Abstract
Background
Over the past decade, minimally invasive cardiac surgery (MICS) has emerged as an accepted approach for the management of cardiac disease that requires a surgical solution. We report the results of an 8-year, single-institution experience with MICS.
Methods
Between January 1, 2000 and December 31, 2007, a total of 910 patients underwent MICS. Major cases included aortic valve procedures (71, 7.8%), coronary artery bypass grafting (96, 10.5%), atrioseptal defect repair (103, 11.3%), and mitral valve procedures (507, 55.7%). Major outcomes of interest included the complication and mortality rates.
Results
The mean age of the patients was 57 ± 15 years; the mean ejection fraction was 55% ± 11%; and the mean body mass index was 26.1 ± 4.9. Overall, 782 cases (85.9%) were performed through a mini-thoracotomy. Most of the cases were accomplished through central cannulation (765, 84.0%), and venous drainage was most commonly performed in a bicaval fashion (percutaneous superior vena cava and percutaneous inferior vena cava). The mean aortic cross-clamp and cardiopulmonary bypass (CPB) times were 58.1 ± 44.9 and 101.9 ± 66.8 min, respectively. Conversion to full sternotomy occurred in 10 patients, and the median length of stay in hospital was 6 days. The overall complication rate was 8.8%, and the 30-day mortality rate was 2.9%. In the multivariate logistic regression analysis, risk factors associated with in-hospital complications included age, CPB time, arterial cannulation location, conversion from off-CPB to on-CPB, hepatic insufficiency, and diabetes. In the multivariate hazards regression analysis, risk factors associated with mortality included postoperative stroke, renal failure, and sternal wound infection; CPB time; and previous surgery.
Conclusions
In our experience, minimally invasive approaches are effective and reproducible for a variety of cardiac operations, with acceptable operating time durations, morbidity, and mortality.
Introduction
Over the past decade, minimally invasive cardiothoracic surgery (MICS) has emerged as an accepted approach for the management of cardiac surgical disease [1–5]. The growth of MICS has been driven in part by major improvements in technology combined with a desire both by surgeons and patients for minimally invasive approaches to the treatment of cardiac diseases that require a surgical solution [6, 7].
There is no formal consensus on what constitutes MICS. The term generally refers to conventional cardiothoracic surgical operations performed through incisions other than the traditional full median sternotomy [8]. Since MICS was first introduced during the 1990s, multiple alternative access approaches have been described in the literature including partial sternotomy, limited access thoracotomy, totally endoscopic approach, catheter-based hybrid approach, and subxiphoid and subdiaphragmatic approaches [9–11].
Despite the diversity of minimally invasive access options, the potential benefits are significant and generalizable. MICS has been associated with decreased surgical trauma, shorter lengths of stay, decreased hospital costs, and overall improvements in patient satisfaction and quality of life [12, 13]. In addition, single-institution studies have demonstrated that these benefits are achieved without compromising the quality of the operation [14–16].
As MICS continues to gain acceptance and become an established approach in cardiac surgery, continued analysis of outcomes is necessary to ensure that the limited incision does not compromise the surgical outcome and potentially to aid in patient selection. This study reviews a single institution’s 8-year experience with MICS.
Methods
From January 1, 2000 to December 31, 2007, a total of 910 MICS procedures were performed at Columbia University Medical Center. For this study, MICS was defined as per New York State reporting guidelines as any cardiac surgical operation performed through an incision other than a full median sternotomy. Data on patient demographics, operative parameters, and both short- and long-term morbidity and mortality were retrospectively gathered from an institutional review board (IRB)-approved, Health Insurance Portability and Accountability Act (HIPAA)-authorized internal cardiac surgical database.
Follow-up mortality data were provided through April 24, 2009. Major outcomes of interest included conversion to full median sternotomy, cross-clamp time, cardiopulmonary bypass (CPB) time, length of hospital stay, complication rate, and mortality rate.
Continuous variables were reported as the mean ± standard deviation and were compared using the Student’s t-test. Cases in which there was no aortic cross-clamp applied and cases done without CPB were excluded from the calculation of mean cross-clamp and CPB times, respectively. Categoric variables were reported as percentages and compared using the chi-squared test. Long-term survival rates were calculated using the Kaplan–Meier method, and statistical significance was calculated by the log-rank test. Multivariate logistic regression (backward stepwise, remove P > 0.15) was used to assess the simultaneous effects of multiple variables on in-hospital complications after MICS. Multivariate Cox proportional hazards regression (backward stepwise, remove P > 0.15) was used to assess the simultaneous effects of multiple variables on mortality. Model discrimination was assessed by calculating the area under the receiver operating curve (AUC). For both models, only data from the four most common procedures: aortic valve repair or replacement (AVR/r), coronary artery bypass grafting (CABG), atrioseptal defect (ASD) repair, and mitral valve repair or replacement (MVR/r) were used in the regression analysis. For all analyses, the conventional P < 0.05 was used to determine the level of statistical significance. All reported P values are two-sided. All data were analyzed using the statistical software package Stata 10 (Stata, College Station, TX, USA).
Results
A total of 910 patients underwent elective MICS from January 2000 to December 2007. Baseline demographic data are listed in Table 1 The mean age of the study population was 57 ± 15 years, with 23.5% of patients >70 years of age and 5.3% >80 years of age. There was a nearly equal distribution of men and women. Patients had a variety of preoperative medical conditions, with the most common being diabetes (9.7% of patients). The mean body mass index (BMI) was 26.1 (<25 in 46.4%, 25–35 in 49.0%, >35 in 4.6%). Notably, 6.5% of patients had had previous cardiac surgery.
Table 1.
Demographic data
| Characteristic | Data |
|---|---|
| Age, mean ± SD | 57.2 ± 15.1 |
| >70 Years | 214 (23.5%) |
| >80 Years | 48 (5.3%) |
| Ejection fraction, mean ± SD | 55.0% ± 10.9% |
| BMI, mean ± SD | 26.1 ± 4.9 |
| Sex (male) | 478 (52.6%) |
| Renal failure | 4 (0.44%) |
| Endocarditis | 16 (1.76%) |
| Immune system deficiency | 17 (1.87%) |
| Peripheral vascular disease | 24 (2.64%) |
| COPD | 46 (5.05%) |
| Previous cardiac surgery | 59 (6.5%) |
| Cerebrovascular accident | 73 (8.02%) |
| Myocardial infarction | 74 (8.13%) |
| Congestive heart failure | 76 (8.35%) |
| Diabetes | 88 (9.67%) |
| Smoker | 237 (26.04%) |
BMI body mass index, COPD chronic obstructive pulmonary disease
The distribution of cases is displayed in Fig. 1. The four most common operations performed in the series were AVR/r (71, 7.8%), CABG (96, 10.5%), ASD repair (103, 11.3%), and MVR/r (507, 55.7%). For the entire series the mean aortic cross-clamp time was 58.1 ± 44.9 min, and the mean CPB time was 101.9 ± 66.8 min.
Fig. 1.
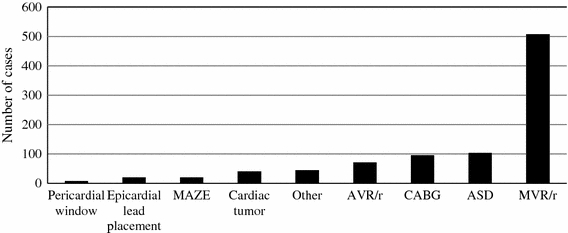
Distribution of cases. MAZE: Maze procedure; AVR/r: aortic valve repair or replacement; CABG: coronary artery bypass graft; ASD: atrial septal defect; MVR/r: mitral valve repair or replacement
The distribution of incision types is displayed in Fig. 2. Most cases were performed through a mini-thoracotomy (782, 85.9%). The distribution of cannulation techniques is displayed in Fig. 3. Arterial cannulation was most commonly performed in a central aortic fashion (765, 84.0%). Venous drainage was most commonly performed in a bicaval fashion (765, 84.0%). The most common venous drainage configuration was percutaneous SVC and percutaneous IVC (446, 49.0%).
Fig. 2.
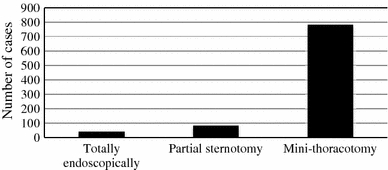
Distribution of incision types
Fig. 3.
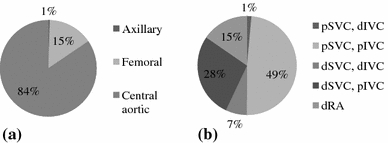
a Distribution of arterial cannulation. b Distribution of venous drainage. p: percutaneous; d: direct; SVC: superior vena cava; IVC: inferior vena cava; RA: right atrium
Conversion to a full median sternotomy occurred in 10 patients (1.1%). The median length of hospital stay was 6 days. The in-hospital complication rate was 8.8% (80 patients). Figure 4 displays the distribution of in-hospital complications. The four most common complications were unplanned reoperation (10, 1.1%), renal failure (15, 1.6%), bleeding that required reoperation (20, 2.2%), and respiratory failure (36, 4.0%). There were no acute aortic dissections in the series.
Fig. 4.
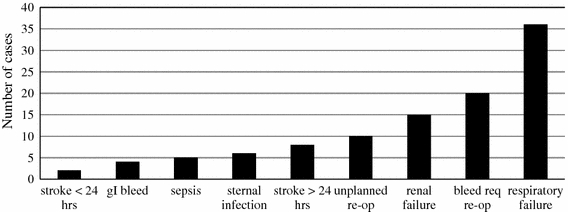
Distribution of in-hospital complications following minimally invasive cardiac surgery (MICS). gI: gastrointestinal; re-op: reoperation; req: requiring
In the multivariate logistic regression analysis, where the outcome of interest was the presence of any in-hospital complication, age, CPB time, arterial cannulation configuration, conversion from off-CPB to on-CPB, hepatic insufficiency at baseline, and diabetes at baseline were all significant risk factors (Table 2). Using central cannulation as the reference group, both peripheral and axillary cannulation was associated with increased odds of complications after MICS. The calculated AUC for the model was 0.73.
Table 2.
Multivariate logistic regression analysis of risk factors for in-hospital complicationsa
| Risk factor | Odds ratio | P | 95% CI |
|---|---|---|---|
| CPB time (min) | 1.009 | <0.001 | 1.005–1.013 |
| Age | 1.025 | 0.023 | 1.004–1.046 |
| Diabetes | 2.338 | 0.033 | 1.069–5.113 |
| Cannulation approach | |||
| Peripheral/central | 2.981 | <0.001 | 1.682–5.293 |
| Axillary/central | 13.866 | 0.009 | 1.901–100.818 |
| Hepatic insufficiency | 35.792 | 0.004 | 3.124–410.067 |
| Conversion from off-CPB to on-CPB | 42.492 | 0.003 | 3.589–503.058 |
CI confidence interval, CPB cardiopulmonary bypass
aEstimates were adjusted for all other variables in the table
Among all cases in the series at 30 days after the index operation, there were 26 deaths (2.9%). Regardless of the type of MICS procedure performed, there were no significant differences in long-term survival, which remained above 85% for all procedures (Fig. 5). In the multivariate Cox proportional hazards regression analysis, CPB time, previous cardiac surgery, sternal wound infection, postoperative renal failure, and postoperative stroke were all associated with increased risk of mortality following MICS (Table 3)
Fig. 5.
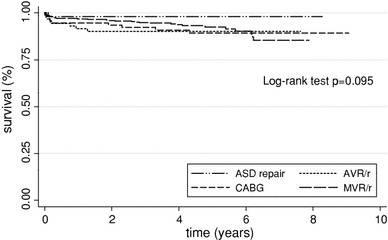
Kaplan-Meier survival curves by major MICS procedure
Table 3.
Multivariate hazards regression analysis of risk factors for mortalitya
| Risk factor | Hazards ratio | P | 95% CI |
|---|---|---|---|
| CPB time (min) | 1.010 | <0.001 | 1.006–1.014 |
| Previous cardiac surgery | 4.376 | 0.005 | 1.580–12.120 |
| Sternal infection | 9.369 | 0.030 | 1.246–70.458 |
| Postoperative renal failure | 13.758 | <0.001 | 3.172–59.676 |
| Postoperative stroke | 15.098 | <0.001 | 3.518–64.790 |
aEstimates were adjusted for all other variables in the table
Discussion
Whereas minimally invasive approaches have become well established in many fields of surgery, the adoption of MICS began in earnest only over the past 10–15 years. Minimally invasive approaches in cardiac surgery were initially delayed by concerns over intracardiac air, limited access approaches for major operations, and limitations in cannulation techniques. Since the mid-1990s, improvements in technology have led to the rapid adoption of MICS for a variety of cardiac operations. Moreover, in select centers, MICS has now become the standard approach for valvular surgery.
One consequence of the adoption of MICS as the standard approach in centers that have achieved superior outcomes with this technique is a lack of equipoise to randomize patients to traditional sternotomy for the purpose of prospectively compare the two approaches. Thus, single-institution, retrospective analyses of MICS outcomes have served an important role in demonstrating the safety and effectiveness of this approach. Previous studies have demonstrated that MICS has the potential to decrease the length of hospital stay, reduce postoperative pain, decrease surgical trauma, and produce outcomes equivalent to those attained by standard sternotomy.
One limitation in this study is the retrospective nature of the analysis and lack of a sternotomy control group for appropriate comparison. In addition, no long-term follow-up on functional status was available nor were there any long-term echocardiographic measures. Despite these limitations in this study, we have added to the growing, although still limited, literature on MICS by demonstrating that during an 8-year period with more than 900 patients MICS is an effective and reproducible approach for a variety of cardiac operations. MICS was safely performed on patients with a diverse array of baseline medical conditions, including nearly 25% of patients in the series >70 years of age and more than 5% of patients >80 years of age. In addition, nearly 18% of patients in the series had a BMI > 30, demonstrating the feasibility of MICS in both the elderly and the obese.
The mean cross-clamp and CPB times of 58.1 and 101.9 min, respectively, demonstrate that MICS can be accomplished within reasonable operating times. Moreover, with regard to short-term outcomes, MICS was performed successfully with only a 1% rate of conversion to full sternotomy and a 30-day mortality rate of less than 3%. Notably, stroke occurred less than 24 h after surgery in only two patients, and it occurred more than 24 h after surgery in only eight patients.
Standard arterial cannulation at our institution has been performed via the central aortic approach. In a multivariate logistic regression analysis, we demonstrate that there is an increased risk of major in-hospital complications associated with the use of both peripheral and axillary aortic cannulation versus central aortic cannulation. Importantly, risk factors associated with major in-hospital complications after MICS (e.g., age, CPB time, diabetes, hepatic insufficiency) were not unlike those that have been demonstrated in patients undergoing cardiac surgery through sternotomy [17, 18].
With regard to long-term outcomes, all major MICS procedures were associated with survival rates of more than 85% at 7 years, and there was no significant difference in survival among these MICS procedures, demonstrating that MICS can be safely performed for a variety of cardiac operations. In the multivariate hazards regression analysis, postoperative sternal wound infection, stroke, renal failure, previous surgery, and length of bypass time were all associated with increased risk of mortality. Again, similar to the risk factors identified for in-hospital complications, the risk factors associated with mortality were not unlike those reported in the literature for traditional sternotomy [19, 20]. The similarity between risk factors reported in our analysis and those reported in the literature for full sternotomy demonstrate comparability in risk assessment between MICS and sternotomy. Thus, the decision to pursue MICS should be guided more by the surgeon’s experience than patient-specific characteristics.
During an era where rapid technologic growth is occurring in the arenas of robotic surgery and percutaneous valve procedures, MICS represents an effective approach for a variety of cardiac operations. New technologic approaches should be encouraged but often require specialized skills beyond those of MICS, limiting their generalizability. In addition, the long-term outcomes of percutaneous valve surgery have yet to be studied. In our extensive series, minimally invasive approaches were effective and reproducible with acceptable operating time duration and low morbidity and mortality rates.
Acknowledgment
This work was supported in part by NIH Training Grant 5T32HL007854-14 (Dr. Iribarne).
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00268-009-0311-0
Contributor Information
Alexander Iribarne, Email: ai2141@columbia.edu.
Michael Argenziano, Phone: +1-212-3055888, Email: ma66@columbia.edu.
References
- 1.Goldstein DJ, Oz MC. Current status and future directions of minimally invasive cardiac surgery. Curr Opin Cardiol. 1999;14:419–425. doi: 10.1097/00001573-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Maehara T. Future of minimally invasive cardiac surgery. Ann Thorac Cardiovasc Surg. 2001;7:259–260. [PubMed] [Google Scholar]
- 3.McKeown PP. Minimally invasive cardiac surgery. Ann Thorac Surg. 1999;67:600–601. [PubMed] [Google Scholar]
- 4.Glenville B. Minimally invasive cardiac surgery. BMJ. 1999;319:135–136. doi: 10.1136/bmj.319.7203.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gayes JM. The minimally invasive cardiac surgery voyage. J Cardiothorac Vasc Anesth. 1999;13:119–122. doi: 10.1016/S1053-0770(99)90072-1. [DOI] [PubMed] [Google Scholar]
- 6.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg. 1997;226:421–426. doi: 10.1097/00000658-199710000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack M, Landreneau R. Minimally invasive cardiac surgery. Semin Laparosc Surg. 1996;3:259–267. doi: 10.1053/SLAS00300259. [DOI] [PubMed] [Google Scholar]
- 8.Cohn LH, Chitwood WR, Dralle JG, et al. Course guidelines for minimally invasive cardiac surgery: STS/AATS Ad Hoc Committee on New Technology Assessment. American Association for Thoracic Surgery. J Thorac Cardiovasc Surg. 1998;116:889–890. doi: 10.1016/S0022-5223(98)00459-0. [DOI] [PubMed] [Google Scholar]
- 9.Coulson AS, Bakhshay S, Bakhshay SA. Influence of minimally invasive cardiac surgery on thoracotomy approach to mitral valve surgery. Int Surg. 1998;83:91–92. [PubMed] [Google Scholar]
- 10.Yozu R, Shin H, Maehara T. Minimally invasive cardiac surgery by the port-access method. Artif Organs. 2002;26:430–437. doi: 10.1046/j.1525-1594.2002.06983.x. [DOI] [PubMed] [Google Scholar]
- 11.Pike NA, Gundry SR. Robotically assisted cardiac surgery: minimally invasive techniques to totally endoscopic heart surgery. J Cardiovasc Nurs. 2003;18:382–388. doi: 10.1097/00005082-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Kypson AP. Recent trends in minimally invasive cardiac surgery. Cardiology. 2007;107:147–158. doi: 10.1159/000094736. [DOI] [PubMed] [Google Scholar]
- 13.Landolfo KP. Minimally invasive cardiac surgery. Ann Surg. 2003;238:S110–111. doi: 10.1097/01.sla.0000097776.21375.ec. [DOI] [PubMed] [Google Scholar]
- 14.Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg. 2004;240:529–534. doi: 10.1097/01.sla.0000137141.55267.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aybek T, Dogan S, Risteski PS, et al. Two hundred forty minimally invasive mitral operations through right minithoracotomy. Ann Thorac Surg. 2006;81:1618–1624. doi: 10.1016/j.athoracsur.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.McClure RS, Cohn LH, Wiegerinck E, et al. Early and late outcomes in minimally invasive mitral valve repair: an eleven-year experience in 707 patients. J Thorac Cardiovasc Surg. 2009;137:70–75. doi: 10.1016/j.jtcvs.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 17.Hannan EL, Kilburn H, Jr, O’Donnell JF, et al. Adult open heart surgery in New York State: an analysis of risk factors and hospital mortality rates. JAMA. 1990;264:2768–2774. doi: 10.1001/jama.264.21.2768. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein AD, Parsonnet V. Bedside estimation of risk as an aid for decision-making in cardiac surgery. Ann Thorac Surg. 2000;69:823–828. doi: 10.1016/S0003-4975(99)01424-1. [DOI] [PubMed] [Google Scholar]
- 19.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/S1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 20.Tu JV, Jaglal SB, Naylor CD. Multicenter validation of a risk index for mortality, intensive care unit stay, and overall hospital length of stay after cardiac surgery; Steering Committee of the Provincial Adult Cardiac Care Network of Ontario. Circulation. 1995;91:677–684. doi: 10.1161/01.cir.91.3.677. [DOI] [PubMed] [Google Scholar]


