica, MA), followed by immunoblotting with anti-Plk1 antibody (Cell Signaling). Reciprocal immunoprecipitation of Plk1 was performed as described below and immunoprecipitates were probed for pan-phosphotyrosine.
Immunoprecipitation and in vitro kinase assay
Immunoprecipitation and in vitro kinase assays were carried out with slight modifications as described previously by Ha et al. (15). Cells were seeded at 2.4 × 106 cells per 150 mm dishes. Following treatment, cells were harvested and lysed by sonication in TGN (50 mM Tris (pH 7.5), 50 mM glycerophosphate, 150 mM NaCl, 10% glycerol, 1% Tween 20, 1 mM NaF, 1 mM NaVO4, 1 mM phenylmethylsulfonyl fluoride, 2 ug/ml pepstatin A, 5 ug/ml leupeptin, 10 ug/ml aprotinin, and 1 mM dithiothreitol (DTT), containing 1 tablet of complete mini protease inhibitor (Roche, Switzerland)/10 ml) buffer. Whole-cell extracts were precleared with protein A-agarose (Calbiochem, Gibbstown, NJ) and Plk1 was immunoprecipitated with anti-Plk1 monoclonal antibody and protein A-agarose. Plk1 immunoprecipitates were incubated with 5 μg α-casein (Sigma) in kinase buffer [20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 150 mM KCl, 10 mM MgCl2, 1 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 0.5 mM dithiothreitol and 5 mM NaF] with the addition of 10 μM adenosine triphosphate and 4 μCi [γ-32P]adenosine triphosphate for 15 min at 30°C. Reactions were stopped by addition of sodium dodecyl sulfate loading buffer (100 mM Tris–HCl, pH 6.8, 4% sodium dodecyl sulfate, 20% glycerol, 0.2% bromophenol blue, 200 mM dithiothreitol and 11.7% β-mercaptoethanol) and heating for 5 min at 95°C. Proteins were resolved on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes and analyzed by both autoradiography for 32P and immunoblotting for Plk1 after decay of two half-lives. The relative quantity of immunoreactive and 32P-labeled proteins was determined by densitometry.
Immunofluorescence
Cells were seeded at 1.2–2 × 104 per well in eight-well chamber slides (Nunc International, Rochester, NY). Following treatment, cells were rinsed with phosphate-buffered saline (PBS) and fixed with 3.7% paraformaldehyde, as we have described previously (16). The cells were permeabilized with 0.1% Triton-X 100 and blocked in a buffer containing 1% bovine serum albumin/1% heat-inactivated fetal bovine serum. The cells were then incubated with Plk1 antibody, washed with PBS, reblocked and incubated with goat anti-mouse IgG secondary antibody conjugated to Alexa 488 (Molecular Probes, Carlsbad, CA). Following incubation, the cells were washed with PBS, stained with Hoechst nuclear dye (1:1000) (Molecular Probes) and mounted. The cells were visualized at ×63 using a Zeiss laser scanning microscope with ZEN software (Hercules, CA), at excitation wavelengths of 488 and 405 nm for anti-Plk1 and Hoechst, respectively.
Transfection
All transfections were performed with the Amaxa nucleofector system as described previously (16). Myc-tagged kinase-inactive mutant K82R Plk1 and constitutively active mutant T210D Plk1 were cloned into a pRcCMV vector and were a kind gift from Dr Erich Nigg (Max-Planck Institute, Germany). HLFs were transfected with 1–2 μg wt Plk1, T210D Plk1, K82R Plk1 or vector control. After 24–48 h, cells were treated with the respective agents and analyzed. Plasmid expression was confirmed at 24, 48 and 72 h posttransfection. The average transfection efficiency achieved was between 80 and 90%.
Yeast strains
The yeast strains used were SJR0751 (MATΔ ade2-101oc his3Δ200 ura3ΔNco lys2ΔBgl leu2-R), a kind gift of Dr Sue Jinks-Robertson (19). YCplac111-GAL1-HA-EGFP and YCplac111-GAL1-HA-EGFP-PLK plasmids were a kind gift from Dr KS Lee (National Cancer Institute, Bethesda, MD). Yeast transformation was carried out by the lithium acetate method. Stock cultures were stored at −80°C in glycerol-containing YEPD [200 μl 50% glycerol, 700 μl YEPD (1% yeast extract, 2% Bacto-peptone and 2% glucose) and 100 μl culture]. Prior to experimental procedures, stock cultures were streaked on YEPD agar plates and grown for 2–4 days in a 30°C incubator until colonies were visible. Plates were subsequently stored at 4°C and used for ∼4 weeks. Yeasts were maintained on YEP medium (1% yeast extract and 2% Bacto-peptone) containing 2% glucose, 2% raffinose or 2% galactose (G) (dependent on plasmid expression as described below) and essential amino acids and nutrients to select for plasmid maintenance.
Saccharomyces cerevisiae treatment and clonogenic survival
Individual colonies were picked from stock plates and grown in 5 ml YEP-raffinose in a shaker at 250 r.p.m. for >18 h at 30°C. Overnight cultures (0.5 ml) were subcultured in 4.0 ml of YEPD or YEP-galactose (YEPG) and were grown to mid-log phase (∼2 × 107 cells/ml) for 2 h. Cultures were then incubated for 2 h with the indicated concentration of Cr(VI). The 10-fold increased Cr(VI) concentrations used in the yeast studies are equitoxic to those used for HLFs. After treatment, cells were collected by centrifugation and washed with PBS. Approximately 400 cells were plated onto YEPD or YEPG and allowed to grow for 3 days. Colonies were counted and data were expressed as percent survival of control (untreated) cultures.
CAN1 mutagenesis assay
Yeasts were grown and treated as described for clonogenic survival. Following treatment, cells were washed with PBS and counted. Yeasts were then plated on synthetic complete agar plates minus arginine (SC-arg), but containing L-canavanine (60 mg/ml), a toxic analogue of arginine. Cultures (1 × 107 cells) were grown on SC-arg plates at 30°C for up to 7 days. In parallel, ∼200 cells were grown on normal YEPD or YEPG to account for plating viability. L-canavanine-resistant colonies were counted ∼5 to 7 days after plating. Spontaneous mutation frequencies were determined by the method of the median. Mutation frequency was determined relative to plating viability.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 4.00 as described in ref. 16.
Results
Plk1 is required for the PTP inhibitor to enhance clonogenic survival after Cr(VI) exposure
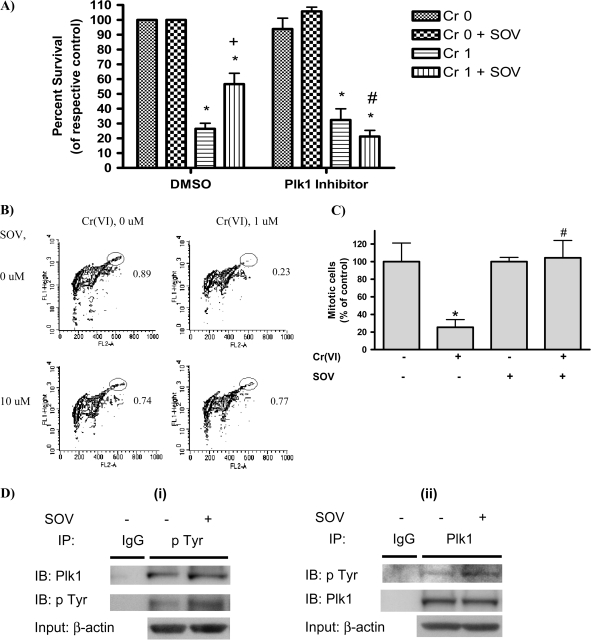
It is known that Plk1 activity is necessary for mitotic progression following recovery from DNA damage (9) and that Plk1 silencing results in growth inhibition and apoptosis in some cancer cells (20). Moreover, Plk1 activation has been demonstrated downstream of tyrosine phosphorylation-regulated pathways (7,8). Therefore, we postulated that inhibition of Plk1 would abrogate the enhanced clonogenic survival induced by PTP inhibition after Cr(VI) exposure. As shown in Figure 1A, Cr(VI) (1 μM) decreased clonogenic survival of HLFs by 73.5%. However, PTP inhibition with 10 μM SOV partially reversed Cr(VI)-induced clonogenic lethality and increased survival (2.1-fold) after Cr(VI) treatment. However, Plk1 inhibition with GW843682X (17) abrogated the ability of the PTP inhibitor to increase clonogenic survival after Cr(VI) treatment and restored Cr(VI) clonogenic lethality to 84.5%. The Plk1 inhibitor had no effect on clonogenic survival either alone or in the sole presence of Cr(VI).
Fig. 1.
Plk1 is required for the PTP inhibitor to enhance clonogenic survival after Cr(VI) exposure. (A) HLF cells were incubated with either dimethyl sulfoxide (DMSO) (vehicle) or GW843682X (Plk1 inhibitor) with or without 1 μM Cr(VI) for 24 h in the presence or absence of 10 μM SOV. Cells were washed and reseeded to allow for colony formation. Colonies were stained with crystal violet and counted 12 days later. The data are expressed as percent of respective control in the absence of Cr(VI) and are the means + standard error of four independent experiments. Asterisks indicates a statistically significant difference from the respective control at P < 0.05. Plus sign indicates a statistically significant difference in the presence versus the absence of SOV after 1 μM Cr(VI) treatment at P < 0.05. Hash symbol indicates a statistically significant difference in the presence versus the absence of the Plk1 inhibitor after 1 μM Cr(VI) + SOV treatment at P < 0.05. (B) HLF cells were coincubated with the indicated agents for 24 h, washed, collected and fixed. Mitotic cells were determined by flow cytometry with an antibody to phospho-histone H3 (pH3), followed by Alexa 488-conjugated secondary antibody. Contour plots show staining of pH3 as an index of mitosis (FL1-Height) and propidium iodide fluorescence (FL2-A) as an index of DNA content. The mitotic population is delineated by the elliptical gate. The numbers next to the mitotic population represent the respective mean of four experiments and are the percentage of mitotic cells within the total population. (C) Graphical representation of data in Figure 1B. Data are the proportion of mitotic cells after Cr(VI) exposure, expressed as a percentage of that in the non-Cr(VI)-treated control and are the means ± standard error of four independent experiments. Asterisk indicates a statistically significant difference from the respective control at P < 0.05. Hash symbol indicates a statistically significant difference between the samples treated with and without SOV at P < 0.05. (D) (i) Immunoprecipitation (IP) for phosphotyrosine was carried out using Catch and Release Phosphotyrosine, clone 4G10 Immunoprecipitation Kit according to the manufacturer's instruction (Millipore). HLFs at 24 h postseeding were treated with 10 μM SOV or medium alone for 1 h and protein lysates were added to the spin column prepacked with immunoprecipitation capture resin. Then, anti-phosphotyrosine 4G10 antibody or normal mouse IgG was added to the column and incubated at room temperature for 30 min. A protein lysate from medium-treated cells was used for mouse IgG immunoprecipitation. Proteins were eluted from the column with a denatured elution buffer followed by immunoblotting (IB) with anti-Plk1 or anti-phosphotyrosine 4G10 antibody. An immunoblot with anti-β-actin antibody is also shown for equal protein loading for the column input. Data are representative of three independent experiments for phosphotyrosine immunoprecipitation and the increase in pTyr in the presence of SOV compared with the absence of SOV is statistically significant at P < 0.05. (ii) The Plk1 immunoprecipitation was carried out as described above by utilizing a Catch and Release immunoprecipitation system with anti-rabbit Plk1 antibody (Cell Signaling). Data are representative of one experiment for Plk1 immunoprecipitation.
We have reported the ability of Cr(VI) to induce prolonged cell cycle arrest for a period of up to 7 days at both G1/S and G2/M checkpoints (15). We investigated the effect of PTP inhibition on mitotic progression in response to Cr(VI) exposure (1 μM for 24 h). G2/M checkpoint function, as reflected by suppression of entry into mitosis, was measured by flow cytometric assessment of histone H3 Ser 10-phosphorylation, which is selectively phosphorylated during mitosis (21). In control cells, 0.89% of the total cell population underwent mitosis (Figure 1B). Treatment with Cr(VI) blocked mitotic progression, as evident by a 3.8-fold decrease in the proportion of cells expressing phosphorylated histone H3 as compared with control (Figure 1C). In sharp contrast, Cr(VI) treatment in the presence of the PTP inhibitor did not block mitotic progression. Pretreatment with the PTP inhibitor increased the number of cells entering M phase by 3.3-fold after Cr(VI) exposure, which was similar to that observed in the control cells in the absence of Cr(VI) treatment (Figure 1B and C). PTP inhibition alone had no effect on G2–M transition, as the number of mitotic cells was comparable with that of control cells. We have shown previously that pretreatment with the PTP inhibitor does not alter uptake of Cr(VI) or the level of Cr(VI)-induced DNA damage (6).
Our data suggested that the enhanced clonogenic survival induced by PTP inhibition after Cr(VI) exposure is a result of enhanced mitotic progression. Furthermore, Plk1 was necessary for the PTP inhibitor-mediated increased clonogenic survival after Cr(VI) exposure (Figure 1A). It has been postulated that Plk1 functions downstream of receptor tyrosine kinases and a Plk1 consensus tyrosine phosphorylation site has been proposed, but not confirmed, at Tyr 217. Therefore, we investigated the ability of the PTP inhibitor to enhance tyrosine phosphorylation of Plk1. Immunoblots of Plk1 from phosphotyrosine immunoprecipitates showed a significant 2.8-fold increase in tyrosine phosphorylated Plk1 as early as 1 h after SOV treatment (Figure 1D(i)). Reciprocal immunoprecipitation of Plk1 showed a 1.5-fold increase in tyrosine phosphorylation (Figure 1D(ii)). There was no change in the level of tyrosine phosphorylated Plk1 upon SOV treatment for 24 h (data not shown).
Plk1 is required for the PTP inhibitor-induced mitotic progression after Cr(VI) exposure
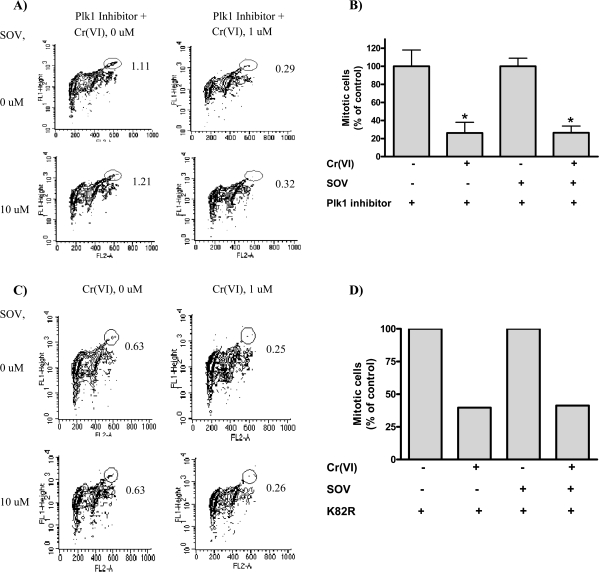
Because Plk1 has been shown to play a central role in the resumption of the G2/M checkpoint after DNA damage (9,22), we sought to determine whether Plk1 was required for G2/M checkpoint bypass induced by PTP inhibition after Cr(VI) exposure. The Plk1 inhibitor, GW843682X (17), had no effect either alone or in the presence of the PTP inhibitor on mitotic progression as measured by histone H3 phosphorylation (Figure 2A and as compared with Figure 1B). Furthermore, GW843682X did not alter the effect of Cr(VI) on mitosis: cotreatment with the Plk1 inhibitor and Cr(VI) resulted in a 4-fold decrease in mitotic cells (Figure 2A and B). In contrast, Plk1 inhibition abrogated the ability of the PTP inhibitor to bypass the Cr(VI)-induced cell cycle checkpoint. As seen in Figure 2A and B, the 4-fold decrease in mitotic cells after Cr(VI) exposure, which was reversed by the PTP inhibitor alone, was maintained in the combined presence of the PTP inhibitor and the Plk1 inhibitor.
Fig. 2.
Plk1 is required for the PTP inhibitor-induced mitotic progression after Cr(VI) exposure. (A) HLF cells were coincubated with or without the Plk1 inhibitor, GW843682X (GlaxoSmithKline R&D), SOV and Cr(VI) for 24 h, washed, collected and fixed. Mitotic cells were determined by histone H3 phosphorylation as described in the legend of Figure 1 and are the mean of four independent experiments. (B) Quantitative data of above experiment. Data are the means ± standard error of four independent experiments. Asterisk indicates a statistically significant difference from the respective control at P < 0.05. (C) HLF cells were transiently transfected with the kinase-inactive K82R mutant. Cells were treated with the respective agents for 24 h at 48 h posttransfection and then washed, collected and fixed. Mitotic cells were determined as described in the legend of Figure 1 and are representative of one experiment. (D) Quantitative data of above experiment.
We further confirmed these findings with a genetic approach by using a Plk1 kinase-inactive mutant, K82R (11,23). Transfection with the K82R mutant induced a 4.2- to 5.2-fold increase in Plk1 total protein expression 24–72 h posttransfection, compared with empty vector alone (supplementary Figure 1A is available at Carcinogenesis Online). Moreover, in vitro kinase activity of the K82R mutant was dramatically decreased when compared with the T210D constitutively active Plk1 mutant (supplementary Figure 1B is available at Carcinogenesis Online). Consistent with results obtained utilizing the Plk1 inhibitor, HLFs transiently transfected with K82R were blocked from entering mitosis after Cr(VI) exposure (Figure 2C and D). Interestingly, expression of the K82R mutant had no effect on mitotic progression alone. However, cells expressing the Plk1 kinase-inactive mutant exhibited a ∼4-fold decrease in mitotic progression after both PTP inhibition and Cr(VI) treatment, thus abrogating the effect of the PTP inhibitor on cell cycle checkpoint bypass. Consistent with previous reports, overexpression of the constitutively active Plk1 mutant, T210D (11,24), was sufficient to bypass Cr(VI)-induced cell cycle arrest and increased the number of cells entering mitosis by 3-fold after Cr(VI) exposure, compared with vector-transfected HLFs (supplementary Figure 1C–E is available at Carcinogenesis Online).
PTP inhibition during Cr(VI) exposure is associated with alterations in Plk1 activity, expression and localization
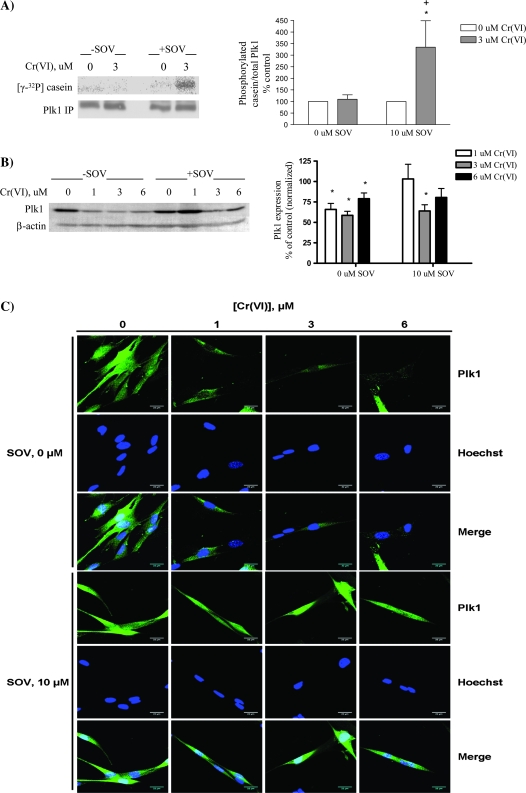
Since Plk1 was necessary for the PTP inhibitor to promote mitotic progression and enhance survival after Cr(VI)-induced genotoxic stress, we studied Plk1 expression and activation (in vitro) after Cr(VI) exposure in the presence and absence of PTP inhibition. Plk1 kinase activity, as expressed as the amount of α-casein phosphorylated by Plk1 and normalized to total Plk1 immunoprecipitated, was unchanged by either treatment with Cr(VI) or in the presence of the PTP inhibitor alone (Figure 3A). Notably, there was a 3.5-fold induction of Plk1 kinase activity after PTP inhibition and Cr(VI) coexposure. This increased kinase activity was attributed specifically to the activation of Plk1 as no Plk1-immunoreactive protein was present when preimmune serum was used for immunoprecipitation (supplementary Figure 1B is available at Carcinogenesis Online).
Fig. 3.
PTP inhibition during Cr(VI) exposure is associated with alterations in Plk1 activity, expression and localization. HLF cells were treated with the respective agents for 24 h, and cellular protein was extracted. (A) Plk1 was immunoprecipitated and used in an in vitro kinase assay with α-casein as a substrate. Proteins from each reaction were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and Plk1 phosphorylation was detected by 32P incorporation. Plk1 protein in the immunoprecipitates was analyzed by western blotting. Data are expressed as percentage of control, in the absence of Cr(VI), and are the means ± standard error of four independent experiments. Asterisk indicates a statistically significant difference from the respective control at P < 0.05. Plus sign indicates a statistically significant difference between the samples treated with and without SOV at P < 0.05. (B) Total protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Plk1 was detected by immunoblotting, and expression was normalized to that of β-actin. Data are expressed as percentage of control, in the absence of Cr(VI), and are the means ± standard error of four independent experiments. Asterisk indicates a statistically significant difference from the respective control at P < 0.05. (C) HLF cells were seeded in chamber slides and incubated with the indicated concentrations of Cr(VI) ± SOV. Cells were fixed and stained with Plk1 antibody followed by Alexa 488-conjugated fluorochrome. Cells were then stained with Hoechst nuclear dye and visualized at ×63 using a Zeiss laser scanning microscope with ZEN software, at excitation wavelengths of 488 and 405 nm for anti-Plk1 and Hoechst, respectively. Data represented in Results are the average of three independent experiments where at least 40 cells per experimental condition were scored per experiment. Images are representative of one independent experiment. Row 1: anti-Plk1 in the presence of Cr(VI); row 2: corresponding Hoechst staining of cells in row 1; row 3: merged images of row 1 and 2; row 4: anti-Plk1 in the presence of Cr(VI) and SOV; row 5: corresponding Hoechst staining of cells in row 4; row 6: merged images of rows 4 and 5.
We next examined Plk1 protein expression after Cr(VI) exposure in HLFs since it has been shown that Plk1 protein expression decreases in response to DNA damage (25). Indeed, there was a significant decrease in Plk1 protein expression after exposure to Cr(VI) for 24 h (Figure 3B). PTP inhibition alone had no significant effect on Plk1 total protein expression, which was similar to that of the untreated control. Plk1 protein expression was also decreased after Cr(VI) exposure in the presence of the PTP inhibitor; however, this was significant only with 3 μM Cr(VI). Since PTP inhibition had no consistently significant effect on Cr(VI)-induced Plk1 protein expression, we next investigated Plk1 subcellular localization.
It has been reported that localization of Plk1 from the cytoplasm to the nucleus is critical for mitotic progression (26). Since Plk1 in vitro kinase activity was increased after PTP inhibition in the presence of Cr(VI), we examined the subcellular localization of Plk1 by immunofluorescence in HLFs treated with increasing concentrations of Cr(VI) in the presence and absence of PTP inhibition. As shown in Figure 3C, Plk1 was distributed throughout the nucleus and cytoplasm in untreated control cells. However, nuclear Plk1 staining was markedly decreased with increasing concentrations of Cr(VI) [∼73% of cells at 1 μM Cr(VI) and 90% at 3 and 6 μM Cr(VI) displayed decreased nuclear Plk1], indicative of either nuclear exit or degradation of Plk1 after Cr(VI) exposure (Figure 3C, row 1). Indeed, merged images (Figure 3C, row 3) of Hoechst-stained nuclei (Figure 3C, row 2) with Plk1 staining (Figure 3C, row 1) show decreased nuclear Plk1 with increasing concentrations of Cr(VI). HLFs treated with the PTP inhibitor alone showed similar subcellular distribution of Plk1 to that of the control cells, but PTP inhibition dramatically altered Plk1 localization in response to Cr(VI). The presence of the PTP inhibitor reversed the loss of nuclear Plk1 (Figure 3C, row 4 and row 6), i.e. nuclear localization of Plk1 after Cr(VI) exposure was maintained in the presence of the PTP inhibitor (∼87% of the cells exhibited nuclear Plk1 distribution).
PTP inhibition abrogates the Cr(VI)-induced increased phosphorylation of Cdk1 on Tyr15
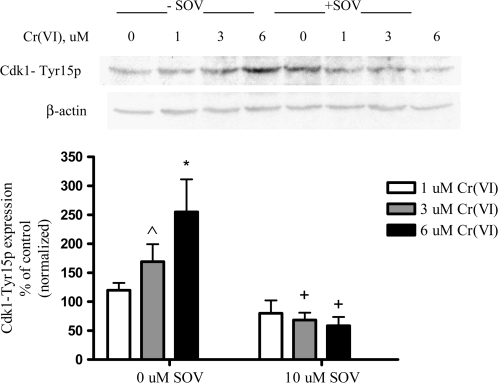
Cdk1 phosphorylation status is associated with G2/M progression and can be regulated by Plk1 after DNA damage (27,28). In response to DNA damage, the inhibitory phosphorylation of Cdk1 on Tyr15 is maintained in order to arrest cells at the G2/M checkpoint, and consequently, the dephosphorylation of this residue is a critical step in mitotic progression (27,28). Since the bypass of the cell cycle checkpoint after Cr(VI) exposure and PTP inhibition corresponded to an increase in Plk1 kinase activity and nuclear localization (Figure 3), we investigated the phosphorylation status of Cdk1 on Tyr15 under these conditions. Cr(VI) induced a concentration-dependent increase (1.7- to 2.5-fold) in the level of Cdk1-Tyr15 phosphorylation (Figure 4). PTP inhibition alone had no effect on Cdk1-Tyr15 phosphorylation in the absence of Cr(VI) (data not shown). In sharp contrast, PTP inhibition significantly abrogated the Cr-induced increase in Cdk1-Tyr15 phosphorylation.
Fig. 4.
PTP inhibition decreases phosphorylation of Tyr15 on Cdk1. (A) HLF cells were treated with the respective agents for 24 h, and cellular protein was extracted. Total protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Cdk1 tyr15p was detected by immunoblotting, and expression was normalized to that of β-actin. Data are expressed as percentage of control, in the absence of Cr(VI), and are the means ± standard error of three independent experiments. Asterisk indicates a statistically significant difference from the respective control at P < 0.05. Cap symbol indicates a difference from the respective control at P = 0.0827. Plus sign indicates a statistically significant difference in the presence versus the absence of SOV at P < 0.05.
Plk1 enhances survival of wt Saccharomyces cerevisiae after Cr(VI) treatment
Taken together, our data suggest that Plk1 activation is a key determinant for the PTP inhibitor-induced G2/M checkpoint bypass and enhanced clonogenic survival in normal mammalian cells after Cr(VI) exposure. Therefore, we investigated if Plk1 activation alone was sufficient to enhance survival after Cr(VI) exposure. We utilized wt S.cerevisiae overexpressing mammalian Plk1, which allowed for stable inducible expression of Plk1 throughout the course of our studies.
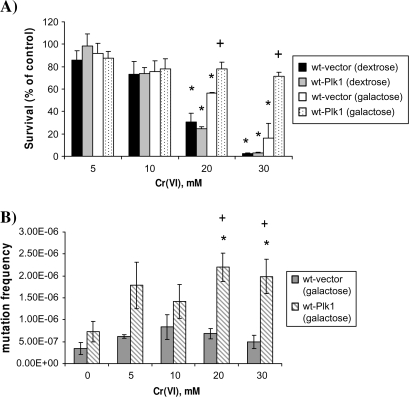
Saccharomyces cerevisiae were stably transformed with plasmids that expressed human Plk1 under control of the Gal1 promoter (29). Culturing the yeast in galactose for inducible conditions or dextrose for non-inducible conditions controlled the expression of these plasmids and Plk1 expression was verified by immunoblotting (supplementary Figure 2 is available at Carcinogenesis Online). As can be seen in Figure 5A, Cr(VI) induced a concentration-dependent decrease in survival. Moreover, yeast transformed with Plk1, but grown under non-inducible conditions (dextrose), displayed a similar sensitivity toward Cr(VI) lethality as wt yeast grown in dextrose. Yeast transformed with a control vector, and grown under inducible conditions (galactose), also displayed sensitivity to Cr(VI). In sharp contrast, yeast transformed with Plk1 under inducible conditions displayed increased survival at both 20 mM Cr(VI) (2.5-fold increase compared with respective control) and 30 mM Cr(VI) (15-fold increase compared with respective control).
Fig. 5.
Plk1 enhances survival of wt Saccharomyces cerevisiae after Cr(VI) treatment. (A) Transformed yeasts were grown in YEP-raffinose to start culture (∼24 to 48 h) and then cultured overnight in YEPG or YEPD. Cells were then subcultured in fresh YEPD (no Plk1 expression) or YEPG (Plk1 expression) to mid-log phase growth (∼2 × 107 cells/ml). Cultures were incubated for 2 h with the indicated concentration of Cr(VI). After treatment, ∼400 cells were plated onto YEPD or YEPG plates and allowed to grow for 3 days. Colonies were counted and data were expressed as percent survival of control (untreated) cultures. Data are the mean + standard error of three independent experiments. Asterisk indicates statistically significant difference from the respective control at P < 0.05. Plus sign indicates a significant difference between yeast expressing Plk1 and not expressing Plk1 at the specified concentration of Cr(VI) at P < 0.05. (B) Yeast transformants (wt-vector and wt-Plk1) were grown in YEP-raffinose to start culture (∼24 to 48 h) and then cultured overnight in YEPG. Cells were then subcultured in fresh YEPG to mid-log phase growth (∼2 × 107 cells/ml). Cultures were incubated for 2 h with the indicated concentration of Cr(VI). Following treatment, yeasts were washed, counted and plated on synthetic complete agar minus arginine (SC-arg), but containing L-canavanine (60 mg/ml), a toxic analogue of arginine, and were grown at 30°C for 7 days. In parallel, ∼200 cells were grown on normal YEPG to account for plating viability. L-canavanine-resistant colonies were counted and mutation frequency was determined relative to plating viability. Spontaneous mutation frequencies were determined by the method of the median. Data are mean ± standard error of three independent experiments. Asterisk indicates statistically significant difference from the respective untreated control at P < 0.05. Plus sign indicates a significant difference between yeast expressing Plk1 and not expressing Plk1 at the specified concentration of Cr(VI) at P < 0.05.
Plk1 expression increases Cr(VI)-induced mutagenesis in wt Saccharomyces cerevisiae
We postulated that the Plk1-induced increase in survival of wt S.cerevisiae treated with Cr(VI) was associated with an increase in Cr(VI)-induced mutagenesis. To test this, control vector and Plk1-transformed S.cerevisiae were exposed to increasing concentrations of Cr(VI) and cells were plated on L-canavanine-containing medium. L-canavanine is a toxic arginine analog that is taken up by yeast cells through the CAN1 transporter. Consequently, mutations occurring in the CAN1 gene can lead to L-can resistance. Vector-transformed S.cerevisiae showed no significant increase in mutation frequency after Cr(VI) exposure. In sharp contrast, Plk1-overexpressing yeast showed a significant increase in Cr-induced mutation frequency at both 20 and 30 mM (Figure 5B).
Discussion
Activation of survival signaling pathways is critical in the promotion of cell proliferation and the acquisition of an unrestricted growth phenotype, which are hallmarks of neoplastic transformation (30). There is considerable evidence that protein tyrosine phosphorylation is responsible for the maintenance of proliferative signals involved in the early stages of neoplasia (3). Studies by us and others have shown that PTP inhibitors, such as SOV, promote activation of key survival signaling pathways by increasing tyrosine phosphorylation of a number of substrates (5,6,16,31,32) We reported that PTP inhibition enhances clonogenic survival and Cr(VI)-induced mutation frequency in normal diploid mammalian cells, through a mechanism involving an override of Cr(VI)-induced growth arrest (6). Several studies performed in cancer cells have reported that Plk1 is necessary for cell cycle progression after DNA damage (17,33) and that Plk1 depletion induces growth inhibition and apoptosis (20). Therefore, we investigated the role of Plk1 as a fundamental regulator of cell cycle progression and survival after Cr(VI)-induced DNA damage in normal (i.e. non-transformed and non-cancerous) cells. Our data implicate Plk1 activity and localization in the early stages of carcinogenesis.
It has been postulated that Plk1 functions downstream of receptor tyrosine kinases (8). In support of this, the murine Sak serine–threonine kinase (homologous to the Plk/Polo family members) has been shown to be tyrosine phosphorylated by Tec (7). It has been suggested that the kinases Pak1, Ste20-like kinase, Stk10 and protein kinase A can phosphorylate the kinase domain of Plk1 on Thr210, Ser137 and Ser49 (24,34,35). However, the identity of upstream tyrosine kinases in the regulation of human Plk1 activity remains to be determined. Masuda et al. (8) demonstrated that STI571, which selectively inhibited the tyrosine kinase activity of Bcr–Abl, reduced the kinase activity of Plk1 in K562 myelogenous leukemia cells, suggesting that tyrosine kinases may be located upstream of signaling cascades that regulate Plk1 activity. Consistent with this, our data suggest an increase in Plk1 tyrosine phosphorylation after treatment with the PTP inhibitor. Furthermore, our data indicate a necessity for PTP-regulated signaling pathways in Plk1 activation and nuclear localization, as well as mitotic progression after DNA damage. The identification of tyrosine phosphorylation-regulated pathways upstream of Plk1 is a subject of ongoing investigation in our laboratory.
The current investigation highlights a potential PTP inhibitor-induced G2/M checkpoint bypass by Plk1 after genotoxic Cr(VI) exposure. We show that PTP inhibition is associated with an increase in Plk1 activity. It is known that Plk1 in vitro and in vivo activity decreases after DNA damage induced by ultraviolet, infrared radiation and adriamycin (11,12,36). The decreased Plk1 activity may be cell type-specific and/or context dependent on the basal activation state since we do not observe a decrease in Plk1 kinase activity with Cr(VI) treatment. Interestingly, we found a significant increase in Plk1 in vitro kinase activity in the combined presence of the PTP inhibitor and Cr(VI), under conditions in which a marked change in Plk1 localization, as well as a significant decrease in Cdk1-Tyr15p was observed. Therefore, increased Plk1 activity is involved in the PTP inhibitor-induced mitotic progression after Cr(VI) exposure.
The nuclear localization of Plk1 is necessary for mitotic progression (26,37). Plk1 accumulates in both nucleus and cytoplasm during S and G2 phases of the cell cycle and the nuclear translocation of Plk1 is regulated by a nuclear localization signal sequence (26). Disruption of the Plk1 nuclear localization signal sequence results in a G2/M arrest (26), indicating the importance of Plk1 nuclear localization for mitotic progression. Our data are consistent with this report in that Plk1 is absent from the nucleus after Cr(VI) exposure. Moreover, PTP inhibition relocates Plk1 to the nucleus in the presence of Cr(VI). Therefore, our data support the concept that Plk1 nuclear localization is required for the enhanced G2–M transition induced by PTP inhibition.
Entry into mitosis is dependent on Cdk1 activation (38). In response to DNA damage, the inhibitory phosphorylation of Cdk1 on Tyr15 is maintained in order to arrest cells at the G2/M checkpoint, and consequently, the dephosphorylation of this residue is a critical step in mitotic progression (27,28). To provide further insight into the cell cycle arrest induced by Cr(VI) and the bypass of this checkpoint by PTP inhibition, we investigated the phosphorylation status of Cdk1 on Tyr15. After Cr(VI) exposure, we observe an increase in Cdk1-Tyr15 phosphorylation (Figure 3), which is indicative of Cdk1 inhibition and G2/M cell cycle arrest. Interestingly, our data indicate a decrease in Cdk1 Tyr 15 phosphorylation after PTP inhibition and Cr(VI) treatment, consistent with mitotic progression.
We investigated the necessity of Plk1 in PTP-regulated survival signaling using both pharmacological and genetic approaches in normal human cells. We utilized the Plk1 inhibitor, GW843682X, which is at least 100-fold selective for Plk1 against other non-Plk kinases tested, as described by Lansing et al. (17). This inhibitor has been reported to inhibit Plk3; however, Plk3 was not detected in the HLFs used in the present study (data not shown). We observed that Plk1 inhibition with GW843682X had no effect on normal cell cycle progression in keeping with other reports (17,33). However, GW843682X abrogated the ability of PTP inhibition to bypass the G2/M checkpoint after Cr(VI) exposure. These results were confirmed by transient transfection of HLFs with the Plk1 kinase-inactive mutant, K82R, suggesting the necessity of Plk1 in the PTP inhibitor-induced bypass of the G2/M checkpoint after Cr(VI) exposure. Taken together, our data highlight an association between protein tyrosine phosphorylation-mediated survival signaling and Plk1 after DNA damage.
The PTP inhibitor-induced bypass of the G2/M checkpoint was not related to a difference in Cr-DNA binding, as PTP inhibition had no effect on either Cr uptake or Cr-DNA adduct levels (6). However, Cr(VI) is a complex genotoxicant and we cannot rule out the possibility that PTP inhibition may alter the ratio and/or repair of specific lesions generated by this DNA-damaging agent. Cr-induced replication arrest is directly caused by Cr binding to DNA (39,40). For instance, Cr(VI) reduction leads to Cr-monoadducts to both DNA bases and sugar-phosphate backbone, strand breaks, oxidized bases, DNA–protein cross-links, abasic sites, Asc-Cr(VI) adducts and DNA–Cr–DNA interstrand cross-links (39). We and others have identified the role of ataxia telangiectasia mutated (ATM) and the excision repair pathways in repair of Cr-induced DNA lesions (15,18). Therefore, it remains possible that the effect of PTP inhibition on the Cr-induced G2/M checkpoint may be related to activation of DNA damage response/repair pathways. Consistent with this, recent studies suggest a requirement for Plx1 (Xenopus Plk1 orthologue) in DNA replication after stalled replication forks by suppressing the ATM/ATM and Rad3-related-dependent intra-S-phase checkpoint (41). Of note, ATM is required for Cr(VI)-induced terminal growth arrest, whereas Plk1 is inhibited by DNA damage that is dependent on ATM/ATM and Rad3-related (36). Consequently, it is plausible that PTP inhibition (through Plk1 activation), or Plk1 overexpression, facilitates bypass of the intra-S-phase checkpoint after Cr(VI) exposure. However, in our studies, Cr(VI) induced an intra-S-phase checkpoint only at concentrations that are much higher than those employed in the present study (42). Our ongoing studies are focused on investigating the potential role of Plk1 in the fidelity of DNA repair mechanisms after DNA damage.
The expression of the constitutively active Plk1 (T210D) mutant was sufficient to bypass the DNA damage checkpoint after 24 h Cr(VI) exposure in non-synchronized, normal diploid human cells. Furthermore, Plk1 may function as a prosurvival trigger mechanism in S.cerevisiae, which further confirms the necessity of Plk1 activation in mitotic progression after DNA damage induced by Cr(VI). These data are consistent with the finding that expression of constitutively active Plk1 in osteosarcoma cells resulted in a partial rescue of the G2 block after nocodazole release following adriamycin treatment (11). However, our data are the first to show that Plk1 activation is sufficient for normal human cells to enter mitosis after DNA damage as well as enhance survival and mutagenesis in wt S.cerevisiae. Interestingly, HLFs transiently transfected with the T210D mutant did not exhibit enhanced clonogenic survival after Cr(VI) treatment (data not shown). Taken together, these data suggest the potential need for prolonged Plk1 activation to affect survival after genotoxic stress.
The dysregulation of cell cycle checkpoints after DNA damage could result in genomic instability and progression toward neoplastic transformation. Indeed, aberrant Plk1 activity is found in a variety of tumors, but little is known regarding the role of Plk1 in early stages of the DNA damage response in normal diploid cells and its contribution to carcinogenesis. Our data show that inappropriate activation of Plk1 through upregulation of tyrosine phosphorylation-dependent survival signaling in normal cells facilitates mitotic progression and survival after genotoxic exposure and contributes to genomic instability and mutagenesis. These studies provide new insight into the potential for increased mutagenesis as a result of the loss of checkpoint control by maintenance of tyrosine phosphorylation and highlight a potential role of Plk1 in early-stage neoplastic progression after genotoxic stress.
Supplementary material
Supplementary Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (ES05304 and ES09961 to S.R.P. and CA107972 to S.C.); PhRMA Foundation Predoctoral Fellowship to G.C.
Supplementary Material
Acknowledgments
The authors thank GlaxoSmithKline for the Plk1 inhibitor, GW843682X, Dr Timothy Lansing for helpful discussions, Dr Kyung S. Lee for providing the yeast Plk1 plasmids and for helpful discussions and Dr Erich A.Nigg for providing the Plk1 T210D and K82R plasmids. This work was conducted in partial fulfillment of the requirements for the PhD degree in Pharmacology, Columbian Graduate School of Arts and Sciences, The George Washington University.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ATM
ataxia telangiectasia mutated
- HLF
human lung fibroblast
- PBS
phosphate-buffered saline
- Plk1
Polo-like kinase 1
- PTP
protein tyrosine phosphatase
- SOV
sodium orthovanadate
- wt
wild-type
References
- 1.Kastan MB, et al. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 2.O'Connell MJ, et al. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
- 3.Ostman A, et al. Protein-tyrosine phosphatases and cancer. Nat. Rev. Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 4.Chernoff J. Protein tyrosine phosphatases as negative regulators of mitogenic signaling. J. Cell. Physiol. 1999;180:173–181. doi: 10.1002/(SICI)1097-4652(199908)180:2<173::AID-JCP5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Furlong F, et al. PTPase inhibition restores ERK1/2 phosphorylation and protects mammary epithelial cells from apoptosis. Biochem. Biophys. Res. Commun. 2005;336:1292–1299. doi: 10.1016/j.bbrc.2005.08.260. [DOI] [PubMed] [Google Scholar]
- 6.Bae D, et al. Bypass of hexavalent chromium-induced growth arrest by a protein tyrosine phosphatase inhibitor: enhanced survival and mutagenesis. Mutat. Res. 2008;660:40–46. doi: 10.1016/j.mrfmmm.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita Y, et al. Sak serine-threonine kinase acts as an effector of Tec tyrosine kinase. J. Biol. Chem. 2001;276:39012–39020. doi: 10.1074/jbc.M106249200. [DOI] [PubMed] [Google Scholar]
- 8.Masuda Y, et al. [beta]-Hydroxyisovalerylshikonin induces apoptosis in human leukemia cells by inhibiting the activity of a polo-like kinase 1 (PLK1) Oncogene. 22:1012–1023. doi: 10.1038/sj.onc.1206200. [DOI] [PubMed] [Google Scholar]
- 9.van Vugt MA, et al. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 10.van Vugt MA, et al. Checkpoint adaptation and recovery: back with Polo after the break. Cell Cycle. 2004;3:1383–1386. doi: 10.4161/cc.3.11.1248. [DOI] [PubMed] [Google Scholar]
- 11.Smits VA, et al. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- 12.Tsvetkov L, et al. Phosphorylation of Plk1 at S137 and T210 is inhibited in response to DNA damage. Cell Cycle. 2005;4:166–171. doi: 10.4161/cc.4.1.1348. [DOI] [PubMed] [Google Scholar]
- 13.Toczyski DP, et al. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 14.Syljuasen RG, et al. Adaptation to the ionizing radiation-induced G2 checkpoint occurs in human cells and depends on checkpoint kinase 1 and Polo-like kinase 1 kinases. Cancer Res. 2006;66:10253–10257. doi: 10.1158/0008-5472.CAN-06-2144. [DOI] [PubMed] [Google Scholar]
- 15.Ha L, et al. Chromium (VI) activates ataxia telangiectasia mutated (ATM) protein. Requirement of ATM for both apoptosis and recovery from terminal growth arrest. J. Biol. Chem. 2003;278:17885–17894. doi: 10.1074/jbc.M210560200. [DOI] [PubMed] [Google Scholar]
- 16.Lal MA, et al. AKT1 mediates bypass of the G1/S checkpoint after genotoxic stress in normal human cells. Cell Cycle. 2009;8:1589–1602. doi: 10.4161/cc.8.10.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lansing TJ, et al. In vitro biological activity of a novel small-molecule inhibitor of polo-like kinase 1. Mol. Cancer Ther. 2007;6:450–459. doi: 10.1158/1535-7163.MCT-06-0543. [DOI] [PubMed] [Google Scholar]
- 18.Ha L, et al. Generation of S phase-dependent DNA double-strand breaks by Cr(VI) exposure: involvement of ATM in Cr(VI) induction of gamma-H2AX. Carcinogenesis. 2004;25:2265–2274. doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- 19.Swanson RL, et al. Overlapping specificities of base excision repair, nucleotide excision repair, recombination, and translesion synthesis pathways for DNA base damage in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999;19:2929–2935. doi: 10.1128/mcb.19.4.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bu Y, et al. Silencing of polo-like kinase (Plk) 1 via siRNA causes inhibition of growth and induction of apoptosis in human esophageal cancer cells. Oncology. 2008;74:198–206. doi: 10.1159/000151367. [DOI] [PubMed] [Google Scholar]
- 21.Crosio C, et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 2002;22:874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Vugt MA, et al. Getting in and out of mitosis with Polo-like kinase-1. Oncogene. 2005;24:2844–2859. doi: 10.1038/sj.onc.1208617. [DOI] [PubMed] [Google Scholar]
- 23.Golsteyn RM, et al. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang YJ, et al. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J. Biol. Chem. 2002;277:44115–44120. doi: 10.1074/jbc.M202172200. [DOI] [PubMed] [Google Scholar]
- 25.Martin BT, et al. Polo-like kinase 1: target and regulator of transcriptional control. Cell Cycle. 2006;5:2881–2885. doi: 10.4161/cc.5.24.3538. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi E, et al. Nuclear translocation of plk1 mediated by its bipartite nuclear localization signal. J. Biol. Chem. 2002;277:48884–48888. doi: 10.1074/jbc.M206307200. [DOI] [PubMed] [Google Scholar]
- 27.Roshak AK, et al. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell. Signal. 2000;12:405–411. doi: 10.1016/s0898-6568(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, et al. Polo-like kinase 1 may regulate G2/M transition of mouse fertilized eggs by means of inhibiting the phosphorylation of Tyr 15 of Cdc2. Mol. Reprod. Dev. 2007;74:1247–1254. doi: 10.1002/mrd.20703. [DOI] [PubMed] [Google Scholar]
- 29.Lee KS, et al. Plk is a functional homolog of Saccharomyces cerevisiae Cdc5, and elevated Plk activity induces multiple septation structures. Mol. Cell. Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D, et al. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen WL, et al. Effects of SOV-induced phosphatase inhibition and expression of protein tyrosine phosphatases in rat corneal endothelial cells. Exp. Eye Res. 2005;81:570–580. doi: 10.1016/j.exer.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto J, et al. Sodium orthovanadate enhances proliferation of progenitor cells in the adult rat subventricular zone after focal cerebral ischemia. J. Pharmacol. Exp. Ther. 2006;318:982–991. doi: 10.1124/jpet.106.104562. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, et al. Normal cells, but not cancer cells, survive severe Plk1 depletion. Mol. Cell. Biol. 2006;26:2093–2108. doi: 10.1128/MCB.26.6.2093-2108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr FA, et al. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 35.Ellinger-Ziegelbauer H, et al. Ste20-like kinase (SLK), a regulatory kinase for polo-like kinase (Plk) during the G2/M transition in somatic cells. Genes Cells. 2000;5:491–498. doi: 10.1046/j.1365-2443.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 36.van Vugt MA, et al. Inhibition of Polo-like kinase-1 by DNA damage occurs in an ATM- or ATR-dependent fashion. J. Biol. Chem. 2001;276:41656–41660. doi: 10.1074/jbc.M101831200. [DOI] [PubMed] [Google Scholar]
- 37.Lee KS, et al. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc. Natl Acad. Sci. USA. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter LA, et al. Cyclin B1 and CDK1: nuclear localization and upstream regulators. Prog. Cell Cycle Res. 2003;5:335–347. [PubMed] [Google Scholar]
- 39.O'Brien TJ, et al. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Bridgewater LC, et al. Arrest of replication by mammalian DNA polymerases alpha and beta caused by chromium-DNA lesions. Mol. Carcinog. 1998;23:201–206. [PubMed] [Google Scholar]
- 41.Trenz K, et al. Plx1 is required for chromosomal DNA replication under stressful conditions. EMBO J. 2008;27:876–885. doi: 10.1038/emboj.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Brien TJ, et al. Effects of hexavalent chromium on the survival and cell cycle distribution of DNA repair-deficient S. cerevisiae. DNA Repair (Amst.) 2002;1:617–627. doi: 10.1016/s1568-7864(02)00078-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.