Abstract
The aim of this systematic review was to critically evaluate the evidence on interventions for depression following traumatic brain injury (TBI) and provide recommendations for clinical practice and future research. We reviewed pharmacological, other biological, psychotherapeutic, and rehabilitation interventions for depression following TBI from the following data sources: PubMed, CINAHL, PsycINFO, ProQuest, Web of Science, and Google Scholar. We included studies written in English published since 1980 investigating depression and depressive symptomatology in adults with TBI; 658 articles were identified. After reviewing the abstracts, 57 articles met the inclusion criteria. In addition to studies describing interventions designed to treat depression, we included intervention studies in which depressive symptoms were reported as a secondary outcome. At the end of a full review in which two independent reviewers extracted data, 26 articles met the final criteria that included reporting data on participants with TBI, and using validated depression diagnostic or severity measures pre- and post-treatment. Three external reviewers also examined the study methods and evidence tables, adding 1 article, for a total of 27 studies. Evidence was classified based on American Academy of Neurology criteria. The largest pharmacological study enrolled 54 patients, and none of the psychotherapeutic/rehabilitation interventions prospectively targeted depression. This systematic review documents that there is a paucity of randomized controlled trials for depression following TBI. Serotonergic antidepressants and cognitive behavioral interventions appear to have the best preliminary evidence for treating depression following TBI. More research is needed to provide evidence-based treatment recommendations for depression following TBI.
Key words: depression, psychiatry, review, traumatic brain injury, treatment
Introduction
Traumatic brain injury (TBI) is a major cause of disability worldwide, particularly with declining mortality rates (Thurman and Guerrero, 1999). In the U.S., an estimated 1.4 million people sustain a TBI annually, and approximately 3.17 million Americans live with TBI-related disabilities (Zaloshnja et al., 2008). Rates are similar for other industrialized nations (Bruns and Hauser, 2003). Data aggregated from Europe and the U.K. suggest that 235 per 100,000 people sustain a TBI severe enough to warrant hospitalization each year (Tagliaferri et al., 2006). The societal cost of TBI, including direct medical costs and indirect costs, has been estimated at $60 billion in the year 2000 in the U.S. alone (Finkelstein et al., 2006). These statistics do not include the toll incurred in the conflicts in Iraq and Afghanistan, which by any count is expected to comprise large numbers of persons with TBI and post-traumatic stress disorder (Hoge et al., 2008; Tanielian and Jaycox, 2008).
Long-term disability from TBI has primarily been attributed to neurobehavioral factors (Kraus and McArthur, 1999; NIH consensus development panel, 1999; Rosenthal et al., 1998b), and frequently includes difficulty remaining employed, maintaining social relationships, and fulfilling many other social roles (Hibbard et al., 1998; Kreutzer et al., 2003; Sander et al., 1996). In addition to the cognitive sequelae that contribute to these limitations, debilitating psychiatric problems such as depression, anxiety, and alcohol abuse are common among persons with TBI (Brooks et al., 1986; Deb et al., 1999; Hibbard et al., 1998; Kolakowsky-Hayner et al., 2002; Seel et al., 2003a; Seel et al., 2003b; van Zomeren and van den Burg, 1985).
Major depressive disorder (MDD) appears to be the most prevalent psychiatric disorder after TBI, with a point prevalence rate over 25% (Rutherford, 1977; Schoenhuber and Gentilini, 1988; van Zomeren and van den Burg, 1985). The reported period prevalence of MDD within the first year is 33%–42% (Jorge et al., 1993b; 2004), and within the first 7 years is 61% (Hibbard et al., 1998). Data from a recent prospective study of 559 subjects hospitalized after TBI revealed a prevalence rate of 52% for probable MDD within the first year after injury (Fann et al., 2003). The increased risk of depression is not limited to those with moderate to severe TBI; it is also present among those with mild TBI (Fann et al., 2004; Hoge et al., 2008). There is also an increased risk of suicide subsequent to TBI, with one study noting that 10% reported suicidal ideation at 1 year post-TBI, and 15% attempted suicide by 5 years post-injury (Brooks et al., 1986).
Depression is an important problem due to its effects on health, productivity, and quality of life. Depression is associated with a threefold decrease in adherence to medical regimens in patients with chronic illness (DiMatteo et al., 2000). In persons with neurological and medical conditions, depression may exacerbate neuropsychological impairment and slow the pace of cognitive recovery (Chen et al., 1996; Jorge et al., 1993a; Levin and Kraus, 1994; Mayberg, 1994; Miller et al., 1990; Robinson et al., 1985; Schoenhuber and Gentilini, 1988). Depression following TBI is associated with worse global outcomes (Federoff et al., 1992), worse social functioning during the first year post-injury (Jorge et al., 1993b; Schoenhuber and Gentilini, 1988), and lower health-related quality of life (Christensen et al., 1994; Rutherford, 1977), even after controlling for medical, demographic, and neuropsychological factors. Depressed survivors of TBI with MDD lasting more than 6 months exhibit deterioration in social functioning and performance of activities of daily living (Bourdon et al., 1992). Depressed TBI patients also report more severe post-concussive symptoms (e.g., headache, blurred vision, dizziness, and memory impairment) compared to non-depressed TBI patients (Fann et al., 1995; Rutherford, 1977).
Depression may result in part from direct or secondary injury to brain tissue. Studies of depression after neurological insult have implicated frontal lobe–basal ganglia circuits and anterior ascending monoaminergic pathways (Levin and Kraus, 1994; Rosenthal et al., 1998b). The frontal and temporal poles are preferentially affected by the focal and diffuse injury caused by TBI (Miller et al., 1990). Dorsolateral frontal, temporal, and left basal ganglia lesions have been associated with onset of depression after TBI (Chen et al., 2008; Fedoroff et al., 1991; Jorge et al., 2004). Depressed patients with TBI, stroke, and Parkinson's disease all show decreased glucose metabolism in the orbital-inferior frontal and anterior temporal cortices (Mayberg, 1994). Psychosocial factors are clearly important as well, and multiple causes of depression may interact in ways that are poorly understood. Increased vulnerability to MDD after TBI is associated with a prior history of MDD (Fann et al., 2004; Koponen et al., 2002), as well as unemployment, low income, and minority status (Seel et al., 2003b). As a result of the multi-factorial biological and psychosocial contributors to depression after TBI, basic questions remain about which treatment approaches might be most effective.
Treatment of MDD has a strong and evolving evidence base documented by numerous systematic reviews and meta-analyses (Cuijpers et al., 2007b; Cuijpers et al., 2007a; Furukawa, 2003; Moncrieff, 2004). The evidence for depression treatments after neurological insult is scarcer, although at least one Cochrane review has examined treatments for depression after stroke (Hackett et al., 2008). In contrast, information on the potential effectiveness of pharmacological or behavioral treatments of depression after TBI is lacking. A 2006 review of pharmacological treatments for neurobehavioral sequelae of TBI, including mood disorders, concluded that there was limited evidence to support or refute the effectiveness of psychotropic medications used in the general population to treat depression after TBI (Warden et al., 2006). Regarding non-pharmacological treatment, Rosenthal and colleagues commented in a 1998 comprehensive review that psychotherapy was frequently done with depressed persons with TBI, but no recommendations could be formulated because the published research was limited to uncontrolled case studies (Rosenthal et al., 1998).
The purpose of the present systematic review is to provide updated information on the evidence for pharmacological, other biological (e.g., electroconvulsive therapy), and psychotherapeutic or rehabilitation treatments for depression after TBI. Based on the current evidence, we identify gaps in the literature and make recommendations for clinical care and future research.
Methods
Search criteria
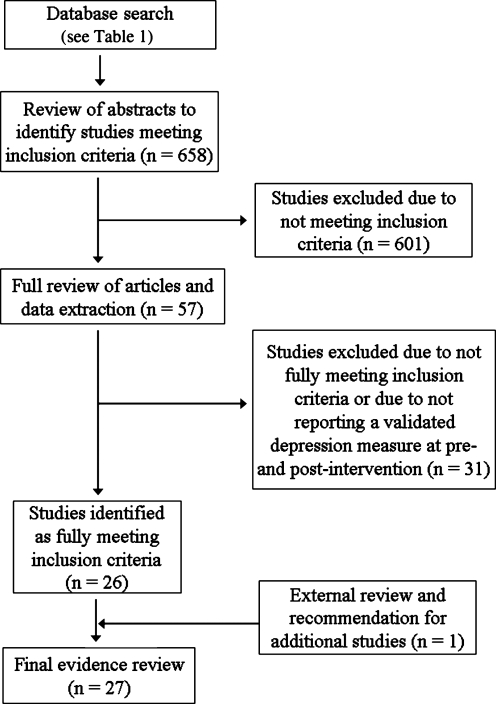
The criteria used to search for published studies for this systematic review included peer-reviewed studies: (1) investigating depression and depressive symptomatology; (2) in an adult population that included those with TBI; (3) published since 1980; and (4) written in English. For the initial search all study types such as review papers and meta-analyses were included. All study design types were also included. A diagram of the study selection is shown in Figure 1.
FIG. 1.
Study selection.
Searches were conducted in PubMed, CINAHL, PsycINFO, ProQuest, Web of Science, and Google Scholar. The specific search terms used were “depression,” “major depression,” “major depressive disorder,” “traumatic brain injury,” and “brain injury,” as well as the names of instruments commonly used to measure symptoms of depression. A complete list of the search terms for each database is included in Table 1. This comprehensive search located 658 articles on the topic of TBI and depression.
Table 1.
Documented Search Protocols for Treating Depression in Persons with TBI
| Database | Search terms |
|---|---|
| PubMed | Traumatic brain injury and Beck Depression Inventory or Zung Self-Rating Depression Scale or Center for Epidemiologic Studies or Patient Health Questionnaire or Structured Clinical Interview for DSM or Hamilton Rating Scale for Depression or Hospital Anxiety and Depression Scale or Minnesota Multiphasic Personality Inventory or Diagnostic Interview Schedule or Brief Symptom Inventory or Short Form-36 Health Survey or Neurobehavioral Functioning Inventory or Composite International Diagnostic Interview or Present-State Exam or major depression or depressive disorder |
| In PubMed, terms are searched as both keywords and subject headings simultaneously, and abbreviations are used for the scale names when appropriate | |
| The term older adult mood and health did not add results and was omitted from the final search | |
| CINAHL | Brain injuries and Center for Epidemiological Studies Depression Scale or Beck Depression Inventory or Self-Rating Scale or Minnesota Multiphasic Personality Inventory or Hamilton Rating Scale for Depression or Hospital Anxiety and Depression Scalea or Brief Symptom Inventory or Short Form-36 Health Survey (SF-36) or Neurobehavioral Functioning Inventorya or Present-State Exama or depression) |
| aThese terms did not have associated CINAHL subject headings and were searched as keywords; other terms were searched as CINAHL subject headings; scale abbreviations were used when appropriate | |
| The abbreviated terms DIS, CIDI, SCID, and PHQ-9 did not add results and were omitted from the final search | |
| PsycINFO | Traumatic brain injury and Beck Depression Inventory or Zung Self-Rating Depression Scale or Minnesota Multiphasic Personality Inventory or Hamilton Rating Scale for Depressiona or Hospital Rating Scale for Depressiona or Brief Symptom Inventorya or short form-36 health surveya or Neurobehavioral Functioning Inventorya or Composite International Diagnostic Interviewa or aPresent-State Exama or major depression |
| aThese terms did not have associated PsycINFO subject headings and were searched as keywords; other terms were searched as PsycINFO subject headings; scale abbreviations were used when appropriate | |
| The abbreviated terms DIS, SCID, CESD, and PHQ-9 did not add results and were omitted from the final search | |
| ProQuest Health and Medical Complete Library | Traumatic brain injury and Beck Depression Inventory or Zung Self-Rating Depression Scale or Center for Epidemiologic Studies or Patient Health Questionnaire or Structured Clinical Interview for DSM or Hamilton Rating Scale for Depression or Hospital Anxiety and Depression Scale or Minnesota Multiphasic Personality Inventory or Diagnostic Interview Schedule or Brief Symptom Inventory or Short Form-36 Health Survey or Neurobehavioral Functioning Inventory or Composite International Diagnostic Interview or Present-State Exam or major depression or depressive disorder |
| Terms were searched as keywords; scale abbreviations were used when appropriate | |
| Web of Science | Traumatic brain injury and Beck Depression Inventory or Zung Self-Rating Depression Scale or Center for Epidemiologic Studies or Patient Health Questionnaire or Structured Clinical Interview for DSM or Hamilton Rating Scale for Depression or Hospital Anxiety and Depression Scale or Minnesota Multiphasic Personality Inventory or Diagnostic Interview Schedule or Brief Symptom Inventory or Short Form-36 Health Survey or Neurobehavioral Functioning Inventory or Composite International Diagnostic Interview or Present-State Exam or major depression or depressive disorder |
| Terms were searched as keywords; scale abbreviations were used when appropriate | |
| Google Scholar | For this database, we completed multiple searches: |
| Search 1: traumatic brain injury, major depression | |
| Search 2: traumatic brain injury, depressive disorder | |
| Search 3: traumatic brain injury, depression | |
| The search was limited to medicine, pharmacology, and veterinary science |
CESD, Center for Epidemiologic Studies Depression Scale; CIDI, Composite International Diagnostic Interview; DIS, Diagnostic Interview Schedule; PHQ-9, Patient Health Questionnaire-9 depression scale; SCID, Structured Clinical Interview for DSM.
Criteria and methods for inclusion
After the search for published articles, more specific inclusion criteria were created to find the most relevant articles. The inclusion criteria were initially created to identify studies where the focus was on treating depression in those with TBI. Due to the paucity of studies returned with these criteria, the search was expanded to identify treatment studies in which depressive symptoms were reported as a secondary outcome. The specific criteria included studies with:
Any treatment modality: pharmacological, psychotherapeutic (e.g., individual or group psychotherapy, counseling, psycho-educational approaches), rehabilitation-based (e.g., comprehensive/holistic rehabilitation), exercise, electroconvulsive therapy (ECT), or transcranial magnetic stimulation;
Depression as a primary outcome: i.e., participants selected for depression and treatment focused on depression;
Depressive symptoms as a secondary outcome: i.e., participants not necessarily selected for depression, treatment not necessarily focused on depression, but depressive symptoms were measured and reported both pre- and post-intervention; and
Sample is composed of those with TBI, or the sample is not exclusively TBI, but results on the TBI subsample are reported separately.
Using these criteria, abstracts from the 658 articles found in the database search were reviewed by two trained reviewers at the University of Washington Model Systems Knowledge Translation Center (MSKTC). Discrepancies were resolved by consensus of the reviewers. If reviewers were unable to determine if the article met the criteria from the abstract the full article was reviewed. If a study did not meet the criteria, it was excluded from further review. After reviewing the abstracts, 57 articles appeared to meet the inclusion criteria.
Data extraction and outcome results
Two MSKTC reviewers independently extracted data from each of the 57 articles, including the research design and sample information, and the details of the interventions, outcome measures, and main outcomes. The data were compiled in an MS ACCESS database specifically developed for systematic reviews. Differences between the two reviewers' data extraction were reconciled by consensus. For each article the total number of data extraction changes between the two reviewers was recorded. These changes included correcting data in a field, filling in missing data, or moving misplaced data into the correct field. The mean number of changes per article between two reviewers was .85 (less than one change per article), with a range of 0 to 4, indicating strong reviewer and data consistency.
During the data extraction process, articles were excluded if the detailed full review revealed that they did not meet the initial criteria. It was also decided during the full review to exclude studies that did not report quantitative scores on a validated depression diagnostic or severity instrument both pre- and post-intervention. At the end of this full review, 26 of the 57 articles met the final criteria. Three external expert reviewers were asked to review the methods and evidence tables and make further recommendations. On the basis of the external reviews, one additional article meeting the final criteria was identified and included in the current review, for a total of 27 articles.
The level of evidence included in this review was categorized according to the American Academy of Neurology criteria for classifying therapeutic studies (Edlund et al., 2004). A final review of each of the 27 articles was performed by one of the investigators (J.R.F. or T.H.) to rate the evidence of the articles, with consultation between investigators as needed for accurate coding and interpretation. The studies were further categorized into the following groups based on depression inclusion criteria:
Prospectively enrolled depressed patients
Depressed patients retrospectively identified at baseline and results reported for them separately
Pre-post scores on depression measure were reported, but there was no selection for depressed patients as a subgroup
Results
Pharmacological interventions
The existing literature on the efficacy of pharmacologic treatment of depression after TBI is limited to small studies varying widely in design, diagnostic and outcome assessment, severity of brain injury, and time post-injury. This review includes 13 studies examining pharmacotherapy for depression (Table 2). There was one evidence class I study, one class II study, two class III studies, and nine class IV studies. Eleven studies prospectively enrolled depressed patients and examined depression “caseness” at post-intervention, while two examined pre- and post-intervention continuous depression scores only. The largest study had 54 participants, and all but three were uncontrolled trials. The studies enrolled a wide range of TBI severity at varying time points ranging from acutely to several years post-TBI, although specific TBI characteristics were not always reported.
Table 2.
Overview of Pharmacological and Other Biological Intervention Studies for Depression in Persons with TBI (n = 19)
| Authors | AAN evidence level | Depression inclusion criteria | Total no. | TBI sample: n severity acuity | Depression entry criteria | Depression instruments | Design and intervention | Results and conclusions |
|---|---|---|---|---|---|---|---|---|
| Pharmacologic interventions | ||||||||
| Ashman et al., 2009 | I | A | 52 | 52 35.5% mild, 38.7% moderate, 25.8% severe TBI Mean 17.7 ± 13.7 y post-TBI |
DSM-IV MDD (SCID) HAM-D > 17 |
HAM-D | Double-blind RCT of sertraline (25–200 mg/d) or placebo for 10 wk | Among the 41 who completed the trial, HAM-D, Beck Anxiety Inventory, and Life-3 Quality of Life scores improved significantly from pre- to post-treatment, but there were no group differences; 59% in the sertraline group and 32% in the placebo group had a 50% drop in baseline HAM-D score (p = 0.15) |
| Lee et al., 2005 | II | A | 30 | 30 Mild to moderate TBI Within 1 y of TBI |
DSM-IV MDD BDI ≥18 |
HAM-D BDI |
Double-blind RCT of methylphenidate (20 mg/d), sertraline (100 mg/d), or placebo for 4 wk | Both drugs improved HAM-D scores more than placebo; methylphenidate improved cognition, alertness, and PCS more than sertraline |
| Saran, 1985 | III | A | 22 | 10 minor TBI versus 12 non-TBI controls LOC ≤20 min, Hospitalized ≤48 h Normal EEG and CT < 1 yr post TBI |
DSM-III MDD with melancholia | HAM-D SDS |
Open trial of amitriptyline (200–300 mg/d, mean 175 mg/d) for 4 wk; amitriptyline non-responders (n = 10) had 3- to 7-day washout, then a trial of phenelzine (60–90 mg/d, mean 65 mg/d) | No significant improvement (50% drop in HAM-D) observed with either drug among subjects with TBI, whereas all non-TBI controls improved on amitriptyline; controls also improved on SDS affective, psychomotor, and psychological subtests; improvement in headaches was correlated with improvement in depression |
| Wroblewski et al., 1996 | III | A | 10 | 10 Severe TBI, PTA ≥1 wk, Rancho Los Amigos Head Injury Scale cognitive function level 4–6 0.5–2.5 y post-TBI |
DSM-III-R depression of at least 2 months duration | DSM-III-R 9-item symptom checklist; 25-item affect/mood scale | Blind random assignment to desipramine (n = 6) (150–300 mg/d) for at least 1 mo or to placebo (n = 4) with blinded cross-over for placebo group at 1 mo if non-responder | None in the placebo group had significant improvement (50% drop in 9-item DSM-III-R symptom checklist), and all were crossed over to desipramine; 6 of 7 study completers on desipramine had significant improvement; affect/mood scale improved overall (p = 0.001); 2 dropped out and 1 refused diagnostic interview; duration of desipramine treatment ranged from 1.5–3 mo |
| Dinan and Mobayed, 1992 | IV | A | 26 | 13 men with minor closed head injury (LOC <20 min, negative neurological exam, hospitalized <3 d) versus 13 non-head-injured controls (matched for age, sex, duration of symptoms, and HAM-D score) | DSM-III HAM-D >17 |
HAM-D CGI |
Open trial of amitriptyline (100–250 mg/d, TBI group, mean 158 mg/d; control group mean 179 mg/d) for 6 wk | 4/13 persons with TBI versus 11/13 non-TBI controls “significantly improved” on CGI; significantly more controls responded (50% drop in HAM-D or final score < 12) compared to the TBI group (p < 0.01), though specific HAM-D response rates were not provided; mean HAM-D in the TBI group dropped from 25.0 ± 7.2 to 18.8 ± 6.8 |
| Fann et al., 2000 | IV | A | 15 | 15 Mild TBI Mean 10.6 mo (range 3–24 mo) post-TBI |
DSM-III-R MDD on DIS HAM-D >17 |
HAM-D CGI |
Non-randomized, single-blind, placebo run-in trial of sertraline (25–150 mg/d, mean 75 mg/d) lasting 8 wk | Response rate (50% drop in HAM-D) was 86.7% and remission rate (final HAM-D ≤7) was 66.7%; there were significant improvements in psychological distress, PCS, cognitive functioning, and QOL |
| Horsfield et al., 2002 | IV | C | 5 | 5 Multiple TBIs ranging from mild to severe TBI acuity not noted |
No depression criteria noted Non-specific behavioral, psychiatric, and cognitive complaints No history of antidepressant treatment |
HAM-D | Open trial of fluoxetine (20–60 mg/d) for 8 mo | Significant reduction in HAM-D scores from baseline (18 ± 7.07) to 8 mo (9.8 ± 8.07) (p < 0.05) |
| Kanetani et al., 2003 | IV | A | 10 | 10 Mild to moderate TBI (GCS 12–15, 7 had brain lesions on CT) Mean 152.8 d post-TBI |
DSM-IV (MINI) minor (n = 3) or major (n = 7) depression | HAM-D 21 | Open trial of milnacipran (30–150 mg/d) for 6 wk | Response rate (50% drop in HAM-D) was 66.7%, and remission rate (final HAM-D ≤7) was 44.4%; there was significant improvement in cognition on MMSE. |
| Khateb et al., 2005 | IV | C | 10 | 10 Moderate to severe TBI At least 6 mo, mean 42 ± 33 mo from TBI |
No depression criteria noted Exclusion: “unstable psychiatric disorders” |
HADS | Open trial of donepezil for 3 mo: 5 mg/d for 1 mo, then 10 mg/d for 2 mo | Nonsignificant reduction in HADS depression scores from baseline (6.8 ± 4.4) to 3 mo (5.0 ± 3.0); significant improvement in processing speed, learning, and attention on neuropsychological testing |
| Newburn et al., 1999 | IV | A | 26 | 26 Severity not noted Mean 4.67 years post-TBI |
DSM-III-R MDD HAM-D ≥17 Moderate depression on CGI |
HAM-D CGI |
Open trial of moclobemide (450–600 mg/d), either as a single dose or 3 divided doses, for 3–6 wk | Baseline HAM-D = 23.4 (range 17–29), mean HAM-D reduction was 81%; 23/26 defined as responders (HAM-D < 10 or 50% reduction); irritability scores dropped by 57% and pain scores dropped by 39% |
| Perino et al., 2001 | IV | A | 20 | 20 Severe TBI 11 < 6 mo post-TBI; 9 between 24 and 36 mo post-TBI |
DSM-IV MDD | BPRS CGI |
Open trial of citalopram (20 mg/d) and carbamazepine (600 mg/d) for 12 wk | Significant reductions in BPRS (p < 0.05) and CGI (p < 0.005) scores overall; post-acute subgroup had worse outcomes than group treated early |
| Rapoport et al., 2008 | IV | A | 54 | 54 Mild to moderate TBI < 1 y post TBI |
DSM-IV MDD (SCID) | HAM-D CGI |
Open trial of citalopram at 20 mg/d for 6 wk (n = 29) or 20–50 mg/d for 10 wk (n = 26) | At 6 wks (n = 54), 27.7% responded (50% drop in HAM-D), and 24.1% remitted (HAM-D <7); at 10 wk (n = 26), 46.2% responded and 26.9% remitted |
| Turner-Stokes et al., 2002 | IV | A | 21 | 3 Severity not noted Mean time from admission to acute rehabilitation was 63.3 d |
DSM-IV Major depressive-like episode (n = 1) Depressive features (n = 2) |
BDI-II | Open trial of sertraline (50–100 mg) for 4–6 wk | Marked clinical response in patient with major depressive-like episode (BDI-II dropped from 30 to 10); some improvement in 2 patients with depressive features (BDI-II dropped from 16 to 9 in one patient; the other was untestable) |
| Other biological interventions | ||||||||
| Schoenberger et al., 2001 | II | C | 12 | 12 CHI with reported substantial cognitive difficulties 9 mild, 3 moderate 36 mo to 21 y from CHI |
None | BDI | RCT of Flexys Neurotherapy System of biofeedback (EEG recording with photic feedback) for 25 sessions over 5–8 wk (6 active treatment and 6 wait-list controls) | Significantly greater pre-post improvement on BDI in intervention group (22.5 ± 9.9 to 7.0 ± 5.3) versus control group (16.7 ± 9.8 to 16.2 ± 12.2); within subjects (n = 12) BDI scores significantly improved from pre-treatment (19.3 ± 11.1) to post-treatment (7.9 ± 6.9) and 3-mo follow-up (7.8 ± 6.7) |
| Baker-Price and Persinger, 2003 | III | A | 11 | 11 Closed head injury 4 had LOC Mean 2 y after injury |
Chronic depression diagnosed by professional or self-response to local newspaper, unresponsive to antidepressant | BDI | Open 2-group study of magnetic field (1-microtesla burst once a week for 6 wk); 7 subjects across left frontal lobe (3 dropped out); 7 subjects across bilateral temporal lobes | BDI scores significantly decreased from baseline (19.7 ± 8.6) to 6 wk (14.1 ± 5.2) and 6 wk after end of treatment (15.1 ± 7.6); no difference between regions of application of magnetic field |
| Baker-Price and Persinger, 1996 | IV | A | 4 | 4 Acquired brain injury with mild to moderate impairment on neuropsychological testing 2 within 1 y 1 within 3 y 1 about 6 y from injury |
Persistent or frequently intermittent depression diagnosed by physician, unresponsive to antidepressant | BDI SCL-90 |
Open study of magnetic field (1-microtesla burst, once a week for 5 wk) across bilateral temporal lobes | Significant decrease in BDI over 5 wk: mean 33 ± 9 to 17 ± 9; SCL-90 depression scale dropped by 1.5 SD, but not statistically significant |
| Donnellan, 2006 | IV | C | 1 | Severe TBI with multiple injuries and significant pain Approximately 5 mo after TBI |
None | HADS | Classical Chinese medicine acupuncture points on limbs and thorax (7 treatments over 6 wk) |
No change in pre-post HADS depression (7/21) or anxiety (9/21) score, despite subjective improvement in anxiety and mood, and significant improvement in pain scores |
| Kant et al., 1999 | IV | B | 11 | 11 Mild to severe (4 with multiple CHI) Within 7–48 mo of injury |
DSM-III-R criteria for neuropsychiatric conditions; 4 had MDD (all antedating CHI), 5 had mood disorder secondary to CHI, 1 had chronic delirium, 1 had delusional disorder secondary to CHI; all unresponsive to medications or prior responders to ECT |
MADRS CGI |
Retrospective study of ECT 3 times per week (total no. of treatments ranged from 4–20; mean 10); 8 patients received continuation ECT (total treatments ranged from 3–62; mean 18) | 8 responders (i.e., MADRS ≤15; 50% reduction in pre-ECT MADRS, CGI ≤3); 2 partial responders (1/3 criteria met; both responded after continuation ECT); 1 nonresponder; among 9 with mood disorders, MADRS dropped from 33.4 ± 6.4 to 11.2 ± 8.5 |
| Martino et al., 2008 | IV | A | 1 | 1 Severe TBI 6 y post TBI |
MDD | HAM-D (24-item) | 8 bifrontal ECT treatments, followed by 4 continuation treatments over 6 wk | HAM-D dropped from 25 at baseline to 12 after acute treatment to 7 after continuation treatment; neuropsychological battery showed stable or improved scores on all tests except MMSE and Trail Making Test B |
AAN, American Academy of Neurology; TBI, traumatic brain injury; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM-III, Diagnostic and Statistical Manual of Mental Disorders, Third Edition; MDD, major depressive disorder; BDI, Beck Depression Inventory; BPRS, Brief Psychiatric Rating Scale; CGI, Clinical Global Impressions Scale; CHI, closed head injury; DIS, diagnostic interview schedule; HADS, Hospital Anxiety and Depression Scale; HAM-D, Hamilton Rating Scale for Depression; LOC, loss of consciousness; MADRS, Montgomery-Asberg Depression Rating Scale; MINI, Mini-International Neuropsychiatric Interview; MMSE, mini-mental state examination; PCS, post-concussive symptoms; QOL, quality of life; SCID, Structured Clinical Interview for DSM; SDS, Zung Self-Rating Depression Scale; SCL-90-R, Symptom Checklist-90-Revised; RCT, randomized controlled trial; EEG, electroencephalogram; CT, computed tomography; PTA, post-traumatic amnesia; GCS, Glasgow Coma Scale; SD, standard deviation; ECT, electroconvulsive therapy.
Depression Inclusion Criteria
A. Prospectively enroll depressed patients
B. Depressed patients retrospectively identified at baseline
C. Pre-post depression scores only, with no selection for depressed patients at baseline
The agents that were studied included tricyclic antidepressants (TCAs: amitriptyline and desipramine), monoamine oxidase inhibitors (MAOIs: phenelzine and meclobemide), and selective serotonin reuptake inhibitors (SSRIs: fluoxetine, sertraline, and citalopram). One study each included a dual-action serotonin-norepinephrine reuptake inhibitor (SNRI: milnacipran), a psychostimulant (methylphenidate), a cholinesterase inhibitor (donepezil), and an anticonvulsant (carbamazepine).
Results of intervention
In the only evidence class I pharmacotherapy study, Ashman and colleagues (Ashman et al., 2009) randomized 52 patients to sertraline or placebo (41 patients completed the study). Although there were no statistically significant group differences in response rates or decrease in HAM-D scores over 10 weeks, 59% of the sertraline group were responders (50% decrease in baseline HAM-D), while only 32% of the placebo group were responders, among the completers. The authors did not report final dosage ranges or specifics about adverse effects, though only one subject withdrew due to side effects. Unique characteristics of the study sample included a high rate of low-income and minority patients. Also, the mean time since injury was 17.7 (SD 13.7) years, making it difficult to ascertain the relative contribution of the TBI to their depressive episode.
In the only evidence class II study, Lee and associates (Lee et al., 2005) randomized 30 patients with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 2000) MDD within 1 year of mild to moderate TBI to receive 4 weeks of 20 mg methylphenidate, 100 mg sertraline, or placebo. Both the methylphenidate and sertraline groups had greater improvements in HAM-D scores than the placebo group, though methylphenidate had the added benefit of improving cognition, alertness, and post-concussive symptoms greater than sertraline. The small sample size, short study duration, and attenuated dosage titration limit the ability to draw firm conclusions from this study.
In the other randomized, placebo-controlled study, Wroblewski and associates (Wroblewski et al., 1996) studied the efficacy of desipramine for depression following severe TBI. Three of the original 10 patients dropped out (one with mania and one with seizures), leaving 7 in the final analysis. Six of these 7 patients improved on desipramine; however, 4 patients who were not responding in the placebo group were crossed over to desipramine after only 1 month. Moreover, the severity of depression was mild (3 patients had 4 or less DSM depressive symptoms at baseline), and it was not clear how many patients met criteria for MDD. The small sample size and other limitations of the study limit the ability to draw conclusions about the efficacy of TCAs in patients with TBI, particularly in light of the findings of the uncontrolled TCA studies done by Saran and co-workers (Saran, 1985) and Dinan and Mobayed (Dinan and Mobayed, 1992). These class III and IV studies reported that persons with depression and minor TBI who were treated with amitriptyline did not show improvements comparable to persons with primary depression not coexisting with TBI. However, unlike the work by Wroblewski and colleagues (Wroblewski et al., 1996), these two studies did not utilize serum TCA levels to guide dosage titration, and adverse effects were not reported. In a retrospective review of 68 severely head-injured patients taking TCAs, Wroblewski and associates (Wroblewski et al., 1990) concluded that 19% developed seizures largely due to TCAs. It is worth noting that patients in the study by Saran and colleagues (Saran, 1985) had particularly high levels of depression at baseline. Furthermore, Dinan and Mobayed (Dinan and Mobayed, 1992) excluded patients who had a pre-TBI history of affective disorder, and specific HAM-D response rates were not reported.
The MAOI phenelzine was not found to be efficacious in the 10 amitriptyline non-responders who crossed over to this medication in the study by Saran and colleagues (Saran, 1985). On the other hand, the MAO-A isoenzyme blocker meclobemide was found to be effective in a class IV open study in patients with MDD an average of 4.67 years post-TBI (Newburn, 1999). Irritability and pain scores also improved, and 4 patients dropped out due to gastrointestinal side effects.
Taken together, the 7 studies using SSRIs suggest that these drugs may be efficacious and seem to be well-tolerated in some persons with TBI, although small sample sizes did not allow for adequate subgroup analyses to determine predictors of response. There is the most evidence supporting the use of sertraline and citalopram. Two class IV studies by Fann and colleagues (Fann et al., 2000) and Turner-Stokes and associates (Turner-Stokes et al., 2002) provide preliminary evidence for the efficacy and tolerability of sertraline in persons with DSM-IV MDD. A recent class IV study of citalopram in mild-to-moderate TBI (Rapoport et al., 2008) suggests that this SSRI is also well-tolerated and potentially efficacious, though the rates of response and remission were lower than for sertraline in Fann and co-workers' (Fann et al., 2000) study of mild TBI. The class IV study by Perino and associates (Perino et al., 2001) of combination citalopram and carbamazepine did not allow conclusions to be drawn about the efficacy or either of the medications alone. A class IV study of fluoxetine (Horsfield et al., 2002) suggests the potential efficacy of this SSRI; however, the study did not require patients to be depressed at study entry and had a duration of 8 months. Therefore, more experience with this SSRI is required to assess its acute efficacy in treating major depression after TBI.
SSRIs were well-tolerated in most studies. Sertraline was well-tolerated in the 3 reviewed studies, with gastrointestinal symptoms and headaches reported most frequently in the study by Fann and colleagues (Fann et al., 2000). Stanislav and Childs (Stanislav and Childs, 1999) reported on a patient who developed dystonia while taking sertraline 50 mg after severe TBI (not included in the evidence table due to lack of a depression measure). Dry mouth, nausea, decreased libido, and sedation were the most common side effects of citalopram in Rapoport and colleagues' (Rapoport et al., 2008) study. In a case series by Cassidy and co-workers (Cassidy, 1989), of fluoxetine treatment for MDD with melancholia after moderate to severe TBI (also not included in the evidence table because it relied on subjective reports of depressive symptoms by staff, patients, and family members), 50% complained of sedation, and 3 of 8 patients complained of anxiety.
The one open study (n = 10) that examined the dual-action SNRI (milnacipran), for minor or major depression after mild-to-moderate TBI, also suggests that it may be efficacious (66.7% responded and 44.4% remitted) and well-tolerated (Kanetani et al., 2003). One subject dropped out due to nausea. In a 3-month open study of the cholinesterase inhibitor donepezil in 10 patients at least 6 months following moderate-to-severe TBI (they did not have to be depressed), patients showed significant improvements in processing speed, learning, and attention, but not depression (Khateb et al., 2005).
Other biological interventions
There are limited data supportive of the efficacy and tolerability of ECT, low-intensity magnetic field exposure, biofeedback, and acupuncture for treating depression after TBI. However, the narrowly selected and small samples and divergent TBI severity and proximity characteristics make these results highly preliminary. Moreover, none of the 6 studies reviewed used structured DSM diagnostic interviews for study entry.
The study by Kant and associates (Kant et al., 1999) examining ECT was a retrospective examination of 9 patients with mood disorders following TBI of varying severity and acuity. The 2 patients who partially responded to initial treatment eventually responded to maintenance ECT. The authors recommend adapting ECT by using unilateral electrode placement and a lower frequency of applications. Significant cognitive impairment was not reported in this small case series. The case report by Martino and colleagues (Martino, 2008) describes a successful treatment of MDD 6 years following severe TBI with eight sessions (the time course was not noted) of bifrontal ECT, followed by four continuation treatments. Cognitive measures actually improved over multiple domains other than cognitive flexibility, which decreased slightly.
The two magnetic field stimulation studies (Baker-Price and Persinger, 2003; Baker-Price and Persinger, 1996) used stimulus levels (1 microtesla) much smaller than those used in the currently FDA-approved repetitive transcranial magnetic stimulation depression treatments. These small studies did not include control groups. An evidence class II study using EEG recordings with photic biofeedback in 12 patients with cognitive difficulties did include a waitlist control, showing a significantly greater decrease in depression scores in the biofeedback group (Schoenberger et al., 2001). A case report of classical Chinese acupuncture in a patient with severe TBI, multiple injuries, and severe pain showed improvement in pain, but not depression or anxiety scores (Donnellan, 2006).
Psychotherapeutic and rehabilitation interventions
Eight studies were reviewed that used psychotherapeutic or rehabilitative treatments (Table 3): one class IV controlled case report (Ownsworth, 2005), and seven group studies that provided experimental and/or control interventions to samples ranging from 13–130 participants. Of the group studies, three used random assignment to treatment conditions: one (Powell et al., 2002) met criteria for class I evidence, and two (McMillan, 2002; Tiersky et al., 2005) were considered class II. The remaining studies either lacked experimental control or used weak controls unlikely to minimize bias, and were classified as class IV.
Table 3.
Overview of Psychotherapeutic and Rehabilitation Intervention Studies for Depression in Persons with TBI (n = 8)
| Authors | AAN evidence level | Depression inclusion criteria | Total no. | TBI: n severity acuity | Depression entry criteria | Depression instruments | Design and intervention | Results and conclusions |
|---|---|---|---|---|---|---|---|---|
| Powell et al., 2002 | I | C | 110 | 110 At least moderate TBI (PTA >24 h or neurosurgical intervention); nearly all had PTA >1 wk; majority had PTA >1 mo 3 mo–20 y post-TBI; median 1.37 y |
No depression criteria 15% scored >13 on HADS pretreatment |
BICRO-39 Psychological well-being subscale rated by participants and caregivers given to 75 participants HADS given to 46 participants with sufficient cognitive ability |
RCT with masked outcome assessment Experimental = individualized, goal-planning-oriented multi-disciplinary team treatment in home or community setting, 2–6 h/wk for mean of 28 wk Control = information condition; 1 home visit with individualized resource booklet |
Primary outcome = Barthel Index and BICRO-39 at mean 2-y post-randomization; experimental > control on both measures (p < .005) 68% of experimental and 50% of control group improved on BICRO-39 psych subscale (p < 0.05) 50% of experimental and 54% of control group improved on HADS (ns) |
| McMillan, 2002 | II | C | 145 | 145 TBI (any severity) with attention complaints or deficits on neuropsychological testing; mean PTA ∼1 mo 3–12 mo post-TBI |
No depression criteria | HADS | RCT with masked outcome assessment Experimental = attention control training: five 45-min sessions supervised practice using audio tape for 4 wk; daily independent practice with tape Control 1 = physical fitness training with same amount of therapist contact and independent practice Control 2 = no treatment, no therapist contact |
Primary outcome not specified No significant group differences were found on HADS or other measures. Pre-treatment HADS 7 ± 5 ACT group, 5 ± 4 exercise group, 5 ± 4 control group; post-treatment HADS 5 ± 4 ACT group, 4 ± 4 exercise group, 6 ± 5 control group |
| Tiersky et al., 2005 | II | C | 20 | 20 Mild/moderate TBI (GCS >8, LOC ≤4 h); 40% had no LOC At least 1 y post-TBI; mean 6 y |
No specific depression criteria Inclusion: complaints of emotional distress on SCL-90-R |
SCL-90-R depression scale | RCT with masked outcome assessment Experimental = “comprehensive neuropsychological rehabilitation:” cognitive remediation (attention process training, memory notebook, problem solving) + CBT; two 50-min individualized sessions, w/daily 30-min homework, 3 × /wk × 11 wk Control = wait list/attention control × 11 wk, with a total of 2 or 3 45-min contacts from primary investigator |
Primary outcome = SCL-90-R GSI; experimental < control (p < .05) Depression scale on SCL-90-R, experimental < control (p < 0.05) Authors noted that post-treatment means remained above “caseness” levels |
| Anson and Ponsford, 2006 | IV | C | 33 | 33 Mean PTA 32 d; ∼90% had PTA <1 wk 46 d–7 y post-TBI |
No depression criteria | HADS | Pre-post-treatment design (data were pooled from two groups with different baselines) Experimental = CBT-based coping skills group, 10 sessions (two 90-min sessions/wk) over 5 wk + homework assignments Control = baseline phase × 5 wk |
Primary outcome not specified Change in HADS depression from pre- to post-treatment = ns (% change = 1.3 ± 41) Authors reported that several variables correlated with higher percentage of improvement in depression (e.g., greater self-awareness, less severe injury, higher premorbid intelligence) |
| Bedard et al., 2003 | IV | C | 13 | 13 Severity not noted At least 1 y post-TBI |
No depression criteria Exclusion: “major concurrent mental illness,” suicidal ideation |
BDI-II | Pre-post treatment design Experimental = weekly group × 12 wk based on mindfulness meditation Control = none; data from three treatment drop-outs used as comparison group |
Primary outcome not specified Change on BDI-II pre- (18.4 ± 12.2) to post-treatment (9.7 ± 10.6) = ns Group × time interaction for BDI-II approached significance (p = .06); treatment completers scored lower and treatment dropouts scored higher (worse) at follow-up |
| Gurr and Coetzer, 2005 | IV | C | 20 | 20 (13 for 3-mo follow-up) 9 mild, 3 moderate, 8 severe At least 6 mo post-TBI; range 7–474 mo |
No depression criteria Exclusion: “psychiatric conditions” |
HADS | Pre-post treatment design (data from a pretreatment baseline phase were presented but not analyzed) Experimental = CBT-based treatment focused on headache management; three weekly relaxation groups +6 30-min individual sessions every other week + 1 follow-up session Control = pre-treatment baseline × 10 wk (data presented but not analyzed) |
Primary outcome = headache frequency and intensity; decreased after treatment Change in HADS pre- (9.7 ± 4.1) to post-treatment (8.5 ± 4.6) = ns |
| Ownsworth, 2005 | IV | n/a | 1 | 1 Severe TBI; coma 6 d; PTA 12 d 4.5 y post TBI |
n/a | DASS | Case report with masked outcome assessment 13 weekly psychotherapy sessions with brain injury education and CBT skills with focus on defensive denial, plus attendance at three TBI group sessions |
Primary outcome not specified Pre-treatment depression on DASS extremely severe (34/42); 1 wk post-treatment depression in normal range (2/42) |
| Svendsen et al., 2004 | IV | C | 143 | 38 Severity not noted; eight had “pure frontal injury” per neuroimaging Acuity not noted |
No depression criteria Exclusion: “psychiatric or progressive neurodegenerative illness” |
EBIQ Depression subscale completed by participants and relatives | Pre-post treatment design Experimental = interdisciplinary, holistic day treatment administered in “classes” of 16 participants; daily for 4 mo + 8 mo close monitoring in community Control = none |
Primary outcome not specified EBIQ depression scores converted to z scores by comparing to uninjured controls; pre-treatment z = –1.1; post-treatment z = –0.5 Similar results for self and relative ratings Authors notes that z scores changed significantly pre- to post-treatment, but remained worse than controls |
Depression Inclusion Criteria
A. Prospectively enrolled depressed patients
B. Depressed patients retrospectively identified at baseline
C. Pre-post depression scores only, with no selection for depressed patients at baseline
ACT, Attention Control Training; GSI, General Symptom Index; AAN, American Academy of Neurology; DASS, Depression Anxiety Stress Scale; EBIQ, European Brain Injury Questionnaire; TBI, traumatic brain injury; BDI, Beck Depression Inventory; BICRO-39, Brain Injury Community Rehabilitation Outcome-39 scales; HADS, Hospital Anxiety and Depression Scale; LOC, loss of consciousness; SCL-90-R, Symptom Checklist-90-Revised; RCT, randomized controlled trial; PTA, post-traumatic amnesia; GCS, Glasgow Coma Scale; ns, not significant. n/a, not available; CBT, cognitive behavioral therapy.
There was considerable variability in the treatment models used in this set of studies. The case report and three of the group studies were based in principles of cognitive behavioral therapy (CBT), administered individually (Ownsworth, 2005; Tiersky et al., 2005), in groups (Anson and Ponsford, 2006), or both (Gurr and Coetzer, 2005). All four of these studies described the inclusion of “ingredients” other than pure CBT, including individualized cognitive retraining (Tiersky et al., 2005). Two studies used attention self-control techniques associated with mindfulness meditation (Bedard et al., 2003; McMillan, 2002). One study reported results of holistic, interdisciplinary “milieu” therapy, including individual and group treatment in a day program targeted to a wide range of cognitive and psychosocial outcomes (Svendsen et al., 2004). The remaining study (Powell et al., 2002) used a multidisciplinary team approach to meeting individualized goals, and provided the only treatment of the eight that was explicitly based in the participant's home and community rather than a clinic setting. The latter two studies, focused as they were on holistic and community outcomes, provided more intensive treatment and a higher overall “dose”—more than 100 hours in total—compared to the CBT- and meditation-based treatments, which tended to address more circumscribed goals in 5–15 h of therapy. (The exception in this set was the study of Tiersky and colleagues [Tiersky et al., 2005], which included cognitive remediation in addition to CBT, and provided about 70 h of treatment.) There also appeared to be variation in the degree to which the treatments were based on standardized protocols or manuals; of the group studies, only three specified that a treatment manual was used (Bedard et al., 2003; Gurr and Coetzer, 2005; Tiersky et al., 2005).
It is important to note that although all eight studies included both pre- and post-treatment measures of depressive symptoms (an inclusion criterion for this review), none of them were designed specifically to evaluate treatments for depression. Thus, there was heterogeneity as to whether and how pre-treatment depression was identified, and the degree to which depressive symptoms occurred in the samples. Pre-treatment depression and/or emotional distress was identified as significant in the case study (Ownsworth, 2005), and the study by Tiersky and associates (Tiersky et al., 2005), which used emotional distress as an inclusion criterion. In the study by Svendsen and co-workers (Svendsen et al., 2004), mean pre-treatment scores on the depression subscale of the European Brain Injury Questionnaire (EBIQ) were reportedly higher than those of an uninjured control group recruited for the study. The mean Beck Depression Inventory-II (BDI-II) score in the Bedard and Persinger (Bedard and Persinger, 2003) study (18.4) was consistent with mild depression. In the remaining four studies, mean pre-treatment depression scores were within the normal range (Anson and Ponsford, 2006; Gurr and Coetzer, 2005; McMillan, 2002; Powell et al., 2002). In fact, the study by Bedard and colleagues (Bedard et al., 2003) excluded participants with psychiatric disorders or substance abuse, as did two other class IV studies (Gurr et al., 2005; Svendsen et al., 2004). Substance abuse, which can co-occur with depression, was also excluded in the study by McMillan and associates (McMillan, 2002). Thus, for only one of the group studies (Tiersky et al., 2005) was there any reason to expect a higher rate of depression in the sample than might normally be seen after TBI; and for four others (Bedard et al., 2003; Gurr and Coetzer, 2005; McMillan, 2002; Svendsen et al., 2004), exclusion criteria may have resulted in a lower-than-usual rate of depression.
The fact that depression was not the focus of the psychotherapeutic and rehabilitation study interventions provides an essential context within which to interpret the composition of the treatments reviewed. The CBT-oriented studies used treatment components intended to improve emotional well-being, but not necessarily as a primary focus. For example, the study by Gurr and Coetzer (Gurr and Coetzer, 2005) targeted reduction in frequency and intensity of headaches as a primary outcome. One class II (Tiersky et al., 2005) and two class IV studies (Anson et al., 2006; Bedard et al., 2003) addressed general emotional distress as a primary outcome; this could include depression, as well as anxiety and other complaints. The case study (Ownsworth, 2005) addressed emotional well-being, including depressive symptoms, with a focus on defensive denial. The other class II study (McMillan, 2002) examined a range of cognitive as well as emotional functional outcomes. Of the remaining studies, the class I study (Powell et al., 2002) and one class IV study (Svendsen et al., 2004) examined a wide range of outcomes befitting comprehensive treatment programs, including physical and cognitive as well as emotional symptoms (Svendsen et al., 2004), and multiple measures at the level of function, activity, and societal participation (Powell et al., 2002).
Results of intervention
Three of the four CBT-based studies—the case study by Ownsworth (Ownsworth, 2005), the class II study by Tiersky and colleagues (Tiersky et al., 2005), and a class IV study by Gurr and Coetzer (Gurr and Coetzer, 2005)—reported positive effects of treatment on mood. The study with the strongest design in this subgroup (Tiersky et al., 2005) also used a small sample (n = 20) of participants who clearly had the mildest TBIs of all eight studies. Recruitment was targeted to persons with persistent emotional problems following a loss of consciousness (LOC) of no more than 4 h; 40% reported no LOC. Participants were 5 years post-injury on average; 55% were female, 70% had at least a college education, and 40% were involved in litigation. Although the treatment group experienced significantly more reduction in emotional distress compared to those on a wait-list for a comparable period of time, their post-treatment depression and overall distress levels remained above the “caseness” cutoff on the SCL-90. Gurr and Coetzer (Gurr and Coetzer, 2005) reported positive change on both the primary outcome (post-traumatic headache) and on the Hospital Anxiety and Depression Scale (HADS). However, in this study more than 50% of participants dropped out of treatment, and the results were based only on analysis of pre- to post-treatment scores from 13 who remained. Anson and Ponsford (Anson and Ponsford, 2006) reported that their 5-week group CBT-based treatment had no significant overall effects on coping style, mood, or adjustment, in 33 persons with primarily severe TBI. In a post-hoc analysis, however, these authors suggested that less severe TBI, greater awareness of deficit, and higher premorbid intelligence, were associated with greater reduction of depression, as measured by the HADS, from pre- to post-treatment.
Of the two treatments based on mindfulness meditation, the class II study by McMillan (McMillan, 2002) reported no effects compared to a comparable “dose” of physical exercise training or no-treatment controls on a range of cognitive and emotional outcomes, despite a promising pilot study and a large sample completing treatment in the full trial (n = 130). Bedard and colleagues (Bedard et al., 2003), in a small (n = 10) class IV study, reported marginally significant pre- to post-treatment change on the BDI-II, but results may have been biased by use of three treatment dropouts as a “control” group.
The remaining two studies offered intensive, multifaceted treatments delivered by a rehabilitation team, either in a clinical milieu with individual and group interventions (Svendsen et al., 2004), or in the patient's home and community settings to meet individualized goals (Powell et al., 2002). The sole class I study (Powell et al., 2002) reported no significant effects of such treatment on the proportions of participants who met criteria for depression. However, significant improvement was reported on a primary outcome measure, the Brain Injury Community Rehabilitation Outcome-39 scales (BICRO-39), which includes a scale of psychological well-being. The class IV study (Svendsen et al., 2004) reported that their milieu treatment program, the most intensive of the eight reviewed (day treatment for 4 months, followed by 8 months of close monitoring in the community), was followed by lower scores on the EBIQ depression subscale compared to pre-treatment. Similarly to Tiersky and associates (Tiersky et al., 2005), these authors noted that despite the improvement, post-treatment depression scores remained elevated compared to those of an uninjured control group.
Discussion
This systematic review differs from prior reviews of interventions for TBI (Alderfer et al., 2005; Warden et al., 2006), in that it systematically examines the evidence for the efficacy of both biological and psychosocial interventions on depression outcomes specifically. Although the data on the treatment of depression following TBI have grown over the past decade, the paucity of adequately powered and controlled studies, including randomized controlled trials, limits the ability to establish evidence-based treatment guidelines. Among the 27 studies meeting criteria for inclusion in this review, there were only two evidence class I studies and four evidence class II studies. Only two of the class I or II studies included depression as an inclusion criterion for study entry (Ashman et al., 2009; Lee et al., 2005). The class I pharmacotherapy study (Ashman et al., 2009) showed trends toward superiority of sertraline over placebo in a demographically heterogeneous sample that was temporally far removed from their TBI, but was underpowered to examine predictors of response. The class I psychosocial study (Powell et al., 2002) demonstrated improvements in general psychological well-being, but not depressive symptoms specifically, following a comprehensive, community based, interdisciplinary team intervention targeted to multiple outcomes. The class II studies spanned modalities from pharmacotherapy (Lee et al., 2005) to psychotherapy (Tiersky et al., 2005) to alternative approaches such as biofeedback (Schoenberger et al., 2001) and meditation (McMillan, 2002). While none of these studies provided sufficient evidence for practice guidelines, taken together they do indicate that well-controlled studies are beginning to be applied to the problem of depression after TBI.
The variety of modalities being studied is appropriate to the target problem. As noted earlier, both biomedical and psychosocial factors contribute to MDD in people with TBI. Researchers have observed that especially soon after TBI, MDD may be more biologically determined, for example by pre-injury susceptibility and/or lesion location (Jorge et al., 1993b). Conversely, psychosocial factors, such as impaired close personal relationships and an unstable job situation, can be stronger determinants of MDD, particularly as time since injury increases (Gomez-Hernandez et al., 1997). In theory, pharmacotherapy, psychotherapy, and alternative approaches might be combined and balanced for individual circumstances, risk factors, and time post-injury. However, we identified no studies that examined the efficacy of combined therapies for depression after TBI; presumably, these will need to await stronger evidence of efficacy for single treatments.
The limited data available on pharmacotherapy for depression after TBI do not provide definitive evidence of efficacy for any specific class of medications. The studies reviewed do suggest that we cannot assume that standard antidepressant medications will have the same efficacy and tolerability in persons with TBI as in persons without neurologic insult. However, few adverse effects were reported in the studies reviewed, with the most severe of these (e.g., seizures) and the most dropouts occurring with tricyclic antidepressants. All but two of the pharmacotherapy studies prospectively selected depressed patients, but all were limited in sample size and randomized controlled trials were rare. Studies of other biological interventions were even more limited in sample size, with the best evidence being a RCT of 12 patients assigned to biofeedback or a wait-list control group.
The psychotherapeutic and rehabilitation treatment studies to date are larger in size, but none prospectively selected a depressed sample, developed an intervention specifically to treat depression, or targeted depression as a primary outcome. Moreover, many of these interventions delivered a complex mix of ingredients, making it difficult to determine the active ingredients for depression in the few cases where depressive symptoms were targeted. Fewer than half of the articles describing these treatments identified a treatment manual, possibly further limiting the reproducibility and feasibility of these treatment packages for TBI-related depression.
Our systematic review of the literature highlights several challenges in conducting and synthesizing depression treatment studies in persons with TBI. Study samples are heterogeneous in their TBI severity and acuity, and injury and medical comorbidity. As summarized in Johnston and associates (Johnston et al., 2006), there are inherent difficulties in applying existing standards of evidence to rehabilitation trials. This is particularly true of the complex, experience-based treatments that predominate in rehabilitation over medically-oriented treatments such as pharmacotherapy and surgery. Interventions that involve explicit teaching, behavior change, and/or environmental manipulations cannot typically be hidden from the patient or the treater; thus the removal of bias by using standard blinding procedures, such as placebo treatment, is not straightforward (see also Hart et al., 2008). In contrast to medical treatments that are aimed at specific symptoms, complex rehabilitation interventions usually target multiple or complex outcomes at the levels of activity and participation. Identification of a “primary outcome” for such treatments may be impossible and even inappropriate. These interventions may have goals that vary across participants and are often delivered by members of multiple disciplines working synergistically, complicating the application of evidence standards that do not incorporate such factors. Other limitations of this study include only focusing on studies of adults published in English since 1980, and a lack of meta-analysis due to the heterogeneity of the interventions and paucity of RCTs. Finally, some articles may have been missed because of inconsistent indexing in electronic databases.
Recommendations
Clinical practice
Based on the best evidence available to guide pharmacological treatment, it is advisable to start with low doses of medications with slow titration toward a therapeutic response, being cognizant of adverse effects that may be more common in neurologically-injured patients (e.g., seizures, sedation, and cognitive dysfunction), and using depression measures that have been validated in the TBI population, such as the Patient Health Questionnaire-9 depression scale (PHQ-9) (Fann et al., 2005).
Due to their favorable side-effect profile, SSRIs are usually the first-line antidepressants for TBI patients. There is evidence for the use of sertraline (25–150 mg/d) for depression after TBI, and the Neurobehavioral Guidelines Working Group (Warden et al., 2006) recommends the use of sertraline as a first-line option for treatment of post-TBI depression. Among the SSRIs, sertraline has the most dopaminergic effect, thus potentially having a positive impact on cognition (Fann et al., 2001). Limited evidence also suggests that citalopram (20–50 mg) may be effective and well-tolerated. While more data are needed on the efficacy and tolerability of SNRIs in this population, data from a small study of milnacipran (which is not available in the U.S. or the U.K.) after TBI, and SNRI efficacy data from other populations suggesting higher rates of remission and documenting analgesic effects (Thase, 2008) indicate that SNRIs may be another reasonable option in this population. Evidence of possible reduced efficacy and a higher risk of side effects (e.g., seizures) for TCAs may limit their use in this population. Traditional MAOIs are not recommended due to a lack of efficacy data and potentially serious side effects, particularly when dietary restrictions are not adhered to in a population with a high rate of cognitive difficulties. The safer MAO-A blocker meclobemide may be a viable second-line treatment for cognitively intact patients; however, this medication is not available in the U.S. ECT, with possible adaptation to electrode placement and stimulus frequency acutely post-TBI, appears to be a viable option for treatment-refractory patients, but cognitive side effects need to be monitored closely. Magnetic stimulation, biofeedback, and acupuncture remain experimental interventions at this time.
From the studies reviewed, there is insufficient evidence to support practice recommendations regarding any of the psychotherapeutic or rehabilitation interventions for depression following TBI. This is due not only to inconsistency in the quality of the research designs, but to the earlier noted difficulty in specifying “active ingredients” for depression within these complex treatments, many of which were deliberately multifaceted. To some extent this difficulty is inevitable in studies of complex interventions (Hart, 2009; Medical Research Council, 2000). With these caveats, it is still of interest to note correspondence between the treatments for TBI that reported improved effects on mood in the studies reviewed, and treatment models with demonstrated efficacy for depression in the general population. For example, CBT has shown efficacy comparable to that of antidepressant medication (DeRubeis et al., 2005). Dismantling designs that compare the cognitive components of CBT (e.g., examination and correction of distorted thinking) to its behavioral components (e.g., engaging in more reinforcing activities), have tended to show superiority for the latter (Dimidjian et al., 2006). According to one meta-analysis (Cuijpers et al., 2007a), therapies focusing on behavioral activation, even in simple forms such as activity scheduling, are at least as effective for depression as CBT. Holistic treatment programs for TBI that include activity scheduling and increasing positive interaction with the environment may therefore improve participants' mood, along with functional outcomes and productivity. Other treatment components such as problem-solving and goal-setting training, that are commonly used in multidisciplinary programs for TBI including two reviewed here (Powell et al., 2002; Svendsen et al., 2004), are also mirrored by depression treatments with proven efficacy (e.g., problem-solving therapy and social problem-solving therapy [Cuijpers et al., 2007b]).
Future research
Clearly, the field is in need of large, appropriately controlled pharmacological, psychosocial, alternative, and multi-modal prevention and treatment studies for depression that:
Have statistical power to compare different modalities;
Stratify randomization on prior psychiatric history;
Determine predictors and potential modifiers of efficacy, such as TBI severity, time since injury, history and severity of depression and other neuropsychiatric symptoms, substance abuse, and cognitive functioning; and
Examine important secondary outcomes such as symptom burden, cognition, functional status, family and caregiver burden, community integration, and quality of life.
In addition, the following specific recommendations are made for both current and future trials.
Research trials should attempt to include documentation of participants' pre-injury psychological status to the extent possible. In trials that target participants with severe, persistent emotional problems, it is especially important to try to establish premorbid contributors to current functioning. A minimal effort would include documenting pre-injury psychiatric diagnoses and psychiatric treatments.
In terms of development and validation of outcome measures, more attention should be paid to measures of depression that do not require verbal self-report, particularly for participants with severe cognitive or linguistic impairments. Until such measures are validated, treatment efficacy can most validly be examined using studies with higher-functioning participants (e.g., Tiersky et al., 2005), or in sub-samples of participants with better cognitive function (e.g., Powell et al., 2002).
Considering the prevalence of depression after TBI and the factors that may affect it (level of participation, social involvement, and independence), measures of depression, as well as other emotional outcomes, should be included in studies of holistic or comprehensive rehabilitation following TBI. In addition to group means, it would be helpful for researchers to report the proportions and characteristics of participants within clinically relevant diagnostic categories, and summary statistics on depression measure scores both pre- and post-treatment.
-
More research is needed in psychotherapeutic interventions for depression after TBI, since many of the modifiable risk factors for MDD in this population (ineffective coping, poor problem solving, social isolation, and a lack of pleasant rewarding activities) are potentially amenable to treatment targeting the behavioral level. Specifically:
We recommend further study of CBT-based treatments for depression following TBI. These treatments may well prove to be most appropriate for mild-to-moderate TBI and/or those with higher education and premorbid intellectual function (Anson and Ponsford, 2006; Tiersky et al., 2005). Future trials should include participants with a range of TBI severity and pre-treatment cognitive/functional status to clarify the range of participants who are able to benefit from CBT, and to develop appropriate modifications for persons with cognitive impairment.
It would also be worthwhile to study depression treatments with efficacy in other medical populations that may be even more feasible for use in TBI populations, including behavioral activation and social problem-solving based treatments.
We support efforts to standardize the description and specification of rehabilitation treatments, particularly complex, experience-based interventions. One complaint is that in journal articles, rehabilitation interventions are not described in adequate detail for replication (Dijkers et al., 2002). For complex interventions, it would be unrealistic to include all the details in the abbreviated form required of a journal article, but authors should reveal the extent to which the treatments have been documented and should let readers know how to obtain further information (e.g., on a web site).
Conduct more trials on innovative delivery systems, such as telephone, telehealth, and web-based models, which have the potential of overcoming some of the treatment barriers faced by TBI patients (e.g., with regard to mobility, transportation, accessibility, and finances).
Conduct effectiveness trials of systematic changes in care, such as collaborative stepped care models that integrate seamlessly with non-mental health systems (e.g., rehabilitation and primary care [Gilbody et al., 2006]), compared with usual models of care in diverse health settings for patients with TBI.
Given the influx of TBI related to the war efforts in Iraq and Afghanistan (often referred to as the “signature wound” of these conflicts), researchers should seek to elucidate differences in effectiveness of treating blast- and non-blast-related TBIs, and TBI-related depression that may be complicated by post-traumatic stress disorder.
While the above recommendations should first apply to the treatment of major depression, researchers should also examine the impact of treating other depressive conditions, such as dysthymia and minor depression, and symptom clusters, such as depression with anxiety, fatigue, or pain, that also adversely affect functioning.
Conclusions
The profile of contributing factors that lead to depression in people with TBI suggests that a comprehensive biopsychosocial approach should be applied to depression management. A goal may be that predominantly biologically- or psychosocially-mediated depressed subgroups can be identified, followed by the application of treatments that target these specific substrates. Alternatively, cost-effective treatment may require collaborative, multimodal, or stepped-care treatment models to achieve adequate rates of remission in a population with multiple risk factors and comorbidities. A combination of multidisciplinary brain injury rehabilitation plus psychiatric and psychological treatment modalities may offer the greatest potential for maximizing outcomes in the majority of people with TBI and depression.
Acknowledgments
The authors wish to thank Teresa Ashman, Ph.D., Vani Rao, M.D., and Jonathan Silver, M.D. for their helpful reviews of the methods and evidence tables, and Kurt Johnson, Ph.D., for his helpful review of the manuscript.
Funding for this study was provided by the National Institute on Disability and Rehabilitation Research (NIDRR), Office of Special Education and Rehabilitative Services, U.S. Department of Education, Washington, D.C. for the University of Washington Model Systems Knowledge Translation Center (H133A060070). This work was also supported by NIDRR grants H133A070040 (Moss Traumatic Brain Injury Model System), and H133G070016 (J.R.F.), and NIH grant R21HD053736 (J.R.F.). These funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Alderfer B.S. Arciniegas D.B. Silver J.M. Treatment of depression following traumatic brain injury. J. Head Trauma Rehabil. 2005;20:544–562. doi: 10.1097/00001199-200511000-00006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- Anson K. Ponsford J. Evaluation of a coping skills group following traumatic brain injury. Brain Injury. 2006;20:167–178. doi: 10.1080/02699050500442956. [DOI] [PubMed] [Google Scholar]
- Ashman T.A. Cantor J.B. Gordon W.A. Spielman L. Flanagan S. Ginsberg A. Engmann C. Egan M. Ambrose F. Greenwald B. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch. Phys. Med. Rehabil. 2009;90:733–740. doi: 10.1016/j.apmr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Baker-Price L.A. Persinger M.A. Weak, but complex pulsed magnetic fields may reduce depression following traumatic brain injury. Percept. Mot. Skills. 1996;83:491–498. doi: 10.2466/pms.1996.83.2.491. [DOI] [PubMed] [Google Scholar]
- Baker-Price L. Persinger M.A. Intermittent burst-firing weak (1 microtesla) magnetic fields reduce psychometric depression in patients who sustained closed head injuries: A replication and electroencephalographic validation. Percept. Mot. Skills. 2003;96:965–974. doi: 10.2466/pms.2003.96.3.965. [DOI] [PubMed] [Google Scholar]
- Bedard M. Felteau M. Mazmanian D. Fedyk K. Klein R. Richardson J. Parkinson W. Minthorn-Biggs M.B. Pilot evaluation of a mindfulness-based intervention to improve quality of life among individuals who sustained traumatic brain injuries. Disabil. Rehabil. 2003;25:722–731. doi: 10.1080/0963828031000090489. [DOI] [PubMed] [Google Scholar]
- Bourdon K.H. Rae D.S. Locke B.Z. Narrow W.E. Regier D.A. Estimating the prevalence of mental disorders in U.S. adults from the epidemiologic catchment area survey. Public Health Rep. 1992;107:663–668. [PMC free article] [PubMed] [Google Scholar]
- Brooks N. Campsie L. Symington C. Beattie A. McKinlay W. The five year outcome of severe blunt head injury: A relative's view. J. Neurol. Neurosurg. Psychiatry. 1986;49:764–770. doi: 10.1136/jnnp.49.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns J.J. Hauser W.A. The epidemiology of traumatic brain injury: A review. Epilepsia. 2003;44:2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- Cassidy J. Fluoxetine: A new serotonergically active antidepressant. J. Head Trauma Rehabil. 1989;4:67–69. [Google Scholar]
- Chen C.P. Alder J.T. Bowen D.M. Esiri M.M. McDonald B. Hope T. Jobst K.A. Francis P.T. Presynaptic serotonergic markers in community-acquired cases of Alzheimer's disease: Correlations with depression and neuroleptic medication. J. Neurochem. 1996;66:1592–1598. doi: 10.1046/j.1471-4159.1996.66041592.x. [DOI] [PubMed] [Google Scholar]
- Chen J.K. Johnston K.M. Petrides M. Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch. Gen. Psychiatry. 2008;65:81–89. doi: 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- Christensen B. Ross T. Kotasek R. Rosenthal M. Henry R. The role of depression in rehabilitation outcomes in the acute recovery of patients with TBI. Adv. Med. Psychother. 1994;7:23–38. [Google Scholar]
- Cuijpers P. van Straten A. Wamerdam L. Behavioral activation treatments of depression: A meta-analysis. Clin. Psychol. Rev. 2007a;27:318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Cuijpers P. van Straten A. Wamerdam L. Problem solving therapies for depression: A meta-analysis. Eur. Psychiatry. 2007b;22:9–15. doi: 10.1016/j.eurpsy.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Deb S. Lyons I. Koutzoukis C. Ali I. McCarthy G. Rate of psychiatric illness 1 year after traumatic brain injury. Am. J. Psychiatry. 1999;156:374–378. doi: 10.1176/ajp.156.3.374. [DOI] [PubMed] [Google Scholar]
- DeRubeis R.J. Hollon S.D. Amsterdam J.D. Shelton R.C. Young P.R. Salomon R.M. O'Reardon J.P. Lovett M.L. Gladis M.M. Brown L.L. Gallop R. Cognitive therapy vs. medications in the treatment of moderate to severe depression. Arch. Gen. Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Dijkers M. Kropp G.C. Esper R.M. Yavuzer G. Cullen N. Bakdalieh Y. Quality of intervention research reporting in medical rehabilitation journals. Am. J. Phys. Med. Rehabil. 2002;81:21–33. doi: 10.1097/00002060-200201000-00005. [DOI] [PubMed] [Google Scholar]
- DiMatteo M.R. Lepper H.S. Croghan T.W. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Dimidjian S. Hollon S.D. Dobson K.S. Schmaling K.B. Kohlenberg R.J. Addis M.E. Gallop R. McGlinchey J.B. Markley D.K. Gollan J.K. Atkins D.C. Dunner D.L. Jacobson N.S. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J. Consult. Clin. Psychol. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dinan T.G. Mobayed M. Treatment resistance of depression after head injury: A preliminary study of amitriptyline response. Acta Psychiatr. Scand. 1992;85:292–294. doi: 10.1111/j.1600-0447.1992.tb01472.x. [DOI] [PubMed] [Google Scholar]
- Donnellan C.P. Acupuncture for central pain affecting the ribcage following traumatic brain injury and rib fractures—a case report. Acupunct. Med. 2006;24:129–133. doi: 10.1136/aim.24.3.129. [DOI] [PubMed] [Google Scholar]
- Edlund W. Gronseth G. So Y. Franklin G. Clinical Practice Guideline Process Manual. American Academy of Neurology; St. Paul, MN: 2004. [Google Scholar]
- Fann J.R. Bombardier C.H. Dikmen S. Esselman P. Warms C.A. Pelzer E. Rau H. Temkin N. Validity of the patient health questionnaire-9 in assessing depression following traumatic brain injury. J. Head Trauma Rehabil. 2005;20:501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- Fann J.R. Bombardier C.H. Temkin N.R. Esselman P. Pelzer E. Keough M. Romero H. Dikmen S. Incidence, severity, and phenomenology of depression and anxiety in patients with moderate to severe traumatic brain injury. Psychosomatics. 2003;44:161. [Google Scholar]
- Fann J.R. Burington B. Leonetti A. Jaffe K. Katon W.J. Thompson R.S. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch. Gen. Psychiatry. 2004;61:53–61. doi: 10.1001/archpsyc.61.1.53. [DOI] [PubMed] [Google Scholar]
- Fann J.R. Katon W.J. Uomoto J.M. Esselman P.C. Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. Am. J. Psychiatry. 1995;152:1493–1499. doi: 10.1176/ajp.152.10.1493. [DOI] [PubMed] [Google Scholar]
- Fann J.R. Uomoto J.M. Katon W.J. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics. 2001;42:48–54. doi: 10.1176/appi.psy.42.1.48. [DOI] [PubMed] [Google Scholar]
- Fann J.R. Uomoto J.M. Katon W.J. Sertraline in the treatment of major depression following mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2000;12:226–232. doi: 10.1176/jnp.12.2.226. [DOI] [PubMed] [Google Scholar]
- Fedoroff J.P. Starkstein S.E. Parikh R.M. Price T.R. Robinson R.G. Are depressive symptoms nonspecific in patients with acute stroke? Am. J. Psychiatry. 1991;148:1172–1176. doi: 10.1176/ajp.148.9.1172. [DOI] [PubMed] [Google Scholar]
- Federoff J. Starkstein S.E. Forrester A.W. Geisler F. Jorge R. Arndt S. Robinson R. Depression in patients with acute traumatic brain injury. Am. J. Psychiatry. 1992;149:918–923. doi: 10.1176/ajp.149.7.918. [DOI] [PubMed] [Google Scholar]
- Finkelstein E.A. Corso P.C. Miller T.R. Fiebelkorn I.A. Zaloshnja E. Lawrence B.A. Incidence and Economic Burden of Injuries in the United States, 2000. Oxford University Press; New York: 2006. [Google Scholar]
- Furukawa T. McGuire H. Barbui C. Low dosage tricyclic antidepressants for depression. Cochrane Database Syst. Rev. 2003;3:CD003197. doi: 10.1002/14651858.CD003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbody S. Bower P. Fletcher J. Richards D. Sutton A.J. Collaborative care for depression: A cumulative meta-analysis and review of longer-term outcomes. Arch. Intern. Med. 2006;166:2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- Gomez-Hernandez R. Max J.E. Kosier T. Paradiso S. Robinson R.G. Social impairment and depression after traumatic brain injury. Arch. Phys. Med. Rehabil. 1997;78:1321–1326. doi: 10.1016/s0003-9993(97)90304-x. [DOI] [PubMed] [Google Scholar]
- Gurr B. Coetzer B.R. The effectiveness of cognitive-behavioural therapy for post-traumatic headaches. Brain Inj. 2005;19:481–491. doi: 10.1080/02699050400005176. [DOI] [PubMed] [Google Scholar]
- Hackett M.L. Anderson C.S. House A. Xia J. Interventions for treating depression after stroke. Cochrane Database Syst. Rev. 2008;4:CD003437. doi: 10.1002/14651858.CD003437.pub3. [DOI] [PubMed] [Google Scholar]
- Hart T. Fann J.R. Novack T.A. The dilemma of the control condition in experience-based cognitive and behavioural treatment research. Neuropsychol. Rehabil. 2008;18:1–21. doi: 10.1080/09602010601082359. [DOI] [PubMed] [Google Scholar]
- Hart T. Treatment definition in complex rehabilitation interventions. Neuropsychol. Rehabil. June. 2009;18:1–17. doi: 10.1080/09602010902995945. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Hibbard M.R. Uysal S. Kepler K. Bogdany J. Silver J. Axis I psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehabil. 1998;13:24–39. doi: 10.1097/00001199-199808000-00003. [DOI] [PubMed] [Google Scholar]
- Hoge C.W. McGurk D. Thomas J.L. Cox A.L. Engel C.C. Castro C.A. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Horsfield S.A. Rosse R.B. Tomasino V. Schwartz B.L. Mastropaolo J. Deutsch S.I. Fluoxetine's effects on cognitive performance in patients with traumatic brain injury. Int. J. Psychiatry Med. 2002;32:337–344. doi: 10.2190/KQ48-XT0L-2H14-5UMV. [DOI] [PubMed] [Google Scholar]
- Johnston M.V. Sherer M. Whyte J. Applying evidence standards to rehabilitation research. Am. J. Phys. Med. Rehabil. 2006;85:292–309. doi: 10.1097/01.phm.0000202079.58567.3b. [DOI] [PubMed] [Google Scholar]
- Jorge R.E. Robinson R.G. Arndt S. Are there symptoms that are specific for depressed mood in patients with traumatic brain injury? J. Nerv. Ment. Dis. 1993a;181:91–99. doi: 10.1097/00005053-199302000-00004. [DOI] [PubMed] [Google Scholar]
- Jorge R.E. Robinson R.G. Arndt S.V. Starkstein S.E. Forrester A.W. Geisler F. Depression following traumatic brain injury: A 1 year longitudinal study. J. Affect. Disord. 1993b;27:233–243. doi: 10.1016/0165-0327(93)90047-n. [DOI] [PubMed] [Google Scholar]
- Jorge R.E. Robinson R.G. Moser D. Tateno A. Crespo-Facorro B. Arndt S. Major depression following traumatic brain injury. Arch. Gen. Psychiatry. 2004;61:42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- Kanetani K. Kimura M. Endo S. Therapeutic effects of milnacipran (serotonin noradrenaline reuptake inhibitor) on depression following mild and moderate traumatic brain injury. J. Nippon. Med. Sch. 2003;70:313–320. doi: 10.1272/jnms.70.313. [DOI] [PubMed] [Google Scholar]
- Kant R. Coffey C.E. Bogyi A.M. Safety and efficacy of ECT in patients with head injury a case series. J. Neuropsychiatry Clin. Neurosci. 1999;11:32. doi: 10.1176/jnp.11.1.32. [DOI] [PubMed] [Google Scholar]
- Khateb A. Ammann J. Annoni J.M. Diserens K. Cognition-enhancing effects of donepezil in traumatic brain injury. Eur. Neurol. 2005;54:39. doi: 10.1159/000087718. [DOI] [PubMed] [Google Scholar]
- Kolakowsky-Hayner S.A. Gourley E.V., 3rd Kreutzer J.S. Marwitz J.H. Meade M.A. Cifu D.X. Post-injury substance abuse among persons with brain injury and persons with spinal cord injury. Brain Inj. 2002;16:583–592. doi: 10.1080/02699050110119475. [DOI] [PubMed] [Google Scholar]
- Koponen S. Taiminen T. Portin R. Himanen L. Isoniemi H. Heinonen H. Hinkka S. Tenovuo O. Axis I and II psychiatric disorders after traumatic brain injury: A 30-year follow-up study. Am. J. Psychiatry. 2002;159:1315–1321. doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- Kraus J. McArthur D.L. Incidence and prevalence of, and costs associated with, traumatic brain injury. In: Rosenthal M., editor; Kreutzer J.S., editor; Griffith E., et al., editors. Rehabilitation of the Adult and Child with Traumatic Brain Injury. FA Davis; Philadelphia: 1999. pp. 3–18. [Google Scholar]
- Kreutzer J.S. Marwitz J.H. Walker W. Sander A. Sherer M. Bogner J. Fraser R. Bushnik T. Moderating factors in return to work and job stability after traumatic brain injury. J. Head Trauma Rehabil. 2003;18:128–138. doi: 10.1097/00001199-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Lee H. Kim S.W. Kim J.M. Shin I.S. Yang S.J. Yoon J.S. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum. Psychopharmacol. 2005;20:97–104. doi: 10.1002/hup.668. [DOI] [PubMed] [Google Scholar]
- Levin H. Kraus M.F. The frontal lobes and traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 1994;6:443–454. doi: 10.1176/jnp.6.4.443. [DOI] [PubMed] [Google Scholar]
- Martino C. Krysko M. Petrides G. Tobias K.G. Kellner C.H. Cognitive tolerability of electroconvulsive therapy in a patient with a history of traumatic brain injury. J. ECT. 2008;24:92–95. doi: 10.1097/YCT.0b013e31814faae5. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S. Frontal lobe dysfunction in secondary depression. J. Neuropsychiatry Clin. Neurosci. 1994;6:428–442. doi: 10.1176/jnp.6.4.428. [DOI] [PubMed] [Google Scholar]
- McMillan T. Brief mindfulness training for attentional problems after traumatic brain injury: A randomised control treatment trial. Neuropsychol. Rehabil. 2002;12:117–125. [Google Scholar]
- Medical Research Council. A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. MRC; London: 2000. [Google Scholar]
- Miller J. Pentland B. Berrol S. Early evaluation and management. In: Rosenthal M., editor; Griffith E., editor; Bond M., editor; Miller J., editor. Rehabilitation of the Adult and Child with Traumatic Brain Injury. FA Davis; Philadelphia: 1990. pp. 21–51. [Google Scholar]
- Moncrieff J. Wessely S. Hardy R. Active placebos versus antidepressants for depression. Cochrane Database Syst. Rev. 2004;1:CD003012. doi: 10.1002/14651858.CD003012.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburn G. Edwards R. Thomas H. Collier J. Fox K. Collins C. Meclobemide in the treatment of major depressive disorder (DSM-3) following traumatic brain injury. Brain Inj. 1999;13:637–642. doi: 10.1080/026990599121368. [DOI] [PubMed] [Google Scholar]
- NIH consensus development panel on rehabilitation of persons with traumatic brain injury. Rehabilitation of persons with traumatic brain injury. JAMA. 1999;282:974–983. [PubMed] [Google Scholar]
- Ownsworth T. The impact of defensive denial upon adjustment following traumatic brain injury. Neuro-Psychoanalysis. 2005;7:83–94. [Google Scholar]
- Perino C. Rago R. Cicolini A. Torta R. Monaco F. Mood and behavioural disorders following traumatic brain injury: Clinical evaluation and pharmacological management. Brain Inj. 2001;15:139–148. doi: 10.1080/026990501458371. [DOI] [PubMed] [Google Scholar]
- Powell J. Heslin J. Greenwood R. Community based rehabilitation after severe traumatic brain injury: A randomised controlled trial. J. Neurol. Neurosurg. Psychiat. 2002;72:193. doi: 10.1136/jnnp.72.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M.J. Chan F. Lanctot K. Herrmann N. McCullagh S. Feinstein A. An open-label study of citalopram for major depression following traumatic brain injury. J. Psychopharmacol. 2008;22:860–864. doi: 10.1177/0269881107083845. [DOI] [PubMed] [Google Scholar]
- Robinson R.G. Lipsey J.R. Price T.R. Diagnosis and clinical management of post-stroke depression. Psychosomatics. 1985;26:769–772. doi: 10.1016/S0033-3182(85)72790-9. 775–768. [DOI] [PubMed] [Google Scholar]
- Rosenthal M. Christensen B.K. Ross T.P. Depression following traumatic brain injury. Arch. Phys. Med. Rehabil. 1998;79:90–103. doi: 10.1016/s0003-9993(98)90215-5. [DOI] [PubMed] [Google Scholar]
- Rutherford W.H. Sequelae of concussion caused by minor head injuries. Lancet. 1977;1:1–4. doi: 10.1016/s0140-6736(77)91649-x. [DOI] [PubMed] [Google Scholar]
- Sander A. Kreutzer J. Rosenthal M. Delmonico R. Young M.E. A multicenter longitudinal investigation of return to work and community integration following traumatic brain injury. J. Head Trauma Rehabil. 1996;11:70–84. [Google Scholar]
- Saran A.S. Depression after minor closed head injury: Role of dexamethasone suppression test and antidepressants. J. Clin. Psychiatry. 1985;46:335–338. [PubMed] [Google Scholar]
- Schoenberger N.E. Shiflett S.C. Esty M.L. Ochs L. Matheis R.J. Flexyx neurotherapy system in the treatment of traumatic brain injury: An initial evaluation. J. Head Trauma Rehabil. 2001;16:260–274. doi: 10.1097/00001199-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Schoenhuber R. Gentilini M. Anxiety and depression after mild head injury: A case control study. J. Neurol. Neurosurg. Psychiatry. 1988;51:722–724. doi: 10.1136/jnnp.51.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seel R.T. Kreutzer J.S. Depression assessment after traumatic brain injury: An empirically based classification method. Arch. Phys. Med. Rehabil. 2003a;84:1621–1628. doi: 10.1053/s0003-9993(03)00270-3. [DOI] [PubMed] [Google Scholar]
- Seel R.T. Kreutzer J.S. Rosenthal M. Hammond F.M. Corrigan J.D. Black K. Depression after traumatic brain injury: A national institute on disability and rehabilitation research model systems multicenter investigation. Arch. Phys. Med. Rehabil. 2003b;84:177–184. doi: 10.1053/apmr.2003.50106. [DOI] [PubMed] [Google Scholar]
- Stanislav S.W. Childs N.L. Dystonia associated with sertraline. J. Clin. Psychopharmacol. 1999;19:98–100. doi: 10.1097/00004714-199902000-00018. [DOI] [PubMed] [Google Scholar]
- Svendsen H.A. Teasdale T.W. Pinner M. Subjective experience in patients with brain injury and their close relatives before and after a rehabilitation programme. Neuropsychol. Rehabil. 2004;14:495–515. [Google Scholar]
- Tagliaferri F. Compagnone C. Korsic M. Servadei F. Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. (Wien.) 2006;148:255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- Tanielian T., editor; Jaycox L.H., editor. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Center for Military Health Policy Research; Santa Monica, CA: 2008. [Google Scholar]
- Thase M.E. Are SNRIs more effective than SSRIs? A review of the current state of the controversy. Psychopharmacol. Bull. 2008;41:58–85. [PubMed] [Google Scholar]
- Thurman D. Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- Tiersky L.A. Anselmi V. Johnston M.V. Kurtyka J. Roosen E. Schwartz T. DeLuca J. A trial of neuropsychologic rehabilitation in mild-spectrum traumatic brain injury. Arch. Phys. Med. Rehabil. 2005;86:1565–1574. doi: 10.1016/j.apmr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Turner-Stokes L. Hassan N. Pierce K. Clegg F. Managing depression in brain injury rehabilitation: The use of an integrated care pathway and preliminary report of response to sertraline. Clin. Rehabil. 2002;16:261–268. doi: 10.1191/0269215502cr489oa. [DOI] [PubMed] [Google Scholar]
- van Zomeren A.H. van den Burg W. Residual complaints of patients two years after severe head injury. J. Neurol. Neurosurg. Psychiatry. 1985;48:21–28. doi: 10.1136/jnnp.48.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D.L. Gordon B. McAllister T.W. Silver J.M. Barth J.T. Bruns J. Drake A. Gentry T. Jagoda A. Katz D.L. Kraus J. Labbate L.A. Ryan L.M. Sparling M.B. Walters B. Whyte J. Zapata A. Zitnay G. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J. Neurotrauma. 2006;23:1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- Wroblewski B.A. Joseph A.B. Cornblatt R.R. Antidepressant pharmacotherapy and the treatment of depression in patients with severe traumatic brain injury: A controlled, prospective study. J. Clin. Psychiatry. 1996;57:582–587. doi: 10.4088/jcp.v57n1206. [DOI] [PubMed] [Google Scholar]
- Wroblewski B.A. McColgan K. Smith K. Whyte J. Singer W.D. The incidence of seizures during tricyclic antidepressant drug treatment in a brain-injured population. J. Clin. Psychopharmacol. 1990;10:124–128. doi: 10.1097/00004714-199004000-00009. [DOI] [PubMed] [Google Scholar]
- Zaloshnja E. Miller T. Langlois J.A. Selassie A.W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]