Abstract
Objective:
Therapeutic hypothermia instituted within 6 h of birth has been shown to improve neurodevelopmental outcomes in term newborns with moderate–to–severe hypoxic–ischemic encephalopathy (HIE). The majority of infants who would benefit from cooling are born at centers that do not offer the therapy, and adding the time for transport will result in delays in therapy, that may lead to suboptimal or no neuroprotection for some patients. Our objective was to evaluate the effect of our center's experience with therapeutic hypothermia on neonatal transport.
Study Design:
Retrospective review of all cases of therapeutic hypothermia at a single neonatal intensive care unit from 2005 to 2009.
Result:
Of 50 infants with HIE treated with hypothermia, 40 were outborn and 35 were cooled on transport. The majority of patients were passively cooled by the referring clinicians, then actively cooled by our transport team. Overcooling to <32 °C occurred in 34% of patients, but there were no significant differences in admission vital signs or laboratory values between overcooled and appropriately cooled infants. The average time after birth of initiation of passive cooling was 1.4 h and active cooling was 2.7 h compared with the time of admission to our unit of 5.9 h.
Conclusion:
We discuss the important aspects of our program, including the education of referring and receiving clinicians and avoidance of overcooling.
Keywords: hypothermia, hypoxic-ischemic encephalopathy, neontal transport, neuroprotection, birth asphyxia
Introduction
Hypoxic–ischemic encephalopathy (HIE) affects approximately 1 per 1000 term newborns, with an estimated 20% mortality and 25% neurodevelopmental impairment in survivors.1, 2 Until recently, treatment for neonates with HIE was limited to supportive care and anticonvulsants for seizure control. In 2005, three multicenter randomized controlled trials were published showing that induction of mild hypothermia resulted in significantly improved neurodevelopmental outcome in neonates ⩾36 weeks gestation with acute perinatal HIE.3, 4, 5, 6
Timing of induction of hypothermia after an asphyxial brain injury is critical for optimum neuroprotection. Studies in animal models suggest that hypothermia should be started within 6 h of an acute asphyxial event, during the latent phase before the onset of secondary cerebral energy failure. Studies in near-term fetal sheep with a cerebral ischemic insult showed optimum neuroprotection if hypothermia was induced within 90 min of reperfusion. Delayed hypothermia continued to show some benefit if started <5.5 h after reperfusion but not if delayed until 8.5 h, at which time animals were experiencing post-asphyxial seizures.7, 8, 9 There is also evidence that the duration of the latent phase between primary and secondary energy failure is inversely proportional to the severity of the ischemic insult, suggesting that the therapeutic window for starting hypothermia therapy may be even shorter than 6 h in profound asphyxial insults.10 On the basis of these and other studies, most clinical trials of induced hypothermia for asphyxiated neonates have begun treatment within 6 h of birth.
Asphyxiated neonates have complex medical needs and should be cared for in intensive care units that can provide multidisciplinary subspecialty evaluation and treatment. Most neonates who meet the criteria for hypothermia therapy will be born in hospitals that do not provide this high level of care. As the time to transport patients to regional neonatal intensive care units (NICUs) offering hypothermia therapy may exceed the therapeutic window for neuroprotection, some centers have implemented cooling during neonatal transport. In the pilot study of Eicher et al. published in 2005, in which our center participated, outborn infants randomized to hypothermia were cooled with ice packs during transport. Two more recent clinical trials, TOBY (total body cooling) and ICE (Infant Cooling Evaluation), cooled some neonates during transport. The TOBY trial allowed for cooling to 33 to 34 °C during neonatal transport, unless it was likely that the infant would be transferred by 3 h after birth. Passive cooling was achieved by turning off the radiant warmer and, if necessary, active cooling with gel packs was used to achieve the target temperature.11 A similar approach to inducing hypothermia during transport was used in the ICE trial.12 Passive cooling during neonatal transport (withholding external heat sources) was described in a case report of two infants with HIE whose rectal temperatures on arrival to the tertiary NICU were 34.3 and 33.4 °C.13 In a recent report, passive cooling was used in 18 babies during transport to a regional hypothermia center.14 On arrival, only four babies had temperatures in the desired range of 33 to 35 °C, whereas eight had admission temperature in the 35 to 37 °C range, and six were overcooled. Passive overcooling can be a significant problem, particularly in severely asphyxiated neonates in whom temperature is only intermittently monitored.15 These reports highlight the need for education, experience, and perhaps new protocols for achieving target neuroprotective temperatures during neonatal transport.
Hypothermia for perinatal HIE is a relatively new, evolving therapy and research into long-term outcomes and other issues is ongoing. In 2006, the National Institutes of Child Health and Human Development issued a statement recommending that centers choosing to offer hypothermia therapy do so only in the context of protocols published in larger clinical trials and with systematic data collection.16 In 2008, we published our first 2 years' experience with implementing a hypothermia for HIE program in our NICU,17 and in the current report, we describe our 4 year's experience with therapeutic hypothermia during neonatal transport.
Methods
In March 2005, the University of Virginia (UVA) NICU instituted a protocol for therapeutic hypothermia for neonates with moderate to severe HIE. The eligibility criteria are similar to those used in published randomized controlled trials, including infants ⩾36 weeks gestation and ⩽6 h of age at the start of therapy with at least one indicator of an acute perinatal hypoxic–ischemic event and at least two indicators of moderate or severe encephalopathy on physical examination. Exclusion criteria are severe sepsis with shock or active bleeding. Parents receive verbal and written information about HIE and hypothermia therapy, but signed parental consent is not required.
The UVA NICU serves as a regional referral center for 10 hospitals and ∼40% of admissions are outborn. The median one-way travel distance to our referring hospitals is 64 miles (range 3 to 160 miles) and 95% of transports are accomplished by ground. Several NICUs beyond our referral region that do not offer hypothermia therapy also refer patients to our center for cooling. As many outborn patients qualifying for hypothermia therapy would not arrive at the UVA NICU before 6 h of age, it was decided that therapy would begin at the referring hospital and continue during transport. After developing written protocols for hypothermia therapy on transport, education sessions were held for referring clinicians through our Perinatal Continuing Education Program.18 Additional training sessions were held with the UVA Neonatal Emergency Transport System clinicians, which includes specially trained NICU nurses, respiratory therapists and emergency medical technicians. Training sessions focused on (1) rationale for hypothermia and clinical trial results, (2) eligibility and exclusion criteria, (3) neurological examination findings of moderate to severe HIE and (4) practical aspects of passive and active cooling before and during the transport. Passive cooling by the referring clinicians was recommended in the first 2 years of our program. Since 2007, level III NICUs, which refer patients for hypothermia therapy, have begun active cooling while awaiting arrival of our transport team, but all other centers continue to use passive cooling only.
The typical sequence of events for initiating hypothermia therapy for outborn infants referred to our center is as follows:
Outreach education to referring clinicians includes a recommendation to turn off the radiant warmer as soon as the diagnosis of acute perinatal moderate to severe HIE is considered (with temperature monitoring at least every 15 min).
When an infant is born with suspected acute perinatal HIE, the referring clinician calls the neonatology attending or fellow to arrange transfer.
The UVA Neonatal Emergency Transport System team is dispatched.
Criteria for an acute perinatal hypoxic–ischemic event and physical examination evidence of moderate or severe encephalopathy are discussed.
If the infant meets the criteria for therapeutic hypothermia, a recommendation is made to continue passive cooling and closely monitor the rectal temperature (at least every 15 min, or continuously if possible). Active cooling in primary care hospitals before arrival of the transport team is discouraged.
The UVA transport team arrives at the referring hospital and initiates continuous rectal temperature monitoring.
If necessary, cool gel packs (∼10 °C, wrapped in a cloth) are briefly applied to the head and/or trunk until the rectal temperature is <34 °C (active cooling).
The infant is placed in a room temperature transport incubator.
The rectal temperature is continuously monitored during transport and adjustments are made to maintain a target temperature of 33 to 34 °C. Adjustments include placing a receiving blanket on the baby if the rectal temperature drops below 33 °C, or a wrapped cool gel pack if the temperature rises above 34 °C. The air temperature of the transport incubator is increased, if the patient's rectal temperature continues to fall despite covering with a blanket. A warm gel pack is applied if the temperature drops below 31 °C and removed once the temperature rises above 32 °C.
A database of patient information is maintained on all patients undergoing hypothermia therapy in our center. The UVA Institutional Review Board has approved the review of de-identified patient information for publication. For this report, we reviewed clinical data on all patients undergoing therapeutic hypothermia in the first 4 years of the program, from March 2005 to February 2009. Vital signs and laboratory values of outborn infants on arrival to UVA were compared between infants who were overcooled (minimum temperature before admission ⩽32 °C, n=12) and those who remained closer to target temperature (minimum temperature >32 °C, n=23), by unpaired, two-tailed t-tests using GraphPad Prism version 4.03 (GraphPad Software, La Jolla, CA, USA), with significance set at P<0.05. Data are presented as mean±s.d. unless otherwise indicated.
Results
A total of 50 infants underwent hypothermia therapy for moderate to severe HIE in the UVA NICU from March 2005 to February 2009. 40 patients (80%) were born at referring hospitals. The UVA Neonatal Emergency Transport System team transported 39 of these patients (all by ambulance) and one patient was transported by helicopter by the referring hospital's neonatal transport team. Of the 40 outborn patients, 35 underwent passive and/or active cooling before and during the transport. Altogether, 25 patients had passive cooling initiated by the referring clinicians (turning off the radiant warmer). For 23 of these patients, active cooling (applying cool packs) was initiated by our transport team at the referring hospital and continued during transport, and the other 2 patients continued with passive cooling during transport (one with a very short transport time and one transported by helicopter by the referring hospital team). 10 patients at level III referring NICUs had active cooling initiated by the referring neonatologists before the arrival of our transport team. Five outborn patients were not cooled until arrival at our center, either because hypothermia was not considered until the infant was assessed at our NICU (four patients, three in the first year of the program) or the transport time was very short (one patient). One patient who qualified for hypothermia therapy was excluded because of shock and disseminated intravascular coagulation with active bleeding.
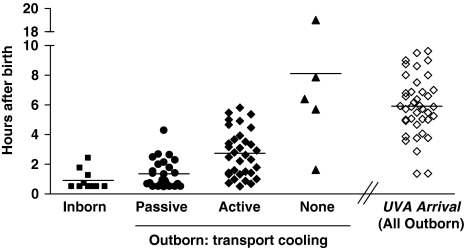
Figure 1 compares the cooling start time for inborn and outborn patients and the hours of age on arrival to the UVA NICU. The time of arrival of the UVA transport team to the referring hospital was 2.9±1.4 h after birth (range 0.37 to 5.5 h). The first rectal temperature recorded by our transport team after arrival at the referring hospital was 34.7±2.0 °C. The patients who were actively cooled at the referring NICU (n=10) had rectal temperature 33.9±2.1 °C when our transport team arrived at the referring hospital, compared with 34.4±1.9 °C in the infants passively cooled (n=25) and 36.6±0.3 °C in those undergoing conventional thermal management (n=5). The time from birth to achieve core temperature <34 °C was 2.6±1.8 h for inborn infants (n=10), 3.9±1.6 h for infants cooled before and during transport (n=35), and 9.8±6.2 h for outborn infants not cooled until the arrival at our unit (n=5). As shown in Figure 1, one patient admitted in the first year of the hypothermia program was not cooled until 19 h of age due to a late decision to initiate therapy.
Figure 1.
Cooling start time and the University of Virginia (UVA) arrival time. A total of 50 infants underwent hypothermia therapy at the UVA Neonatal Intensive Care Unit from March 2005 to February 2009. Cooling start time is shown for inborn infants (hours after birth, n=10, filled squares). Of the 40 outborn infants, 35 were cooled before and during transport, and 5 were not cooled until arrival at UVA (filled triangles). Cooling was classified as passive (no external heat source, filled circles) or active (application of cool gel packs, filled diamonds). Some infants underwent passive followed by active cooling and both start times are included. The arrival time at UVA for all 40 outborn infants is shown (open diamonds). Horizontal lines represent mean hours after birth.
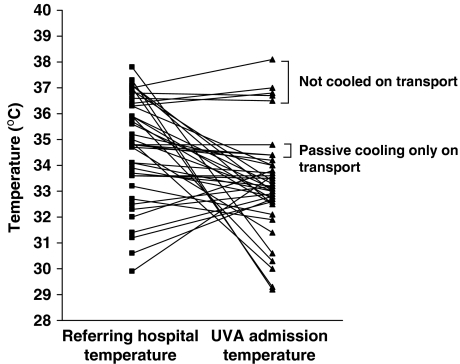
Figure 2 shows the rectal temperature for outborn patients when the transport team arrived at the referring hospital and the temperature on arrival to our unit. The UVA admission rectal temperature range was 29.2 to 34.4 °C for the 33 infants actively cooled on transport, 34.4 to 34.8 °C for the two infants passively cooled on transport and 36.5 to 38.1 °C for the five infants not cooled on transport.
Figure 2.
Comparison of temperature of 40 outborn neonates with hypoxic–ischemic encephalopathy (HIE) before and after neonatal transport. The rectal temperatures of 40 outborn infants on arrival of the University of Virginia (UVA) transport team to the referring hospital and on arrival of the patients to the UVA Neonatal Intensive Care Unit. Five patients were not cooled until after arrival to UVA, two patients underwent passive cooling only and thirty-three patients underwent active cooling during transport.
Admission characteristics of the 50 infants in our therapeutic hypothermia program March 2005 to February 2009 are summarized in Table 1. Of the 35 infants cooled before and during transport, 23 (66%) maintained temperatures in the range 32 to 35 °C over the course of the entire transport. Five infants (14%) had at least one core temperature <30 °C, and the duration of temperature <30 °C was 1.0±0.6 h. Seven infants (20%) had at least one core temperature between 30 and 32 °C, and the duration of temperature in this range was 1.6±1 h. No cardiac arrhythmias other than sinus bradycardia were noted in any patient. When comparing patients who were overcooled to <32 °C (n=12) with those who maintained temperature in the 32 to 35 °C range (n=23), there was a trend toward lower birth weight in those infants who were overcooled (2948±722 g vs 3284±693 g, P=0.18). There were no significant differences in admission vital signs or laboratory tests between the groups, although there was a trend toward lower platelet counts (143±45 vs 193±18, P=0.06) and higher blood sugar (189±101 vs 139±65, P=0.08) in overcooled infants vs appropriately cooled infants, respectively.
Table 1. Admission characteristics and laboratory values of outborn and inborn infants.
| Minimum temperature <30 °C, n=5 | Minimum temperature 30–32 °C, n=7 | Minimum temperature 32.1–35 °C, n=23 | Minimum temperature ⩾35.1 °C (no transport cooling), n=5 | All outborn, n=40 | Inborn, n=10 | |
|---|---|---|---|---|---|---|
| Gestational age (weeks) | 38.8±1.1 | 38.1±2.7 | 38.8±1.1 | 38.0±2.6 | 38.5±1.9 | 37.9±2.2 |
| Birth weight (g) | 2914±415 | 2973±917 | 3284±693 | 3853±971 | 3228±750 | 3197±845 |
| Degree of encephalopathy (moderate/severe) | 2/3 | 3/4 | 16/7 | 3/2 | 24/16 | 4/6 |
| Age at start of cooling, passive or active (h) | 1.9±1.3 | 2.5±1.5 | 3.0±1.6 | 8.1±6.5 | 3.5±3.2 | 0.9±0.7 |
| Age at arrival of transport team (h) | 2.6±1.2 | 2.7±1.5 | 3.0±1.4 | 2.8±1.8 | 2.9±1.4 | N/A |
| Age at arrival to UVA (h) | 6.1±1.0 | 5.8±2.2 | 6.1±2.0 | 5.8±2.9 | 5.9±2.1 | N/A |
| Age when temperature reached <34 °C (h) | 3.2±1.7 | 3.4±0.4 | 4.3±2.0 | 9.8±6.1 | 4.7±3.2 | 2.6±1.8 |
| HR (bpm) | 111±23 | 104±16 | 113±20 | 147±31 | 116±24 | 143±13 |
| Blood pressure (mean, mm Hg) | 55±7 | 49±6 | 50±11 | 53±5 | 51±11 | 43±7 |
| Platelet count (k μl−1) | 136±32 | 146±56 | 193±84 | 234±110 | 183±82 | 207±81 |
| PT (s) | 21.9±5.1 | 27.0±8.9 | 24.9±10.7 | 21.6±8.8 | 24.5±9.7 | 26.0±5.8 |
| INR | 1.8±0.5 | 2.3±0.9 | 2.0±1.2 | 2.0±1.1 | 2.1±1.1 | 2.2±0.5 |
| AST (U l−1) | 121±80 | 398±597 | 575±1082 | 260±209 | 451±866 | 316±391 |
| ALT (U l−1) | 40±22 | 343±714 | 211±273 | 110±165 | 208±423 | 91±108 |
| Creatinine (mg per 100 ml) | 0.8±0.2 | 1.1±0.1 | 0.9±0.3 | 1.3±0.6 | 1.0±0.3 | 0.9±0.1 |
| Glucose (mg per 100 ml) | 191±88 | 188±116 | 139±65 | 73±16 | 142±82 | 143±90 |
Abbreviations: N/A, not applicable; UVA, University of Virginia; HR, heart rate; PT, prothrombin time; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
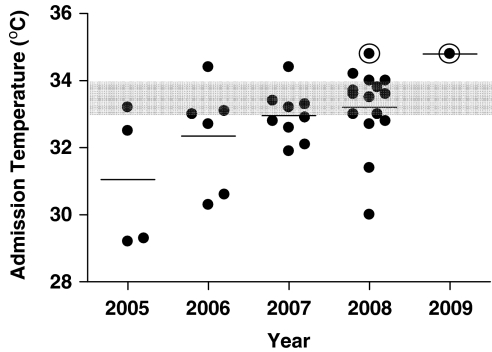
Figure 3 shows, by year, the UVA admission temperature for patients cooled on transport. The mean admission temperature increased every year and by 2008 two-thirds of patients fell in the target range of 33 to 34 °C.
Figure 3.
The University of Virginia (UVA) admission temperature for 35 outborn infants cooled on transport. The rectal temperatures of 35 outborn infants cooled during transport, recorded on admission to the UVA Neonatal Intensive Care Unit. Gray-shaded area represents target temperature (33 to 34 °C). Two patients with only passive cooling during transport (indicated with an open circle) had admission temperatures of 34.4 and 34.8 °C. All others were transported with active cooling.
Discussion
The timing of initiation of therapeutic hypothermia is critical, with animal studies showing optimum neuroprotection, if hypothermia is induced within ∼6 h of an acute asphyxial insult. In our center, 80% of newborns eligible for hypothermia therapy from 2005 to 2009 were born at the referring hospitals, and nearly half of the outborn patients arrived at our center >6 h after birth. Initiating hypothermia therapy at the referring hospital resulted in an average time savings of ∼3 h, which in some cases may result in improved outcomes. In this report, we highlight our efforts to educate health-care providers about passive and active cooling of neonates with HIE and share suggestions for avoiding overcooling. Key points in the implementation of a hypothermia therapy on transport program are summarized in Table 2.
Table 2. Therapeutic hypothermia on neonatal transport.
| 1. Decision to implement based on the geographics of the referral base and neonatal transport team capabilities |
| 2. Establish protocols and organize education sessions (including neurological assessment): referring clinicians, transport clinicians and receiving clinicians |
| 3. Equipment: system for continuous rectal temperature monitoring throughout cooling and transport, cool gel packs, receiving blankets and transport incubator |
| 4. Consider passive cooling and/or targeting temperature 34–35 °C in start-up phase to avoid overcooling |
| 5. Maintain flow sheets and database for recording clinical data for quality assessment and improvement |
Educating referring clinicians about when and how to initiate therapeutic hypothermia is a cornerstone to a successful program. We recommend that in cases of acute perinatal distress with suspected HIE, the radiant warmer should be turned off and the baby's temperature closely monitored during initial stabilization and neurological assessment. If the neurological examination is not consistent with moderate to severe encephalopathy, the baby can then be transitioned to routine thermal care. If the baby meets criteria for therapeutic hypothermia, passive cooling is continued until the arrival of our transport team. Asphyxiated, encephalopathic newborns are notoriously poor at thermoregulation and some will passively cool to a core temperature in the 33 to 34 °C range if left uncovered at room temperature.15 In fact, in some cases in our series, passive cooling resulted in temperatures below the target range. On the other hand, passive cooling did not achieve optimum neuroprotective temperatures in many patients, with 40% of patients having rectal temperature >35 °C on arrival of our transport team and 20% with temperature >37 °C.
Active cooling of neonates with HIE can be accomplished with brief application of a wrapped cool gel pack. In general this, is done by our neonatal transport team, but several level III NICUs referring patients to our center have begun active cooling before the arrival of our team. In either case, it is important to continuously monitor rectal temperature and remove the cool pack when temperature falls below 34 °C to avoid overcooling. In our experience, active cooling resulted in at least one recorded temperature <30 °C in 14% of patients and <32 °C in 34%. Most patients who were overcooled had only intermittent rather than continuous rectal temperature monitoring, and had ice packs (∼0 to 4 °C) applied rather than cool gel packs (∼7 to 10 °C). Although we did not see statistically significant differences in admission vital signs or laboratory values in overcooled compared with appropriately cooled patients, the number of patients in each group was too small to draw any conclusions about the detrimental effects of overcooling. There were trends toward lower platelet counts and higher blood sugar in the overcooled groups, which, if borne out in larger numbers of patients, could be a result of the hypothermia or simply an indicator of a more severe degree of asphyxia. We did find that overcooled patients were more likely to be classified as severe, rather than moderate, encephalopathy, possibly contributing to a more deranged thermoregulatory state. In any case, efforts should be made to avoid overcooling neonates, and we found that use of continuous rectal temperature monitoring and judicious use of cooling packs has resulted in more patients arriving at our center in the target temperature range in recent years (Figure 3). Neonatal transport teams instituting a new protocol for induced hypothermia should consider beginning with either passive cooling or active cooling targeting a slightly higher temperature to avoid overcooling during the start-up phase.
As therapeutic hypothermia is increasingly used for neonates with HIE, whether and how to cool patients before arrival to a hypothermia center will be an important issue to resolve. Results of recently completed clinical trials, which cooled some babies during transport to study centers, are likely to provide important information about this practice. We have found that, with experience and education, our referring and transport clinicians can induce and maintain hypothermia for asphyxiated neonates before and during transport. Neuroprotective temperatures are often achieved several hours sooner with this approach, and efforts to develop and evaluate protocols for safe transport of neonates undergoing induced hypothermia are warranted.
The authors declare no conflict of interest.
References
- du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol. 2002;15 (2:151–157. doi: 10.1097/00019052-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta Paediatr. 1995;84 (8:927–932. doi: 10.1111/j.1651-2227.1995.tb13794.x. [DOI] [PubMed] [Google Scholar]
- Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, et al. Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol. 2005;32 (1:18–24. doi: 10.1016/j.pediatrneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32 (1:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365 (9460:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353 (15:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46 (3:274–280. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99 (2:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102 (5:1098–1106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- Iwata O, Iwata S, Thornton JS, De Vita E, Bainbridge A, Herbert L, et al. ‘Therapeutic time window' duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007;1154:173–180. doi: 10.1016/j.brainres.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Azzopardi DV, Strohm B, Edwards AD, Dvet L, Halliday HL, et al. TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361 (14:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P.Cooling for newborns with hypoxic ischaemic encephalopathy Cochrane Database Systemic Reviews 2007. Issue 4. Article no. CD003311. [DOI] [PubMed]
- Anderson ME, Longhofer TA, Phillips W, McRay DE. Passive cooling to initiate hypothermia for transported encephalopathic newborns. J Perinatol. 2007;27 (9:592–593. doi: 10.1038/sj.jp.7211781. [DOI] [PubMed] [Google Scholar]
- Hallberg B, Olson L, Bartocci M, Edqvist I, Blennow M. Passive induction of hypothermia during transport of asphyxiated infants: a risk of excessive cooling. Acta Paediatr. 2009;98 (6:942–946. doi: 10.1111/j.1651-2227.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Thoresen M.Supportive care during neuroprotective hypothermia in the term newborn: adverse effects and their prevention Clin Perinatol 200835(4749–763.vii. [DOI] [PubMed] [Google Scholar]
- Higgins RD, Raju TN, Perlman J, Azzopardi DV, Blackmon LR, Clark RH, et al. Hypothermia and perinatal asphyxia: executive summary of the National Institute of Child Health and Human Development workshop. J Pediatr. 2006;148 (2:170–175. doi: 10.1016/j.jpeds.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Zanelli SA, Naylor M, Dobbins N, Quigg M, Goodkin HP, Matsumoto JA, et al. Implementation of a ‘Hypothermia for HIE' program: 2-year experience in a single NICU. J Perinatol. 2008;28 (3:171–175. doi: 10.1038/sj.jp.7211896. [DOI] [PubMed] [Google Scholar]
- Kattwinkel J, Cook LJ, Nowacek G, Bailey C, Crosby WM, Hurt H, et al. Regionalized perinatal education. Semin Neonatol. 2004;9 (2:155–165. doi: 10.1016/j.siny.2003.08.005. [DOI] [PubMed] [Google Scholar]