Abstract
The CD1 locus encodes a family of major histocompatibility complex (MHC) antigen-like glycoproteins which associate with beta 2-microglobulin and are expressed on immature thymocytes and Langerhans cells. Three CD1 molecules have been identified by monoclonal antibodies and molecular cloning: CD1a, -b, and -c. We have isolated a cDNA coding for a fourth CD1 molecule from a human thymocyte library and termed this molecule CD1d. Reported here are the complete nucleotide sequence and genomic organization of CD1d. They predict that this molecule is related to the previously identified CD1a, -b, and -c molecules and to MHC class I molecules, with three external domains, a transmembrane domain, and a short cytoplasmic tail. The sequence of CD1d is the most divergent among the CD1 molecules in the membrane-distal alpha 1 and alpha 2 domains and in the 5' untranslated region. In contrast, all four CD1 molecules are highly homologous in the membrane-proximal alpha 3 domain, which is likely involved in beta 2-microglobulin binding. A comparison of CD1 and MHC class I sequences suggests that these molecules each evolved to interact with a distinct set of cell surface proteins.
Full text
PDF

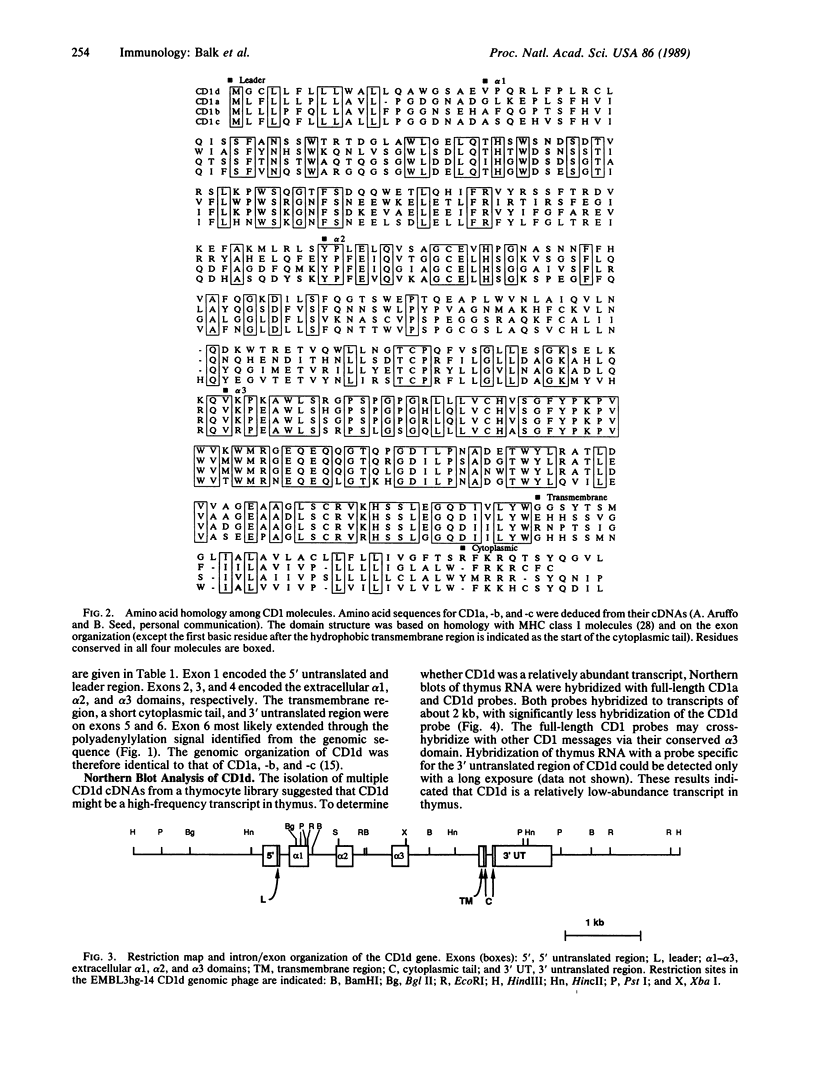


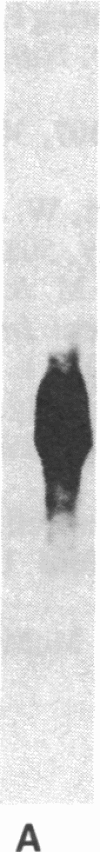
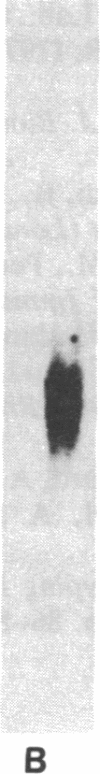
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiot M., Bernard A., Raynal B., Knapp W., Deschildre C., Boumsell L. Heterogeneity of the first cluster of differentiation: characterization and epitopic mapping of three CD1 molecules on normal human thymus cells. J Immunol. 1986 Mar 1;136(5):1752–1758. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bernabeu C., van de Rijn M., Lerch P. G., Terhorst C. P. Beta 2-microglobulin from serum associates with MHC class I antigens on the surface of cultured cells. Nature. 1984 Apr 12;308(5960):642–645. doi: 10.1038/308642a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Calabi F., Milstein C. A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature. 1986 Oct 9;323(6088):540–543. doi: 10.1038/323540a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fithian E., Kung P., Goldstein G., Rubenfeld M., Fenoglio C., Edelson R. Reactivity of Langerhans cells with hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2541–2544. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanau D., Fabre M., Schmitt D. A., Garaud J. C., Pauly G., Tongio M. M., Mayer S., Cazenave J. P. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis "nonclassical" major histocompatibility complex class I molecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987 May;84(9):2901–2905. doi: 10.1073/pnas.84.9.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn-Perles B., Wietzerbin J., Caillol D. H., Lemonnier F. Delineation of three subsets of class I human T antigens (HTA) on Molt-4 cells: serologic and regulatory relationship to HLA class I antigens. J Immunol. 1985 Mar;134(3):1759–1765. [PubMed] [Google Scholar]
- Kefford R. F., Calabi F., Fearnley I. M., Burrone O. R., Milstein C. Serum beta 2-microglobulin binds to a T-cell differentiation antigen and increases its expression. Nature. 1984 Apr 12;308(5960):641–642. doi: 10.1038/308641a0. [DOI] [PubMed] [Google Scholar]
- Knowles R. W., Bodmer W. F. A monoclonal antibody recognizing a human thymus leukemia-like antigen associated with beta 2-microglobulin. Eur J Immunol. 1982 Aug;12(8):676–681. doi: 10.1002/eji.1830120810. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Tsu T. T., Clark E. A. Covalent association between human thymus leukemia-like antigens and CD8(Tp32) molecules. J Immunol. 1985 Jun;134(6):4250–4254. [PubMed] [Google Scholar]
- Martin L. H., Calabi F., Lefebvre F. A., Bilsland C. A., Milstein C. Structure and expression of the human thymocyte antigens CD1a, CD1b, and CD1c. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9189–9193. doi: 10.1073/pnas.84.24.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. H., Calabi F., Milstein C. Isolation of CD1 genes: a family of major histocompatibility complex-related differentiation antigens. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9154–9158. doi: 10.1073/pnas.83.23.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small T. N., Knowles R. W., Keever C., Kernan N. A., Collins N., O'Reilly R. J., Dupont B., Flomenberg N. M241 (CD1) expression on B lymphocytes. J Immunol. 1987 May 1;138(9):2864–2868. [PubMed] [Google Scholar]
- Snow P. M., Van de Rijn M., Terhorst C. Association between the human thymic differentiation antigens T6 and T8. Eur J Immunol. 1985 May;15(5):529–532. doi: 10.1002/eji.1830150520. [DOI] [PubMed] [Google Scholar]
- Terhorst C., van Agthoven A., LeClair K., Snow P., Reinherz E., Schlossman S. Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell. 1981 Mar;23(3):771–780. doi: 10.1016/0092-8674(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Milstein C. A small polypeptide different from beta2-microglobin associated with a human cell surface antigen. Nature. 1979 May 17;279(5710):243–244. doi: 10.1038/279243a0. [DOI] [PubMed] [Google Scholar]
- van de Rijn M., Lerch P. G., Knowles R. W., Terhorst C. The thymic differentiation markers T6 and M241 are two unusual MHC class I antigens. J Immunol. 1983 Aug;131(2):851–855. [PubMed] [Google Scholar]