Abstract
We describe for the first time the chemopreventive effects of S-(−)equol and R-(+)equol, diastereoisomers with contrasting affinities for estrogen receptors (ERs). S-(−)equol, a ligand for ERβ, is an intestinally derived metabolite formed by many humans and by rodents consuming diets containing soy isoflavones. Whether the well-documented chemopreventive effect of a soy diet could be explained by equol's action was unclear because neither diastereoisomers had been tested in animal models of chemoprevention. Sprague–Dawley rats (n = 40–41 per group) were fed a soy-free AIN-93G diet or an AIN-93G diet supplemented with 250 mg/kg of S-(−)equol or R-(+)equol beginning day 35. On day 50, mammary tumors were induced by dimethylbenz[a]anthracene and thereafter, animals were palpated for number and location of tumors. On day 190, animals were killed and mammary tumors were removed and verified by histology, and the degree of invasiveness and differentiation was determined. S-(−)equol and R-(+)equol plasma concentrations measured on days 35, 100 and 190 by tandem mass spectrometry confirmed diet compliance and no biotransformation of either diastereoisomer. In this model, S-(−)equol had no chemopreventive action, nor was it stimulatory. In contrast, R-(+)equol compared with Controls reduced palpable tumors (P = 0.002), resulted in 43% fewer tumors (P = 0.004), increased tumor latency (88.5 versus 66 days, P = 0.003), and tumors were less invasive but showed no difference in pattern grade or mitosis. Both enantiomers had no effect on absolute uterine weight but caused a significant reduction in body weight gain. In conclusion, the novel finding that the unnatural enantiomer, R-(+)equol, was potently chemopreventive warrants investigation of its potential for breast cancer prevention and treatment.
Introduction
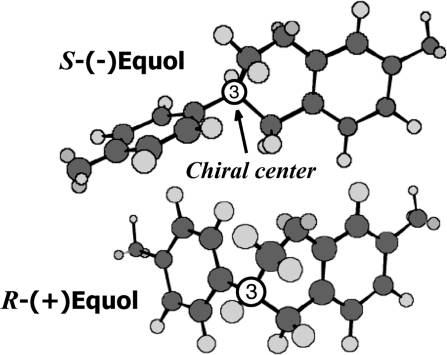
The chemopreventive effect of soy isoflavones is well established from the results of many in vitro studies of human cancer cell lines and in animal models of cancer (1–3). Troll et al. (4) first showed in an X-ray irradiated animal model of breast cancer that exposure to soy resulted in a reduction in the number of tumors formed. Some years later, the anticancer effects of soy protein were confirmed in the dimethylbenz[a]anthracene (DMBA) and the N-methyl-N-nitrosourea chemically induced animal models of mammary cancer (5). A reduction in the number of mammary tumors was observed, in a dose-dependent manner, when animals were fed purified soy protein, and this effect was lost when the soy protein was devoid of its isoflavones (5). Soy contains two principal isoflavones, daidzein and genistein, that naturally occur in soybeans and soy foods in the form of glycoside conjugates (6). Interest in the anticancer effects of soy isoflavones focused mainly on genistein, in part because it was found to be a potent inhibitor of tyrosine kinases (7) and to influence growth factors and other pathways that play a role in carcinogenesis (2,8). Genistein was subsequently shown to have impressive chemopreventive actions on mammary cancer, especially when administered early in life (2,9,10) where it was shown to influence mammary gland morphology and development (11). In contrast to these animal models of chemoprevention, genistein was shown in an athymic mouse model to stimulate the growth of transplanted human MCF-7 breast cancer cells (12,13), findings that have led to confusion regarding the potential risks or benefits of soy and isoflavones for women with breast cancer (3). Notwithstanding the effectiveness of genistein as a chemopreventive agent (9), the fact remains that when soy-based diets are fed to rats, it is the metabolite equol and not genistein that becomes the major circulating isoflavone (14). Therefore, the issue of whether equol could explain the chemopreventive effect of soy has remained a tantalizing one. Until recently, few studies were possible on equol because it was not commercially available in quantities sufficient to test in animal models. This problem has been resolved with the description of several methods for the synthesis of pure equol (15–17). Of further intrigue is the fact that equol can occur in two enantiomeric forms, S-(−)equol and R-(+)equol (18), but this fact seems to have evaded many investigators because most studies of equol have used the commercially available racemic form (Figure 1). A comparison of the in vitro estrogenicity of the two enantiomers shows that S-(−)equol has a relatively high affinity for estrogen receptor (ER)β when compared with estradiol, whereas R-(+)equol has low affinity for either ERs ERα or ERβ (15,19,20). We have recently shown that intestinal bacteria of humans and rats produce exclusively the S-(−)equol enantiomer when fed either soy protein or pure daidzein (20). Our recent development of a method for the large-scale synthesis of enantiomeric pure S-(−)equol and R-(+)equol by chiral chemistry has been a major breakthrough (16), permitting for the first time animal studies on the chemopreventive properties of both equol enantiomers. We now report on the chemopreventive effects of S-(−)equol and R-(+)equol using the classical animal model of DMBA chemically induced breast cancer, a model that was first used to demonstrate the anticancer actions of soy isoflavones (5).
Fig. 1.
Comparison of the conformational structures of the diastereoisomers of the soy isoflavone metabolite equol.
Materials and methods
Animals
Sprague–Dawley rats, purchased from Charles River Breeding Laboratories (Raleigh, NC), were placed on a soy-free AIN-93G diet and bred in-house to obtain female pups for the study. On parturition, litters were reduced to 10 pups per litter retaining all females. All rats were housed in an AAALAC-accredited facility that meets or exceeds the Animal Welfare Act requirements, and the study protocol (#5B09057) was approved by the Children's Hospital Research Foundation's Animal Use Committee.
Chemicals and reagents
S-(−)equol and R-(+)equol were synthesized in a six-step reaction sequence by the enantioselective hydrogenation of a 3,4-chromene structure formed in daidzein after initial protection of the hydroxyl groups by formation of the monomethyl ethers, reduction and elimination of the resulting C-4 hydroxyl group. After asymmetric hydrogenation by specific iridium catalysts, removal of the protecting groups by hydrolysis and recrystalization, the final products S-(−)equol and R-(+)equol were produced to >99.5% enantioselective purity. The exact method of bulk synthesis of these enantiomers is described in US Patent 2007/0027329A1 and PCT WO 2007/016423A2 (16). Nuclear magnetic resonance spectroscopy and chiral high-performance liquid chromatography–mass spectrometry analysis were used to confirm the chemical structure and purity of both enantiomers.
The 10% neutral buffered formalin was obtained from Fisher Scientific, Pittsburgh, PA. Isoflurane (‘Iso Flo’) was obtained from Abbott Laboratories, Abbott Park, IL. All other chemicals were purchased from Sigma Chemicals (St Louis, MO) unless specified otherwise.
Animal diets
The AIN-93G soy-free/isoflavone-free diet was obtained from Dyets (Bethlehem, PA). The macrocomposition of this rodent diet was 18.7% protein, 7% fat and 64.7% carbohydrate and it had a metabolizable energy of 3.97 kcal/g. The S-(−)equol and R-(+)equol-containing diets were prepared by Dyets to provide a content of 250 mg equol enantiomer per kilogram of the AIN-93G diet. The equol composition of each batch was verified by a chiral high-performance liquid chromatography method described previously (20,21).
Study design
To investigate the chemopreventive effects of dietary S-(−)equol and R-(+)equol against chemically induced mammary cancer, female Sprague–Dawley rats bred in-house were fed a soy-free AIN-93G diet from birth to 35 days of age, then separated into three groups. Group 1 (Control group, n = 40) continued on this diet, whereas the two other groups of animals were fed the AIN-93G diet supplemented with 250 mg/kg of either S-(−)equol (Group 2, n = 40) or R-(+)equol (Group 3, n = 41) beginning on day 35 until killing on day 190. Treatment was begun on day 35 to compare findings for equol with previous studies using soy protein or isoflavones in this animal model (5,9,22). It was also intended to represent exposure to equol enantiomers from puberty continuing throughout adult life. A dose of 250 mg equol per kilogram diet was selected to allow comparison of our data with previous studies of genistein and daidzein (9,23) in this model and because at this dose, plasma S-(−)equol concentrations are similar to those for humans consuming modest amounts of soy foods when adjusted for allometric scaling between rodents and humans (23,24). Furthermore, these concentrations compare with those measured in human adults given 10–30 mg equol either as a supplement (25) or as a pure stable-isotopically labeled standard (26) and are lower, but in the range of plasma equol concentrations of most laboratory rats fed commercial soy-containing diets, such as Purina 5008 (14). On day 50 postpartum, the carcinogen, DMBA was administered by gavage (80 mg/kg body wt dissolved in <1.0 ml sesame oil) to induce mammary tumors. Animals were weighed weekly and palpated twice weekly, and the number and location of tumors were recorded until the animals were killed at 190 days of age, when tumors reached 2.5 cm or when tumors were necrotic. At killing, tumor and uterine weights were recorded, and all tumors were fixed in 10% neutral buffered formalin for 48 h, blocked in paraffin, sectioned at 5 μ and stained with hematoxylin and eosin. All suspected mammary tumors were verified histologically, and histological analyses were performed by a certified pathologist blinded to the treatment groups. Classification of tumor tissue has been addressed by a number of researchers, with extensive work comparing human and rat tumors (27–31). Tumor tissue was analyzed utilizing the Nottingham-Bloom-Richardson and Scarff-Bloom-Richardson grading systems as modified for tumors in the DMBA rat model of mammary cancer (32). Thus, tumor pattern was classified as grade 1, 2 or 3 depending on the extent of solid area within the tumor; tubule formation analysis was based on the extent of cribriform structures, and the extent of necrosis and degree of cellular infiltration were evaluated. Tumor proliferation was determined by counting the number of cells in mitosis in 10 fields of hematoxylin and eosin stained tumor sections (×400) from all animals in the three groups. Also degree of invasiveness of tumors from all animals in each group was determined. The degree of tumor invasiveness was assessed by assigning nominal values of ‘0’ to ‘non-invasive’ tumors and ‘1’ to invasive tumors.
Blood samples were collected at days 35, 100 and 190 to determine plasma equol concentrations. For rats 35 days of age, an additional number of animals (n = 6) were included in each group to confirm compliance to the diets. Blood (2–3 ml) was collected by intracardiac stick from rats anesthetized with isoflurane followed by CO2 inhalation and killing. To check diet compliance at 100 days, blood (0.5–1 ml) was collected under anesthesia via tail vein stick from six rats, one animal from six different cages, and the animals were allowed to recover and continue in the tumorigenesis study. At the termination of the study on day 190, blood (2–3 ml) was taken from one animal per cage by intracardiac stick from rats anesthetized with isoflurane followed by CO2 inhalation and killing. All blood samples were allowed to clot at room temperature and centrifuged at 3000 r.p.m. for 10 min and the plasma was removed and stored at −20°C until analyzed.
Analytical techniques
Plasma S-(−)-equol or R-(+)equol concentrations were determined by liquid chromatography tandem mass spectrometry with multiple reaction monitoring (MRM) using stable-isotopic labeled [2,3,4-13C3]equol as the internal standard and in positive ion mode. In summary, the method involved addition of the stable-labeled internal standard to plasma (0.25–0.50 ml), solid-phase extraction, hydrolysis of conjugates by a mixed β-glucuronidase and sulfatase preparation (Helix pomatia), re-extraction by solid-phase adsorption and conversion of equol enantiomers to their dansylated derivatives. Total (sum of conjugated and unconjugated forms) S-(−)equol and R-(+)equol were detected and quantified by monitoring the MRM transition ion m/z 709→170 generated by fragmentation and loss of the dansyl group, and the internal triply labeled standard was detected from the corresponding MRM transition m/z 712→170. For the separate determination of only unconjugated S-(−)equol and R-(+)equol, the same procedure was used with omission of the enzymatic hydrolysis step. The concentrations of S-(−)equol and R-(+)equol in plasma were determined from the peak area ratio of each ion relative to the peak area response from the internal standard and by interpolation of this area ratio against calibration curves plotted for known concentrations of S-(−)equol and R-(+)equol. The intra-assay and inter-assay precisions were 4–8% expressed as coefficient of variation for samples containing S-(−)equol and R-(+)equol at a concentration of 50 ng/ml. The lower limit of quantification of the assay was 1 ng/ml and the limit of detection for both enantiomers was 0.1 ng/ml.
Sample size calculation
The proposed number of animals to use per group was calculated from previous studies (5,11) in which the number of tumors per rat in the Control group was estimated to be 7 ± 3.8 (mean ± SD). We hypothesized that the greatest average tumor reduction per animal (35%) would be observed in those rats exposed to S-(−)equol. To detect this reduction, the number of animals needed per group was calculated based on a two sample t-test at alpha = 0.05 level to detect the specified differences with 80% power. The number of Controls was determined based on the smallest difference we desired to detect (since the same Control animals were used for all comparisons). The minimum number for each group was estimated to be n = 39. Additional animals were used per group as they were available from the litters bred and, therefore, ∼40 animals per group were used.
Data analysis
Tumor data were analyzed by several approaches. The longitudinal measurements of palpable tumors were first analyzed using zero inflated negative binomial distributions, reflecting a high zero count in the data. The group specific means of the additionally palpated tumors were assumed to be increasing over time and modeled by an exponential function of the logarithm of post-DMBA time. The probabilities of inflated zero counts decreasing over time were modeled by a logistic function of the logarithm of post-DMBA time. We used the proc nlmixed procedure of SAS version 9.1.3 for computation. The group mean tumor growth curves were estimated by the cumulative sum of the estimated means of the additionally palpated tumors over time. The mean tumor growth curves were compared among the groups by a permutation test, pairwise. Both treatment groups were compared with the Controls and then treatment groups were compared with each other. A total of 500 resamples were generated by permutation and the difference between the mean tumor growth curves of two groups under comparison was estimated in each resample. We used the permutation test as the SAS proc nlmixed procedure did not allow a flexible modeling of dependency among the longitudinal measurements taken within rats, or across rats or within dams, and it is robust to the distributional assumption of the analysis.
We also analyzed the total number of palpated tumors, the number of tumors per animal identified at dissection, the number of animals per group without tumors and the time to appearance of the first tumor using an independent t-test.
Histological evaluation of the tumor pattern grade, proportion of cribriform and papillary structures, extent of necrosis and cellular infiltration were analyzed by contingency table analysis testing the differences in the distributions of histological parameters across groups. Each parameter was evaluated separately by the Pearson Chi Square test Statistic. Analpha level = 0.05 was assumed to determine statistical significance. The data were re-analyzed using log linear analyses, and results were essentially unchanged. Analysis for tumor proliferation and invasiveness was performed by non-parametric analysis, namely the student’s t-test.
Data for body weight gain curves were analyzed by a non-linear mixed effects model. The method accounts for dependency among the weight measurements taken on the same rat and permits the measurement to grow non-linearly over time. Random coefficient effects both at the dam and the individual rat level were included to allow differential growth of the weight over time among different dams and different rats in the same group. We considered an autoregressive moving average correlation structure to account for the dependency among the weight measurements taken on the same rat. We compared different autoregressive moving average structures using model fit statistics such as Akaike Information Criterion and chose autoregressive moving average (2,2). The body weight measurements were also log transformed to meet the constant variance assumption of the non-linear mixed effects. The goodness of the fit was examined graphically via residual plots.
Results
Plasma equol concentrations
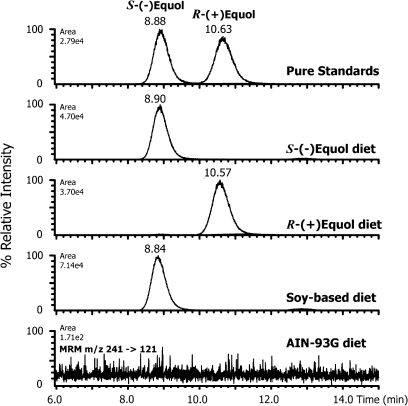
Using chiral-phase high-performance liquid chromatography–mass spectrometry that specifically separates the two enantiomers of equol (18,20), the plasma obtained at day 100 and 190 from animals fed S-(−)equol contained exclusively S-(−)equol, whereas that of animals fed R-(+)equol contained only R-(+)equol. There was no indication of any biotransformation by racemization of either enantiomer following its absorption. Figure 2 compares electrospray liquid chromatography tandem mass spectrometry chromatograms for the MRM transition m/z 242→121 for underivatized equol enantiomers with those obtained following analysis of plasma extracts from animals fed each diet. As a reference, Figure 2 also includes the profile of a plasma sample taken from an animal fed a typical soy-containing laboratory diet (Formulab 5008 closed formula diet incorporating soymeal, Purina Company, St Louis, MO) and confirms that rats naturally produce exclusively S-(−)equol when consuming soy isoflavones (20).
Fig. 2.
Chiral phase liquid chromatography-tandem mass spectrometry profiles of the negative ion MRM transition m/z 242→121 for underivatized pure equol enantiomers compared with extracts of plasma samples collected on day 190 at killing from animals fed an AIN-93G soy free diet, and the same diet supplemented with S-(−)equol or R-(+)equol. As a reference, the profile of a plasma from an animal fed a soy-based commercial Formulab 5008 diet is shown where S-(−)equol is the exclusive enantiomer formed.
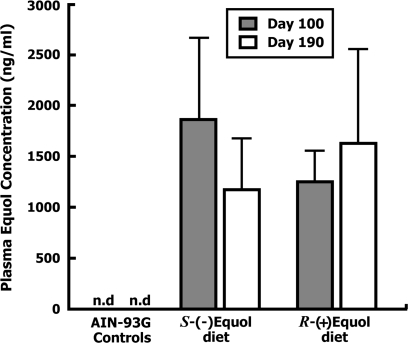
Plasma total equol concentrations (mean ± SD) measured on day 35 of age in a representative number of animals taken from each group were undetectable, or <1 ng/ml, consistent with the fact that all animals were consuming a soy-free diet up to that age. Mean (±SD) plasma equol concentrations at day 100 in six animals from each group were below the limit of quantification for the Control group, 1861 ± 825 ng/ml for animals fed S-(−)equol-containing diet and 1172 ± 380 ng/ml for those fed R-(+)equol diet. The plasma total equol concentrations did not differ significantly between the treatment groups, but the mean concentration in the treatment groups was expectedly higher than the Control group (P < 0.0001). On day 190 of age, the mean total equol concentration measured in plasma samples from one animal per cage, for a total of n = 17–21 animals per group again confirmed that animals fed the soy-free diet (Controls) had undetectable or only traces of equol (n = 21), whereas the plasma total equol concentrations of animals fed the S-(−)equol diet (1250 ± 422 ng/ml, n = 18) and R-(+)equol diet (1627 ± 917 ng/ml, n = 17) maintained a similarly high concentration to the levels measured on day 100 of age (Figure 3). Plasma total equol concentrations at day 190 in treatment groups were significantly greater (P < 0.0001) than that of the Control group.
Fig. 3.
Mean (±SD) plasma total equol concentrations (ng/ml) on day 100 (n = 6 samples per group) and 190 (n = 17–21 samples per group) of age from animals fed the AIN-93G soy-free diet and animals fed the same diet supplemented with 250 mg/kg S-(−)equol or R-(+)equol. n.d., not detected or below the lower limit of quantification of the assay. Both treatments had significantly higher equol levels in plasma compared with Controls (P < 0.001).
Unconjugated S-(−)equol and R-(+)equol concentrations were measured in a limited number of plasma samples (n = 10 per group) collected at the time of killing and were below the level of quantification in the animals from the Control group. The mean (±SEM) plasma unconjugated S-(−)equol concentration in animals fed the S-(−)equol diet was 13.9 ± 2.3 ng/ml, whereas in this group of plasma samples, the total S-(−)equol concentration was 1389.0 ± 266.6 ng/ml. For animals fed R-(+)equol, the corresponding values for unconjugated and total R-(+)equol concentration were 36.8 ± 8.4 ng/ml and 1812 ± 309 ng/ml, respectively. The unconjugated fraction of S-(−)equol and R-(+)equol circulating in plasma, therefore, represented 1.1 and 2.1%, respectively, of the total plasma equol concentrations.
Effect of equol enantiomers on tumorigenesis
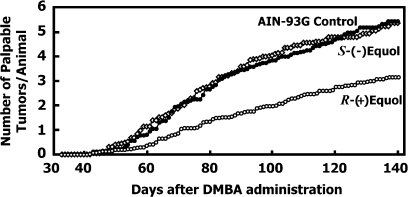
The mean number of palpable tumors per animal plotted over the time-course of the study for each group of animals is shown in Figure 4. There were no significant differences in the number of tumors found in animals fed a diet supplemented with S-(−)equol when compared with animals fed an AIN-93G isoflavone-free diet (P = 0.97). However, animals fed R-(+)equol had a significantly reduced number of palpable tumors over time when compared with Controls (P = 0.002). Furthermore, the number of palpable tumors formed per rat in the R-(+)equol-fed group was significantly lower than that of rats treated with S-(−)equol (P = 0.008).
Fig. 4.
Comparative effect of dietary S-(−)equol and R-(+)equol (250 mg/kg diet) fed from 35 days of age on the numbers of palpable tumors in the Sprague–Dawley rat model of DMBA chemically induced mammary carcinogenesis. The carcinogen was administered on age day 50. R-(+)equol-treated rats had significantly fewer tumors than Controls (P = 0.004).
At killing, additional small tumors were observed in all groups, and histological evaluation of all tumors showed that the R-(+)equol-fed animals had 43% fewer tumors than the control group and this difference was highly statistically significant (P = 0.004).
Tumor incidence and multiplicity
At the termination of the experiment, 5.1% of the animals in the Control group treated with DMBA were free of tumors. The proportion of animals that were free of tumors in the S-(−)equol and R-(+)equol-fed groups was 2.6 and 22.2%, respectively.
Taking into account only those animals that had tumors, the number of tumors/tumor-bearing animal was significantly lower in the animals fed R-(+)equol compared with Controls (3.3 ± 0.4 versus 5.5 ± 0.5, P = 0.004), as well as when compared with the S-(−)equol-fed animals (5.5 ± 0.6, P = 0.009).
At necropsy, the mean (±SEM) tumor weight per animal for R-(+)equol fed rats (5.3 ± 1.1 mg) was significantly reduced (P = 0.04) when compared with Controls (9.9 ± 1.4 mg). However, mean tumor weight per rat of R-(+)equol fed rats was not significantly different than S-(−)equol fed animals (8.9 ± 1.4 mg).
Tumor latency
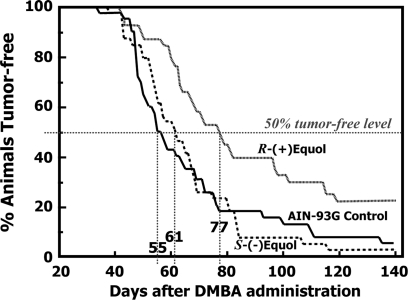
After administration of the carcinogen DMBA, the average time for appearance of the first palpable tumor per animal in the Control group was 66.0 days. The average number of days before the first palpable tumor appeared in those fed the S-(−)equol and R-(+)equol was 63.6 and 88.5 days, respectively. Feeding the R-(+)equol diet resulted in significantly increased tumor latency (P = 0.003). Tumor latency was also increased significantly (P = 0.004) in the R-(+)equol fed animals compared with the S-(−)equol fed animals. Furthermore, the time it took for 50% of the animals to develop tumors was longer in the R-(+)equol fed animals compared with the S-(−)equol fed or AIN-93G fed animals (Figure 5).
Fig. 5.
Kaplan–Meier type plots showing the time-course for the proportion of Sprague–Dawley rats that remained tumor free after administration of DMBA at day 50 of age. Rats were fed an AIN-93G soy-free diet, or an AIN-93G diet supplemented with 250 mg/kg of either S-(−)equol or R-(+)equol from age 35 days onwards. The time (days) it took for 50% of the animals to develop tumors is indicated.
Tumor histology
The majority of the tumors (>98%), independent of treatment diet, were adenocarcinomas, and >90% were of ductal origin. Adenocarcinomas with <25% solid area were assigned Grade I, tumors with 25–75% solid area were Grade 2 and Grade 3 tumors had >75% solid area. Results of that grading analysis, as well as the proportion of cribriform and papillary structures, necrosis and infiltration are given in Table I. In keeping with the results found by Costa (32), most DMBA-induced rat tumors showed a pattern of Grade 1 or Grade 2 with very few fitting the Grade 3 criteria. Results show that in Control tissue more than half (55.2%) of the tumors were Grade 1 with very low (<25%) solid cell mass area, this was similar to the majority of R-(+)equol tumors (54.3%) that also had a very low (<25%) solid cell mass area. S-(−)equol-treated rats had fewer tumors (44.8%) with <25% solid area, and more tumors (63.2%) which were Grade 2 or Grade 3 with >25% solid cell mass areas; however, statistical analysis showed no significant differences between the groups.
Table I.
Classification of tumors from the three treatment groups determining the pattern grade of the tumors, the proportion of tubular structures, the degree of necrosis and cellular infiltration
| Carcinoma pattern grade: amount of solid areas |
Proportion of cribriform and papillary structures |
Necrosis |
Cellular infiltrates |
||||||||||
| Group | Grade 1 < 25% | Grade 2 < 75% | Grade 3 > 75% | 70–100% Crib | 40–60% Crib | 0–30% Crib | Absent | Focal | Extensive | Light | Moderate | Pronounced | |
| Control | Tumor no. | 116 | 76 | 18 | 116 | 76 | 18 | 126 | 73 | 11 | 86 | 102 | 22 |
| % out of 210 | 55.2 | 36.2 | 8.6 | 55.2 | 36.2 | 8.6 | 60.0 | 34.8 | 5.2 | 41.0 | 48.6 | 10.5 | |
| S-equol | Tumor no. | 86 | 85 | 21 | 85 | 87 | 20 | 118 | 62 | 12 | 73 | 90 | 28 |
| % out of 192 | 44.8 | 44.3 | 10.9 | 44.3 | 45.3 | 10.4 | 61.5 | 32.3 | 6.3 | 38.0 | 46.9 | 14.6 | |
| R-equol | Tumor no. | 57 | 44 | 4 | 57 | 44 | 4 | 56 | 46 | 3 | 29 | 60 | 16 |
| % out of 105 | 54.3 | 41.9 | 3.8 | 54.3 | 41.9 | 3.8 | 53.3 | 43.8 | 2.9 | 27.6 | 57.1 | 15.2 | |
Analysis of the morphological features of the tumors was also similar to that described by Costa (32) with the majority of Control and R-(+)equol tumors evidencing 70–100% of the tissue with the cribriform pattern with glandular-like structure surrounding a variably sized lumen. S-(−)equol also had a high proportion (44.3%) but not a majority of the tumors with this pattern; however, again this difference was not significant.
Our evaluation of tumor necrosis did not place the percentage of tumors without necrosis as high as that described in Costa's work (≈90%), however, 50–60% of the tumors in all groups were necrosis free and another ≈30–40% had only focal necrosis. The evaluation of cellular infiltration was similar, with all three groups having 70–80% of the tumors with light to moderate infiltration and fewer tumors (≈30–40%) classified as ‘Light’ compared with ≈70% by Costa's analysis. There were no differences between groups in the extent of necrosis or cellular infiltration. Tumor proliferation counts were higher for controls than both S-(−)equol and R-(+)Equol (mean ± SD were 4.5 ± 4.3, 3.6 ± 3.6, 4.4 ± 4.0, respectively); however, there were no significant differences between the groups.
The degree of invasiveness of the tumors based on our histological score was not significantly different between animals fed the S-(−)equol diet (0.570 ± 0.5) when compared with Controls (0.55 ± 0.5). However, the tumors in animals fed the R-(+)equol diet were much less invasive as evidenced by a lower mean histological score (0.37 ± 0.49, P = 0.03) when compared with Controls. Similarly the extent of invasiveness of tumors was significantly less in the R-(+)equol versus S-(−)equol fed diets (P = 0.015).
Effect of equol enantiomers on the body and uterine weights
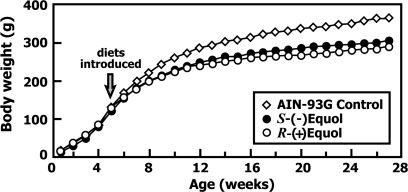
Body weight was monitored throughout the duration of the experiment beginning from day 1 of life (Figure 6). Animals fed the S-(−)equol or R-(+)equol diets beginning on day 35 showed significantly lower weight gains over time when compared with animals in the Control group (P < 0.001 for both groups). The weight gain in animals fed the R-(+)equol diet was also significantly less than those fed S-(−)equol (P = 0.04). Despite a lower weight gain, the animals were considered healthy based upon physical appearance, normal behavior and activity as assessed by standard veterinary criteria. The uterus of each animal was removed at killing and weighed. The mean (±SD) weight of Control uteri was 623 ± 21 mg. No significant differences were found between uterine weights of animals fed the equol-containing diets (586 ± 15 mg and 660 ± 20 mg, respectively, for S-(−)equol and R-(+)equol). However, when the uterine weights were expressed relative to body weight (milligram per gram), the ratios for the three groups were 1.8, 1.9 and 2.3 for Controls, S-(−)equol and R-(+)equol fed rats, respectively, and the ratio was significantly higher (P = 0.001) for R-(+)equol fed rats compared with Controls.
Fig. 6.
Mean body weight gain in animals fed an AIN-93G soy-free diet, or an AIN-93G diet supplemented with 250 mg/kg of either S-(−)equol or R-(+)equol from day 35 of life. Both treatment groups had significantly lower weight gain than Controls (P < 0.001).
Discussion
The two soy-derived isoflavones, genistein and daidzein (6), and the intestinal bacterial metabolite equol (33,34) have chemical structures strikingly similar to estradiol, thus accounting for their affinity for ERs (35–38). However, equol, unlike daidzein and genistein, is unique in chemical structure in having a chiral carbon at position C-3 of the furan ring. It can therefore occur in two distinct enantiomeric forms, S-(−)equol and R-(+)equol, and these differ significantly in conformational structure (Figure 1). Reported studies of equol, although sparse, have mostly used racemic (±)equol because when equol is chemically synthesized it is the racemate that is usually obtained, and this has been the chemical form that has been commercially available. Consequently, until recently little was known about the pharmacological and biological properties of the individual diastereoisomers (15,18,20,39).
Several years ago, we conclusively established that S-(−)equol was the sole enantiomer circulating in human plasma after consumption of soy foods (20) because intestinal microflora are enantiomeric specific in their synthesis of S-(−)equol from daidzein (20). This is also true for rodents fed commercial diets containing soy protein (20). This finding may be of clinical relevance because S-(−)equol shows preferential binding to ERβ (15,19,20), although its transcriptional activity on both ERs has been reported to be similar (15). Furthermore, this preferential binding affinity of S-(−)equol to ERβ is similar to that of the soy isoflavone genistein (37), which has been shown to be chemopreventive in the DMBA animal model of mammary cancer (9,11) and to that of the selective ER modulators, raloxifene and tamoxifen that are clinically proven to be effective in the prevention and treatment of patients with ER-positive breast cancer (40). R-(+)equol on the other hand has been reported to bind slightly better to ERα than ERβ in one report (15), and not in another (20), but its transcriptional potency on ERα (15) and that of the racemate (19) is reported as greater than S-(−)equol. These differences in properties pose intriguing questions regarding the potential role that S-(−)equol and R-(+)equol may play in chemoprevention. Specifically, is the natural S-(−) equol enantiomer (20) chemopreventive, and could this explain the effectiveness of soy-based diets in animal models of mammary cancer (5,22,41,42)? Also, could the unnatural enantiomer R-(+)equol have chemopreventive effects because it has the potential to induce transcription? Our studies are unique in describing for the first time the chemopreventive effects on mammary carcinogenesis of two essentially identical molecules that differ significantly in biological properties—we are unaware of another example where the chemopreventive properties of diastereoisomers have been compared. This was possible because of the ability to synthesize enantiomeric pure S-(−)equol and R-(+)equol in bulk quantity using chiral chemistry (16) and to incorporate the pure compounds into an AIN-93G diet soy-free diet.
Analysis of the diet fed to these animals (data not shown) confirmed that each batch contained the correct equol enantiomer. Furthermore, chiral phase liquid chromatography tandem mass spectrometry analysis of plasma collected on day 100 and at the end of the experiment from animals fed the S-(−)equol or R-(+)equol supplemented diets was found to contain exclusively the ingested enantiomers, thereby confirming that no racemization of the enantiomers had occurred after oral intake (Figure 2). This is consistent with a lack of biotransformation of S-(−)equol and R-(+)equol following oral administration to healthy adults (20,26). Plasma equol concentrations in animals fed an AIN-93G diet were either undetectable or at the lower limit of quantification of the assay in contrast to the high concentrations attained when animals were fed either S-(−)equol or R-(+)equol diets. In the latter case, the mean plasma equol concentrations at both time points it was measured were similar and were in the range reported for rats fed soy-containing diets (14). Overall, these findings confirmed compliance to the diets. Furthermore, the plasma concentrations achieved with a dietary intake of 250 mg/kg diet gave comparable plasma concentrations to humans consuming modest amounts of soy foods when adjusted for allometric scaling between rodents and humans (43) and higher than, but in a similar range to those reported for rats fed the equol precursor daidzein (23). The proportion of S-(−)equol and R-(+)equol circulating in plasma in unconjugated form was only 1.1 and 2.1%, respectively, of the total isoflavones, consistent with the major biotransformation pathway being phase II metabolism by conjugation to glucuronic and to a lesser extent sulphuric acids (26). Whether the higher proportion of unconjugated R-(+)equol circulating could account for the differences observed in chemoprevention of the two enantiomers is uncertain.
A number of different rat models can be used to study factors influencing breast cancer, and each has merits and limitations. We chose to examine the anticancer effect of equol enantiomers in the DMBA chemically induced rat model of mammary cancer because it is a well characterized and extensively used model of chemoprevention, as opposed to chemotherapy, and more importantly because the anticancer effect of soy isoflavones was originally established using this animal model (5). The model has the following merits: (i) mammary cancers can be reproducibly induced with high frequency; (ii) cancers formed are adenocarcinomas that, like most human breast cancers, are mainly of ductal origin (27,30); (iii) there is similarity in ontogeny of the rat and human mammary glands, as well as shared susceptibility of terminal end buds to carcinogens (28–30); (iv) the tumors formed in this classical animal model are estrogen sensitive; and (v) it was used in the development of anti-neoplastic drugs like tamoxifen (40). An alternative model we could have considered using is the ovariectomized athymic mouse model with transplanted human MCF-7 breast cancer cells; however, this metastatic cell line is not representative of primary breast cancer cells (44).
Using the DMBA-chemically induced rat model, our results show that S-(−)equol has no chemopreventive effect when fed to animals at a relatively high dose of 250 mg/kg diet from day 35 of life, just prior to exposure to the carcinogen. The numbers of palpable tumors that developed over the time-course of the study paralleled those observed for animals fed an AIN-93G diet that was free of isoflavones (Figure 4). In contrast, R-(+)equol, at the same daily intake to that of S-(−)equol, resulted in a highly significant 40% reduction (P = 0.004) in tumor burden when compared with the Control group. R-(+)equol and S-(−)equol clearly exhibit contrasting effects in this animal model of chemoprevention. The ineffectiveness of S-(−)equol was also evident from its complete lack of effect on tumor latency, tumor histology and degree of invasiveness of the tumors when compared with the Controls. In contrast, animals fed the R-(+)equol had significantly increased tumor latency and were less invasive when compared with animals fed the Control diet. R-(+)equol showed a more positive histological classification than S-(−)equol in pattern grade and proportion of cribriform structures, however, it appears that if resistance to DMBA initiation of tumorigenesis is overcome, the histological progression follows the pattern typically seen in control rats. The fact that there were no differences between control and R-(+)equol tumors in the mean number of mitotic cells per 10 fields may be accounted for by opposing processes. For example, racemic equol has been shown to increase apoptosis in human breast cancer cell lines (45).
The effect of the equol enantiomers on body weight and growth was particularly striking. Growth curves for animals fed the AIN-93G diet supplemented with 250 mg/kg S-(−)equol and R-(+)equol tracked significantly lower over the time-course of the study than for animals fed an AIN-93G diet alone (Figure 6). All of the animals fed equol-containing diets appeared healthy were normally active and ate and drank freely despite the lower rate of weight gain compared with animals fed the control diet. In fact, in parallel studies (data not shown), rats maintained on a commercial Purina 5008 diet, commonly used in many institutions, had significantly lower weight gains that those fed our control diet, whereas the growth curves of S-(−)equol and R-(+)equol fed animals differed only by <1 and 8%, respectively, from animals fed Purina 5008. Despite the similarity in mean plasma equol concentrations, the weight gain of animals fed R-(+)equol was lower than that of S-(−)equol fed animals (P = 0.04). The background diet used in our study is a soy-free and isoflavone-free diet. Diets containing soy protein tend to lead to reduced body weight gain, an effect that has been attributed to the presence of isoflavones. Lephart et al. (46) found that animals fed a high phytoestrogen diet displayed the lowest body and adipose tissue weights, whereas dietary equol, presumed to be racemic equol, was recently reported to attenuate weight gain in ovariectomized female rats (47). More recently, exposure of female rats to isoflavone aglycons led to a reduced weekly body weight gain, an effect caused by reduced food intake (48). Interestingly, a recent study of Caucasian, Japanese and Hawaiian women found higher soy food intake, and hence isoflavone intake, was associated with a lower body mass index (49). Estrogen administration has long been known to reduce food intake and decrease body weight gain (50,51). These effects have been suggested to be mediated through ERα as evidenced from the lack of estrogen's effect on food intake in the ERα-knockout mouse (52). The differential effects of estradiol and isoflavones on weight gain in male and female animals recently reported seem not to support the notion that this is an estrogen-mediated effect (48). It has been known for >50 years that calorie restriction reduces tumor burden in animal models of breast and other cancers (53–55). It however seems unlikely that the impressive chemopreventive effect of R-(+)equol is the result of reduced body weight because animals fed the identical diet containing its diastereoisomer, S-(−)equol, also had significantly reduced weight gain; yet, no chemopreventive effect was observed.
To our knowledge, little is known about equol's effect on the reproductive tract of the rat (56), but equol was responsible for an endometriosis in sheep, referred to as Clover disease, which devastated the sheep breeding industry in Southwest Australia in the 1940s (57). Other phytoestrogens, including genistein, are known to have effects on the reproductive tract of rodents, e.g. (58–60). However, in most of these studies, isoflavones were injected, which bypasses the normal first-pass metabolism that would alter significantly their pharmacodynamics and metabolism. Chronic feeding of R-(+)equol and S-(−)equol had no significant effects on the absolute uterine weight and this is an important observation in light of the concerns of potential uterotrophic effects of soy isoflavones, especially as there is much interest in developing equol as a nutraceutical or pharmaceutical agent (61,62). However, the uterus weight:body weight ratio was higher in animals fed R-(+)equol, the chemopreventive enantiomer, when compared with control animals or animals fed S-(−)equol and whether this reflects a subtle uterotrophic effect is unclear. Interestingly, when dietary daidzein, which is converted to S-(−)equol in the rat was fed during pregnancy and in the neonatal period at a comparable dose (250 mg/kg diet) to that used in our experiments on equol, there was little or no reproductive toxicity reported (23). Future clinical studies of equol will need to address the issue of effects on the endometrium, whereas animal studies should incorporate dose-response experiments with the equol enantiomers to investigate the potential for toxicity and mechanism of action.
To conclude, we report for the first time a comparison of the chemopreventive effects of the two enantiomeric forms of equol, S-(−)equol and R-(+)equol, two essentially identical molecules that have contrasting affinities for ER. Our studies show that S-(−)equol, the major metabolite of the soy isoflavone daidzein had no chemopreventive action in the classical animal model of DMBA chemically induced mammary cancer, a finding that seems to rule out our earlier notion that this metabolite could be responsible for the anticancer effect of soy diets in rodent models of breast cancer. Nevertheless, an extremely important finding from this study was the lack of stimulatory effect of S-(−)equol on tumor formation, which concurs with the recently reported lack of stimulatory effect of S-(−)equol in the athymic mouse model of implanted human MCF-7 tumor cells (63) and it is in stark contrast to the stimulatory effect of the soy isoflavone genistein in this same model (13). This is reassuring, particularly as S-(−)equol is being clinically developed for the treatment of prevention of other hormone-dependent conditions (61). Furthermore, our novel finding that the unnatural enantiomer, R-(+)equol was chemopreventive and increased tumor latency in this animal model warrants further investigations of its mechanism of action, especially given the wide-ranging biological effects of isoflavones and of its potential pharmacological action in breast cancer prevention or treatment. Finally, our studies also show that both R-(+)equol and S-(−)equol significantly reduces body weight and this tantalizing finding suggests that these enantiomers, independent of their potential in cancer prevention, may play a role in weight control or prevention of obesity, both of which are important risk factors for cancer at all sites.
Funding
National Institutes of Health (R01AT-003313) to K.D.R.S.
Acknowledgments
K.D.R.S. was the Principal Investigator who conceived and designed the studies with N.M.B. N.M.B. performed the carcinogenesis studies with the technical assistance of C.A.B. and S.L.L. The mass spectrometric analysis of equol was performed by L.Z.N. and X.Z. D.W. performed the histological analysis of mammary tumors. M.K. performed the statistical analysis of these data. The manuscript was written by K.D.R.S. and N.M.B. and all co-authors read and approved the manuscript.
Conflict of Interest Statement: K.D.R.S. has intellectual property on equol enantiomers, including patents licensed by Cincinnati Children's Hospital Medical Center, Cincinnati, to industry, and the other authors have nothing to declare.
Glossary
Abbreviations
- DMBA
dimethylbenz[a]anthracene
- ER
estrogen receptor
- MRM
multiple reaction monitoring
References
- 1.Messina MJ, et al. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 2.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J. Nutr. 1995;125:777S–783S. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 3.Messina MJ, et al. Soy for breast cancer survivors: a critical review of the literature. J. Nutr. 2001;131:3095S–3108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 4.Troll W, et al. Soybean diet lowers breast tumor incidence in irradiated rats. Carcinogenesis. 1980;1:469–472. doi: 10.1093/carcin/1.6.469. [DOI] [PubMed] [Google Scholar]
- 5.Barnes S, et al. Soybeans inhibit mammary tumors in models of breast cancer. Prog. Clin. Biol. Res. 1990;347:239–253. [PubMed] [Google Scholar]
- 6.Coward L, et al. Genistein, daidzein, and their β-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J. Agric. Food Chem. 1993;41:1961–1967. [Google Scholar]
- 7.Akiyama T, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 8.Kim H, et al. Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am. J. Clin. Nutr. 1998;68:1418S–1425S. doi: 10.1093/ajcn/68.6.1418S. [DOI] [PubMed] [Google Scholar]
- 9.Lamartiniere CA, et al. Genistein suppresses mammary cancer in rats. Carcinogenesis. 1995;16:2833–2840. doi: 10.1093/carcin/16.11.2833. [DOI] [PubMed] [Google Scholar]
- 10.Murrill WB, et al. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17:1451–1457. doi: 10.1093/carcin/17.7.1451. [DOI] [PubMed] [Google Scholar]
- 11.Lamartiniere CA, et al. Genistein alters the ontogeny of mammary gland development and protects against chemically-induced mammary cancer in rats. Proc. Soc. Exp. Biol. Med. 1998;217:358–364. doi: 10.3181/00379727-217-44245. [DOI] [PubMed] [Google Scholar]
- 12.Allred CD, et al. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- 13.Ju YH, et al. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J. Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 14.Brown NM, et al. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab. Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- 15.Muthyala RS, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Setchell KDR, et al. A method for the enantioselective synthesis of chromenes. 2005 US Patent 2007/0027329A1 and PCT WO 2007/016423A2. [Google Scholar]
- 17.Heemstra JM, et al. Total synthesis of (S)-equol. Org. Lett. 2006;8:5441–5443. doi: 10.1021/ol0620444. [DOI] [PubMed] [Google Scholar]
- 18.Setchell KDR, et al. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 19.Morito K, et al. Interaction of phytoestrogens with estrogen receptors α and β. Biol. Pharm. Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 20.Setchell KDR, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 21.Setchell KDR, et al. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006;136:2188–2193. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 22.Constantinou AI, et al. Inhibition of N-methyl-N-nitrosourea-induced mammary tumors in rats by the soybean isoflavones. Anticancer Res. 1996;16:3293–3298. [PubMed] [Google Scholar]
- 23.Lamartiniere CA, et al. Daidzein: bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol. Sci. 2002;65:228–238. doi: 10.1093/toxsci/65.2.228. [DOI] [PubMed] [Google Scholar]
- 24.Fritz WA, et al. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998;19:2151–2158. doi: 10.1093/carcin/19.12.2151. [DOI] [PubMed] [Google Scholar]
- 25.Setchell KDR, et al. The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women. J. Nutr. 2009;139:2037–2043. doi: 10.3945/jn.109.110874. [DOI] [PubMed] [Google Scholar]
- 26.Setchell KDR, et al. The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am. J. Clin. Nutr. 2009;90:1029–1037. doi: 10.3945/ajcn.2009.27981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo J, et al. Comparative study of human and rat mammary tumorigenesis. Lab. Invest. 1990;62:244–278. [PubMed] [Google Scholar]
- 28.Russo IH, et al. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz[a]anthracene. J. Natl Cancer Inst. 1978;61:1439–1449. [PubMed] [Google Scholar]
- 29.Russo J, et al. Pathology of tumours in laboratory animals. Tumours of the rat. Tumours of the mammary gland. IARC Sci. Publ. 1990;99:47–78. [PubMed] [Google Scholar]
- 30.Russo J, et al. “Biological and Molecular Basis of Breast Cancer”. Heidelberg, Germany: Springer-Verlag; 2004. [Google Scholar]
- 31.Meyer JS, et al. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod. Pathol. 2005;18:1067–1078. doi: 10.1038/modpathol.3800388. [DOI] [PubMed] [Google Scholar]
- 32.Costa I, et al. Histopathologic characterization of mammary neoplastic lesions induced with 7,12 dimethylbenz(alpha)anthracene in the rat: a comparative analysis with human breast tumors. Arch. Pathol. Lab. Med. 2002;126:915–927. doi: 10.5858/2002-126-0915-HCOMNL. [DOI] [PubMed] [Google Scholar]
- 33.Axelson M, et al. The identification of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl)chroman] in human urine. Biochem. J. 1982;201:353–357. doi: 10.1042/bj2010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Setchell KDR, et al. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984;40:569–578. doi: 10.1093/ajcn/40.3.569. [DOI] [PubMed] [Google Scholar]
- 35.Shutt DA, et al. Steroid and phyto-oestrogen binding to sheep uterine receptors in vitro. J. Endocrinol. 1972;52:299–310. doi: 10.1677/joe.0.0520299. [DOI] [PubMed] [Google Scholar]
- 36.Tang BY, et al. Effect of equol on oestrogen receptors and on synthesis of DNA and protein in the immature rat uterus. J. Endocrinol. 1980;85:291–297. doi: 10.1677/joe.0.0850291. [DOI] [PubMed] [Google Scholar]
- 37.Kuiper GG, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 38.Casanova M, et al. Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol. Sci. 1999;51:236–244. doi: 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- 39.Magee PJ, et al. Equol: a comparison of the effects of the racemic compound with that of the purified S-enantiomer on the growth, invasion, and DNA integrity of breast and prostate cells in vitro. Nutr. Cancer. 2006;54:232–242. doi: 10.1207/s15327914nc5402_10. [DOI] [PubMed] [Google Scholar]
- 40.Jordan VC, et al. Tamoxifen, raloxifene, and the prevention of breast cancer. Endocr. Rev. 1999;20:253–278. doi: 10.1210/edrv.20.3.0368. [DOI] [PubMed] [Google Scholar]
- 41.Gotoh T, et al. Chemoprevention of N-nitroso-N-methylurea-induced rat mammary carcinogenesis by soy foods or biochanin A. Jpn. J. Cancer Res. 1998;89:137–142. doi: 10.1111/j.1349-7006.1998.tb00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Constantinou AI, et al. Chemopreventive effects of soy protein and purified soy isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr. Cancer. 2001;41:75–81. doi: 10.1080/01635581.2001.9680615. [DOI] [PubMed] [Google Scholar]
- 43.Setchell KDR, et al. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J. Nutr. 2003;133:1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 44.Soule HD, et al. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 45.Choi EJ, et al. Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem. Biol. Interact. 2009;177:7–11. doi: 10.1016/j.cbi.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 46.Lephart ED, et al. Dietary isoflavones alter regulatory behaviors, metabolic hormones and neuroendocrine function in Long-Evans male rats. Nutr. Metab. (Lond.) 2004;1:16. doi: 10.1186/1743-7075-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rachon D, et al. Effects of dietary equol on body weight gain, intra-abdominal fat accumulation, plasma lipids, and glucose tolerance in ovariectomized Sprague-Dawley rats. Menopause. 2007;14:925–32. doi: 10.1097/GME.0b013e31802d979b. [DOI] [PubMed] [Google Scholar]
- 48.Kishida T, et al. Dietary soy isoflavone-aglycone lowers food intake in female rats with and without ovariectomy. Obesity (Silver Spring) 2008;16:290–297. doi: 10.1038/oby.2007.68. [DOI] [PubMed] [Google Scholar]
- 49.Maskarinec G, et al. Soy intake is related to a lower body mass index in adult women. Eur. J. Nutr. 2008;47:138–144. doi: 10.1007/s00394-008-0707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyons PM, et al. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am. J. Clin. Nutr. 1989;49:1164–1168. doi: 10.1093/ajcn/49.6.1164. [DOI] [PubMed] [Google Scholar]
- 51.Gong EJ, et al. Menstrual cycle and voluntary food intake. Am. J. Clin. Nutr. 1989;49:252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- 52.Geary N, et al. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 53.King JT, et al. The influence of estrogen on cancer incidence and adrenal changes in ovariectomized mice on calorie restriction. Cancer Res. 1949;9:436. [PubMed] [Google Scholar]
- 54.Kritchevsky D, et al. Calories, fat and cancer. Lipids. 1986;21:272–4. doi: 10.1007/BF02536411. [DOI] [PubMed] [Google Scholar]
- 55.Macrae FA. Fat and calories in colon and breast cancer: from animal studies to controlled clinical trials. Prev. Med. 1993;22:750–766. doi: 10.1006/pmed.1993.1069. [DOI] [PubMed] [Google Scholar]
- 56.Medlock KL, et al. Effects of coumestrol and equol on the developing reproductive tract of the rat. Proc. Soc. Exp. Biol. Med. 1995;208:67–71. doi: 10.3181/00379727-208-43833. [DOI] [PubMed] [Google Scholar]
- 57.Bennetts HW, et al. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. J. Agric. Res. 1946;22:131–138. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 58.Folman Y, et al. The interaction in the immature mouse of potent oestrogens with coumestrol, genistein and other uterovaginotrophic compounds of low potency. J. Endocrinol. 1966;34:215–225. doi: 10.1677/joe.0.0340215. [DOI] [PubMed] [Google Scholar]
- 59.Medlock KL, et al. The effects of phytoestrogens on neonatal rat uterine growth and development. Proc. Soc. Exp. Biol. Med. 1995;208:307–313. doi: 10.3181/00379727-208-43861. [DOI] [PubMed] [Google Scholar]
- 60.Awoniyi CA, et al. Reproductive sequelae in female rats after in utero and neonatal exposure to the phytoestrogen genistein. Fertil. Steril. 1998;70:440–447. doi: 10.1016/s0015-0282(98)00185-x. [DOI] [PubMed] [Google Scholar]
- 61.Yee S, et al. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem. Toxicol. 2008;46:2713–2720. doi: 10.1016/j.fct.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 62.Ishiwata N, et al. New equol supplement for relieving menopausal symptoms: randomized, placebo-controlled trial of Japanese women. Menopause. 2009;16:141–148. doi: 10.1097/gme.0b013e31818379fa. [DOI] [PubMed] [Google Scholar]
- 63.Ju YH, et al. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856–863. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]