Abstract
The protein kinase C activator phorbol 12-myristate 13-acetate (PMA) is known to interact with aquaporin 4 (AQP 4), a water-selective transporting protein that is abundant in astrocytes, and has experimentally been found to decrease osmotically-induced cell swelling. The purpose of this study was to examine whether PMA reduces brain edema following focal ischemia induced by middle cerebral artery (MCA) occlusion by modulation of AQP4 expression. Male Sprague-Dawley rats were randomly assigned to either sham surgery (n = 6), or a continuous intravenous infusion of vehicle (1% dimethylsulfoxide), followed by MCA occlusion (n = 18), and administration of PMA at 50 μg/kg (n = 6) or at 200 μg/kg (n = 6) starting 60 min before or 30 min (200 μg/kg; n = 6) or 60 min (200 μg/kg; n = 6) after MCA occlusion. Cerebral blood flow was monitored with laser Doppler over the MCA territory, and confirmed a 70% reduction during occlusion. After a 2-h period of ischemia and 2 h of reperfusion, the animals were sacrificed for assessment of brain water content and sodium and potassium concentration. AQP4 expression was assessed by immunoblotting and quantified by densitometry (n = 24). Statistical analysis was performed by ANOVA followed by Tukey's post-hoc test. PMA treatment at 200 μg/kg significantly reduced brain water concentration in the infarcted area when started 60 min before or 30 min after occlusion (p < 0.001 and p = 0.022, respectively), and prevented the subsequent sodium shift (p < 0.05). PMA normalized the AQP4 upregulation in ischemia (p = 0.021). A downregulation of AQP4 in the ischemic area paralleling the reduction in brain edema formation following PMA treatment suggests that the effect was mediated by AQP4 modulation.
Key words: aquaporin 4, brain edema, middle cerebral artery occlusion, phorbol ester, phorbol 12-myristate 13-acetate, rat
Introduction
In addition to the initial brain damage caused by any type of insult, the resulting brain edema and increase in intracranial pressure contribute substantially to the morbidity and mortality seen in these patients (Chesnut et al., 1993; Marmarou et al., 2000). The specific anatomical and cellular localization of aquaporin 4 (AQP 4) water channels in the central nervous system suggests that this protein plays a crucial role in the cerebral water balance (Venero et al., 1999). Recently, the role of AQP4 in maintaining water homeostasis during the development of post-traumatic or ischemic brain edema has been described, although conflicting results have been obtained using different models (Hu et al., 2005; Ke et al., 2001; Kiening et al., 2002; Lu and Sun, 2003; Manley et al., 2000; Meng et al., 2004; Sato et al., 2000; Sun et al., 2003; Taniguchi et al., 2000; Vizuete et al., 1999).
The exact mechanisms behind AQP4 regulation have yet to be identified. However, direct and indirect evidence suggests that protein kinase C (PKC) is involved in AQP4 regulation (Nakahama et al., 1999; Neely et al., 1999; Yamamoto et al., 2001). PKC serves as a second messenger for G-protein receptors, which are coupled to the phosphoinositide pathway, causing either a transient rise in intracellular Ca2+ through inositol triphoshate, or activation of PKC through diacylglycerol. The phorbol ester and known PKC activator 12-O-tetradecanoylphorbol 13-acetate has been found to decrease AQP4 mRNA in cultured rat astrocytes, but prolonged treatment eliminated the subsequent decrease in AQP4 mRNA (Nakahama et al., 1999; Yamamoto et al., 2001). Since pre-treatment with cycloheximide did not eliminate the reduction of AQP4 mRNA, PKC may be involved in AQP4 regulation on the transcriptional level, rather than affecting de novo protein synthesis. Furthermore, the PKC activator phorbol 12-myristate 13-acetate (PMA) is known to interact with AQP4, and has experimentally been found to increase AQP4 phosphorylation, and to decrease osmotically-induced cell swelling in AQP4-transfected Xenopus laevis oocytes (Han et al., 1998).
Thus in the present study we investigated whether PMA modulates AQP4 expression and reduces brain edema and subsequent electrolyte dysfunction. In order to reduce any effect of blood–brain barrier (BBB) breakdown, we utilized a model of middle cerebral artery (MCA) occlusion, since the early period following ischemia has been found to result in a predominantly cytotoxic edema (Neumann-Haefelin et al., 2000; Yang and Betz, 1994). Specifically, we assessed the brain water, sodium, and potassium content following different doses of an intravenous PMA infusion, started at different time points before or after MCA occlusion. To examine whether the effect of PMA on brain edema development was mediated through AQP4 modulation, we assessed AQP4 expression by immunoblotting with subsequent quantification by densitometry.
Methods
Animals and surgical procedure
The experiments were conducted with the approval of the Institutional Animal Care and Use Committee and National Institutes of Health guidelines. The experiments were carried out on 350–400 g adult male Sprague-Dawley rats. The rats were housed with a 12-h light/dark cycle at 22 ± 1°C with 60% humidity. Pellet food and water were given ad libitum. Surgery was performed after intubation under halothane anesthesia and controlled ventilation (1.3% halothane in 70% nitrous oxide and 30% oxygen). Rectal temperature was maintained at 36.5 ± 0.5°C using a heating lamp. The left femoral artery and vein were cannulated with polyethylene tubing for continuous monitoring of mean arterial blood pressure (MABP), blood sampling, and drug infusion. Adequate ventilation was verified by an arterial blood gas measurement after 1 h of anesthesia.
The regional cerebral blood flow (rCBF) over the supply territory of the right MCA was continuously monitored by laser Doppler flowmetry (LaserFlo; Vasamedics Inc., St Paul, MN) through a burr hole located 1 mm posterior and 5 mm lateral to the bregma with the dura mater intact. The animals were placed in a supine position over the laser Doppler probe, and rCBF as well as MAP were recorded continuously using a data acquisition system (ADInstruments Pty. Ltd., Colorado Springs, CO).
MCA occlusion was induced using the intraluminal suture method as previously described (Belayev et al., 1996) with slight modification. Through a midline neck incision, the bifurcation of the right common carotid artery (CCA) was exposed, and the branches of the external carotid artery (ECA) and internal carotid artery (ICA), including the occipital, lingual, and maxillary arteries, were microsurgically separated and coagulated. The ECA was ligated with a 4-0 silk suture, and after temporary occlusion of the ICA and CCA with vascular mini-clips, a 4-0 monofilament nylon suture with a silicon tip 0.3 mm in diameter was inserted through the ECA stump and secured by a suture. The clips were removed and the filament was advanced through the ICA into the circle of Willis while occluding the pterygopalatine artery with forceps. A rCBF reduction to 70–80% of baseline was observed when the suture was advanced to a distance of 22–24 mm from the carotid bifurcation, thereby verifying proper occlusion of the MCA. After 2 h of occlusion, a 2-h period of reperfusion of the MCA territory was performed by withdrawing the suture into the ECA stump with confirmation by a consequent increase in rCBF.
Study protocol and drug preparation
The objective of these experiments was to assess the effect of different doses of intravenous PMA infusion on brain swelling after MCA occlusion and reperfusion. We also assessed the effect of PMA infusion started at different time points. The animals were randomly assigned to sham surgery, vehicle, or PMA infusion at 50 μg/kg or 200 μg/kg starting 60 min before, and at 200 μg/kg starting either 30 min or 60 min after induction of ischemia, with each group consisting of 6 animals. As previously described (Manabe et al., 1992), a dose of 50 or 200 μg/kg PMA (Sigma-Aldrich Co., St Louis, MO) was dissolved in 1% dimethylsulfoxide (DMSO) as vehicle (Sigma-Aldrich). The drug was intravenously administered using a continuous infusion pump (sp210w syringe pump; KD Scientific, Holliston, MA). To keep the total drug concentration administered constant in the different treatment groups, the infusion rate was adapted to either 480 μL/h, 640 μL/h, or 720 μL/h. After 2 h of MCA occlusion and 2 h of reperfusion, the animals were sacrificed with an overdose of halothane, decapitated, and the brains were removed (Table 1).
Table 1.
Experimental Protocol
| Infusion rate | PMA 15 μM (50 μg/kg) | PMA 50 μM (200 μg/kg) | Vehicle | |
|---|---|---|---|---|
| − 60 min | 480 μL/h | n = 6 | n = 6 | n = 6 |
| MCA occlusion | ||||
| +30 min | 640 μL/h | n = 6 | n = 6 | |
| +60 min | 720 μL/h | n = 6 | n = 6 | |
| Reperfusion | ||||
| +2 h | ||||
| Sacrifice | n = 6 | n = 18 | n = 18 | |
| +4 h |
MCA, middle cerebral artery; PMA, phorbol 12-myristate 13-acetate.
Tissue processing
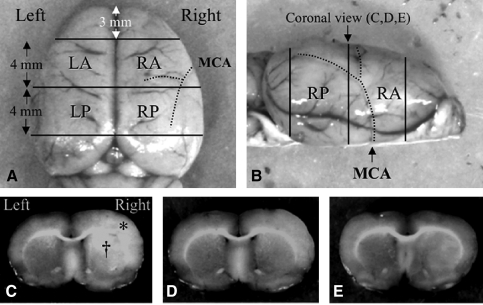
The cerebral tissue was cut into four consecutive 4-mm coronal sections, excluding the most rostral and caudal sections (Fig. 1). After division into right and left hemispheres along the anatomic midline, the four regional samples obtained were analyzed for water content as measured by the wet and dry weight method. The wet weight of each sample was measured using an electronic analytical balance before drying the sample at 95°C for 5 days, and reweighing to obtain the dry weight. The water content of each sample is given as a percentage of total tissue weight. For measurement of brain sodium and potassium concentrations, the dried samples were placed in a furnace and reduced to ashes by heating for 24 h at 400°C. The ash was then extracted using distilled water, and the concentrations of sodium and potassium were determined using a flame photometer (943 nm), with cesium as an internal standard.
FIG. 1.
Tissue processing. (A) Dorsal view and (B) coronal view of the rat brain showing the brain tissues sampled. Coronal sections 4 mm thick were cut through the middle cerebral artery core territory for water and electrolyte assessment. Representative coronal sections 7 mm posterior to the frontal pole were assessed for ischemic injury. Phorbol 12-myristate 13-acetate (PMA) treatment reduced brain edema in a dose-dependent manner. Brains from animals receiving (C) vehicle alone, (D) 50 μg/kg PMA, and (E) 200 μg/kg PMA (* = cerebral cortex; † = caudate putamen; LA, left anterior; LP, left posterior; RA, right anterior; RP, right posterior; MCA, middle cerebral artery).
Immunoblot
After sacrifice, the brains were removed and immediately cut on dry ice into two 2-mm sections, excluding 4 mm of the rostral tissue. Slices were cut into the right and left hemispheres, then the striatum and the parietal cortex were isolated, minced finely at 4°C with a Potter Elvehjem tissue grinder, and each sample was homogenized in 800 μL of radioimmunoprecipitation buffer (50 mM Tris, 150 mM NaCl, 1% NP40, 1% deoxycholic acid, and 0.5% sodium n-dodecyl sulfate [SDS], pH 7.2). This homogenate was centrifuged in a Thermo MicroMax RF centrifuge (Thermo Electron Corp., Needham Heights, MA) at 14,000g for 30 min at 4°C to remove nuclei and mitochondria. From the resultant pellet, gel samples in 2% SDS were made. Supernatant samples were run on 12% Bis-Tris gels (Invitrogen Corp., Carlsbad, CA). After transfer by electroelution to nitrocellulose membranes, the blots were blocked with 5% milk powder in tris buffered saline plus 0.1% Tween 20 (pH 7.5) for 1 h and incubated with primary antibodies (1:1000 monoclonal mouse anti-AQP4; Abcam, Cambridge, MA). The labeling was visualized with horseradish peroxidase-conjugated secondary antibody (1:5000 goat anti-mouse; Rockland Immunochemicals, Inc., Gilbertsville, PA), using an enhanced chemiluminescence system (Amersham, Buckinghamshire, U.K.). Controls were made by replacing primary antibody with non-immune IgG (cyclophilin). Quantification was performed by densitometry.
Statistical analysis
The animals were assigned to the different groups randomly. Tissue processing and quantification of brain edema and AQP4 expression were performed in a blinded fashion. SPSS software (SPSS Inc., Chicago, IL) was used for statistical analysis. The data were analyzed by a randomized one-way analysis of variance (ANOVA) for group variations, followed by Tukey's post-hoc analysis. Statistical significance was set at p < 0.05.
Results
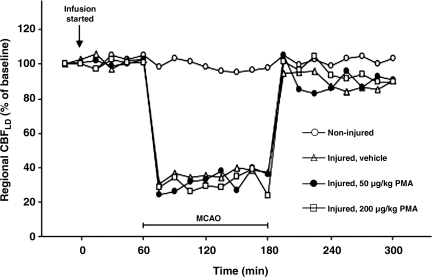
In the overall experiments, including 2 h of MCA occlusion and 2 h of reperfusion, the injury-induced mortality rate was 14%. The MABP and arterial blood gases were kept within physiological limits throughout the experimental procedures, requiring a few adjustments in the halothane concentration and ventilation parameters (Table 2). In the vehicle-infused group, animals demonstrating reduction in MABP related to differing infusion volume rates (480, 640, and 720 μL/h) were excluded from the study. In all the injured animals, rCBF dropped after MCA occlusion to around 30% of the original baseline (Fig. 2). There were no significant differences in rCBF between the injured-vehicle treated group and the injured, 50 μg/kg and 200 μg PMA-treated groups (Fig. 2). The rCBF in the ischemic area increased immediately after reperfusion, to nearly 100% of baseline, and then rapidly decreased to 70–80% for the duration of the experiment.
Table 2.
Physiological Parameters
| |
|
Time course during 5-h infusion |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
Pre-insult (min) |
MCAO (min) |
Reperfusion (min) |
|||||
| Parameters | Baseline | 30 | 60 | 10 | 60 | 120 | 10 | 60 | 120 |
| Sham-operated | |||||||||
| MABP (mm Hg) | 95 ± 4 | 96 ± 5 | 92 ± 5 | 93 ± 4 | 94 ± 7 | 94 ± 8 | 96 ± 7 | 96 ± 6 | 97 ± 2 |
| Rectal temperature (°C) | 36.2 ± 0.5 | 36.4 ± 0.2 | 36.7 ± 0.3 | 36.3 ± 0.4 | 36.5 ± 0.3 | 36.2 ± 0.5 | 36.7 ± 0.4 | 36.4 ± 0.4 | 36.4 ± 0.2 |
| pH | 7.42 ± 0.04 | 7.40 ± 0.04 | 7.40 ± 0.03 | 7.40 ± 0.03 | 7.40 ± 0.03 | 7.41 ± 0.02 | |||
| Paco2 (mm Hg) | 39 ± 6 | 39 ± 3 | 41 ± 6 | 42 ± 5 | 40 ± 9 | 39 ± 3 | |||
| Pao2 (mm Hg) | 133 ± 9 | 145 ± 20 | 134 ± 25 | 128 ± 7 | 135 ± 24 | 132 ± 12 | |||
| Injured, vehicle | |||||||||
| MAP (mm Hg) | 94 ± 8 | 97 ± 9 | 94 ± 10 | 108 ± 8 | 98 ± 5 | 99 ± 5 | 95 ± 9 | 99 ± 6 | 97 ± 6 |
| Rectal temperature (°C) | 36.5 ± 0.3 | 36.7 ± 0.3 | 36.6 ± 0.5 | 36.6 ± 0.4 | 36.3 ± 0.3 | 36.2 ± 0.3 | 36.3 ± 0.4 | 36.4 ± 0.4 | 36.3 ± 0.5 |
| pH | 7.42 ± 0.04 | 7.44 ± 0.02 | 7.40 ± 0.03 | 7.36 ± 0.03 | 7.39 ± 0.04 | 7.40 ± 0.03 | |||
| Paco2 (mm Hg) | 41 ± 5 | 37 ± 2 | 40 ± 2 | 41 ± 4 | 39 ± 4 | 38 ± 4 | |||
| Pao2 (mm Hg) | 142 ± 25 | 140 ± 16 | 130 ± 14 | 134 ± 7 | 129 ± 14 | 133 ± 12 | |||
| Injured, 50 μg/kg PMA | |||||||||
| MAP (mm Hg) | 94 ± 5 | 94 ± 2 | 91 ± 3 | 106 ± 7 | 97 ± 7 | 99 ± 10 | 93 ± 7 | 98 ± 7 | 100 ± 8 |
| Rectal temperature (°C) | 36.3 ± 0.4 | 36.8 ± 0.4 | 30.6 ± 0.1 | 36.3 ± 0.4 | 36.4 ± 0.2 | 36.7 ± 0.2 | 36.8 ± 0.3 | 36.6 ± 0.3 | 36.5 ± 0.3 |
| pH | 7.40 ± 0.03 | 7.4 ± 0.03 | 7.36 ± 0.03 | 7.36 ± 0.03 | 7.37 ± 0.03 | 7.37 ± 0.03 | |||
| Paco2 (mm Hg) | 40 ± 4 | 39 ± 6 | 44 ± 4 | 42 ± 2 | 42 ± 4 | 42 ± 3 | |||
| Pao2 (mm Hg) | 127 ± 21 | 140 ± 18 | 127 ± 11 | 126 ± 13 | 135 ± 26 | 121 ± 9 | |||
| Injured, 200 μg/kg PMA | |||||||||
| MAP (mm Hg) | 93 ± 5 | 97 ± 5 | 92 ± 7 | 110 ± 8 | 99 ± 9 | 99 ± 10 | 95 ± 11 | 99 ± 8 | 98 ± 8 |
| Rectal temperature (°C) | 36.4 ± 0.4 | 36.8 ± 0.3 | 36.4 ± 0.3 | 36.3 ± 0.4 | 36.6 ± 0.2 | 36.7 ± 0.2 | 36.2 ± 0.3 | 36.8 ± 0.2 | 36.5 ± 0.4 |
| pH | 7.41 ± 0.06 | 7.41 ± 0.06 | 7.39 ± 0.06 | 7.38 ± 0.03 | 7.42 ± 0.09 | 7.39 ± 0.03 | |||
| Paco2 (mm Hg) | 40 ± 9 | 42 ± 7 | 33 ± 6 | 41 ± 5 | 40 ± 5 | 41 ± 6 | |||
| Pao2 (mm Hg) | 143 ± 19 | 145 ± 19 | 139 ± 19 | 133 ± 20 | 128 ± 20 | 129 ± 17 | |||
Data are expressed as mean ± SD; n = 6 rats/group; MCAO, middle cerebral artery occlusion; MAP, mean arterial pressure; PMA, phorbol 12-myristate 13-acetate; Paco2, partial arterial carbon dioxide pressure; Pao2, partial arterial oxygen pressure; SD, standard deviation.
FIG. 2.
Changes in regional cerebral blood flow (CBF) during and after middle cerebral artery occlusion in sham controls (open circles), vehicle-alone (open triangles), and animals receiving 50 μg/kg PMA (solid circles), and 200 μg/kg PMA (open squares). Data are expressed as percentage of baseline and are means ± SD; n = 6 rats in each group (SD, standard deviation; PMA, phorbol 12-myristate 13-acetate; MCAO, middle cerebral artery occlusion).
Drug effect on brain edema
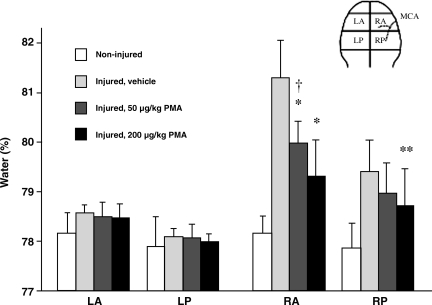
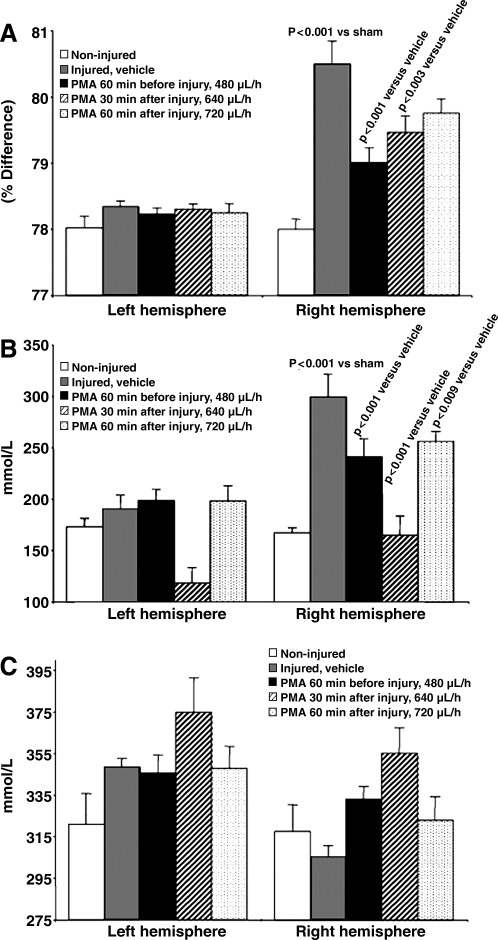
PMA administration strongly reduced brain edema formation as assessed after 2 h of ischemia and 2 h of reperfusion in a dose-dependent manner in the ischemic hemisphere when the infusion was started 60 min before the induction of ischemia (Fig. 3); thus we continued using PMA at the higher dose. The ANOVA of brain water content comparing vehicle and PMA infusion started at different time points following MCA occlusion produced a significant group effect (F4,79 = 11.25, p < 0.001; Fig. 4A). The Tukey post-hoc analysis indicated that PMA treatment started either 60 min before or 30 min after MCA occlusion reduced brain edema in the ischemic area significantly compared to infusion of vehicle alone (p < 0.001 and p = 0.022, respectively), while PMA infusion started 60 min after MCA occlusion failed to be effective (p = 2.67). The ANOVA of brain sodium content comparing vehicle and PMA infusion started at different time points following MCA occlusion produced a significant group effect (F4,79 = 10.01, p < 0.001; Fig. 4B). The Tukey post-hoc analysis indicated that PMA treatment started either 60 min before or 30 or 60 min after MCA occlusion reduced brain sodium accumulation in the ischemic area significantly compared to infusion of vehicle alone (p ≤ 0.004, p ≤ 0.008, and p ≤ 0.045, respectively). The ANOVA of brain potassium content comparing vehicle and PMA infusion started at different time points following MCA occlusion produced a significant group effect (F4,79 = 3.42, p ≤ 0.013; Fig. 4C). The Tukey post-hoc analysis indicated that PMA treatment started either 60 min before or 30 or 60 min after MCA occlusion did not reduce brain potassium loss in the ischemic area significantly compared to infusion of vehicle alone (p = 0.079, p = 0.284, and p = 0.542, respectively).
FIG. 3.
Effect of different doses of PMA on brain water content after MCA occlusion. PMA administration by continuous intravenous infusion significantly reduced brain water content in both the right anterior and posterior coronal sections in a dose-dependent manner. Data are means ± SD; n = 6 rats/group; *p < 0.0001; **p < 0.02 compared with the vehicle-alone group; †p < 0.04 compared with the 200 μg/kg PMA-treated group; SD, standard deviation; PMA, phorbol 12-myristate 13-acetate; MCA, middle cerebral artery; LA, left anterior; LP, left posterior; RA, right anterior; RP, right posterior).
FIG. 4.
Effect of PMA given at different time points before or after MCA occlusion on brain edema and electrolyte content. When started 60 min before or 30 min after MCAO, intravenous PMA infusion significantly reduced ischemia-induced brain edema. Data are expressed as the difference of percentage of water content (A), sodium concentration (B), and potassium concentration (C), between the ischemic and the non-ischemic side (means ± SEM; PMA, phorbol 12-myristate 13-acetate; SEM, standard error of the mean).
Drug effect on AQP4 expression
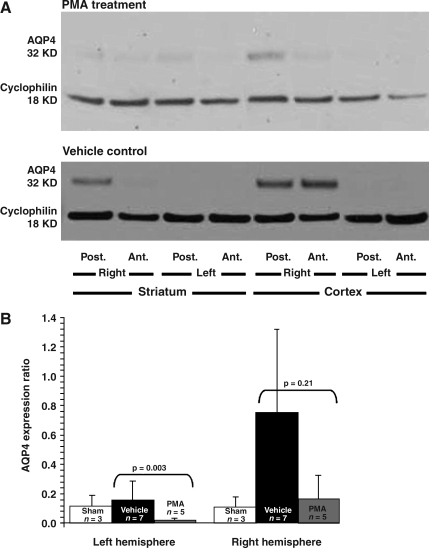
The ANOVA of AQP4 expression comparing vehicle and PMA infusion started 60 min before MCA occlusion produced a significant group effect (F2,21 = 5.922, p = 0.009). Following MCA occlusion, PMA infusion downregulated AQP4 expression when compared to vehicle infusion in the ischemic as well as in the non-ischemic hemispheres (Fig. 5).
FIG. 5.
Effect of PMA on AQP4 expression in the ischemic hemisphere after MCA occlusion. (A) Intravenous PMA infusion started 1 h before MCA occlusion (2 h ischemia, then 2 h reperfusion) reduced the ischemia-induced AQP4 upregulation seen in vehicle-infused animals. (B) AQP4 expression as assessed by immunoblotting and quantified by densitometry revealed significant differences (means ± SEM; AQP4, aquaporin 4; PMA, phorbol 12-myristate 13-acetate; SEM, standard error of the mean; MCA, middle cerebral artery; Post., posterior; Ant., anterior).
Discussion
The results of this study confirm that cerebral edema following cerebral ischemia is reduced by treatment with the irreversible PKC activator PMA in a dose-dependent manner, and it also reduced AQP4 upregulation in animals that had 2 h of ischemia followed by 2 h of reperfusion. We demonstrated that PMA, either started before or 30 min after MCA occlusion, significantly reduced brain water content in the ischemic hemisphere, and prevented the shift of sodium that normally accompanies brain edema. Although PMA slightly blunted the potassium loss associated with brain edema, this effect failed to reach statistical significance. These findings are consistent with the generally-accepted opinion that water and sodium tend to coexist, and move together through the plasma membrane under physiological and pathological conditions (Gotoh et al., 1985; Loo et al., 1996; Wright and Loo, 2000). Alternatively, in addition to the blunting of AQP4 upregulation by PMA, brain sodium channels may have been independently affected (Hourez et al., 2005).
Phorbol esters are tumor-promoting and inflammatory agents (Hussaini et al., 2000; Won et al., 1998). Some reported adverse effects include increased respiratory rate, decreased mean blood pressure, and a decreased absolute granulocyte count. Pulmonary problems include hemorrhagic and interstitial pneumonitis, and diffuse interstitial fibrosis. Some toxic effects have been reported when the drug was administered to dogs, rabbits, and rats (Lafuze et al., 1987; O'Flaherty et al., 1980; Taylor et al., 1985). On the other hand, PMA has also been used with no irreversible toxic effects (Han et al., 1998; Strair et al., 2002), and a species-specific difference in susceptibility to PMA-induced toxicity has been suggested (Manabe et al., 1992). The physiological parameters we monitored continuously throughout our study confirmed that blood pressure and pulmonary function were not affected by PMA treatment. However, more detailed pathological studies are needed to verify that PMA administration does not induce any permanent toxicity.
Changes in injury- or ischemia-induced AQP4 protein and AQP4 mRNA expression have been described in the literature. In cultured rat astrocytes, AQP4 was found to mediate the bidirectional transport of water during hypoxia-ischemia and reoxygenation (Fu et al., 2007). After transient focal brain ischemia in mice, two peaks of maximal hemispheric swelling at 1 h and at 48 h post-ischemia coincided with two peaks of AQP4 expression (Ribeiro Mde et al., 2006). Following experimental cerebral ischemia in the rat, AQP4 mRNA levels have been reported to be decreased at 24 h in ischemic compared to non-ischemic hemispheres (Sato et al., 2000), while in another study, researchers found AQP4 mRNA to be increased in the peri-infarcted area, and it peaked on day 3 (Taniguchi et al., 2000). In experimental traumatic brain injury (TBI) in the rat, AQP4 mRNA was increased at the injury site in a cortical contusion model at 24 h (Sun et al., 2003), whereas in another study AQP4 mRNA was decreased in edematous contusional cortex with impaired BBB integrity (Ke et al., 2001). In another study, AQP4 protein levels were decreased 48 h following controlled cortical impact (Kiening et al., 2002). In humans with TBI, AQP4 levels were reported to be increased between 15 h and 8 days post-injury (Hu et al., 2005). These contradictory findings are difficult to explain. Aside from time-dependent variations in AQP4 regulation, other effects are likely to be involved, like gene transcription, protein synthesis, protein translocation from storage sites to the membrane, or activation by phosphorylation (Madrid et al., 2001). These inconsistencies may also have resulted from the use of various methodological approaches.
We found that the PKC activator PMA blunts ischemia-induced upregulation of AQP4, as well as brain water accumulation and the sodium shift. Our findings are consistent with those of in-vitro studies demonstrating that PKC activators inhibit AQP4 activity (Han et al., 1998), and also to downregulates the expression of AQP4 protein (Yamamoto et al., 2001), and AQP4 mRNA (Nakahama et al., 1999). However, the experimental model used may also affect the changes seen in AQP4 expression. Furthermore, deletion of the AQP4 gene in knockout mice reduced brain edema and the peri-vascular foot process swelling seen after acute water intoxication and ischemic stroke (Manley et al., 2000). The expression of AQP4 mRNA has been shown to increase in the peri-infarcted cortex for 7 days after MCA occlusion, and is maximal on day 3 (Taniguchi et al., 2000). The signals for mRNA expression were predominantly observed in the glial cells and the outer granular layer of the peri-infarcted cortex. In the same study, the increased expression corresponded with the generation and resolution of edema as assessed by MRI. Similarly, Lu and Sun found that after MCA occlusion, AQP4 increased within 15 min, and was coincident with a reduced apparent diffusion coefficient, signifying a predominantly cellular edema (Lu and Sun, 2003; Sun et al., 2003). They concluded that upregulation of AQP4 may play a significant role in acute ischemic brain edema, but has no correlation with vasogenic edema, especially during the stages of intracellular edema and necrosis.
Although we utilized a model of a focal ischemic insult, in which the cytotoxic edema appears to be predominant (Dijkhuizen et al., 1999; Loubinoux et al., 1997), it is possible that a vasogenic component of edema was also present. Studies of brain edema have shown that the BBB remains intact during the first 6 h following MCA occlusion and reperfusion (Hatashita et al., 1988; Iannotti and Hoff, 1983). Therefore, we choose the 4-hour time period for our study. Nevertheless, it is possible that the total increase in water was not entirely due to cellular swelling beyond that which might occur due to water in the extracellular space moving into cells. This concerned us, and in another experiment the integrity of the BBB was evaluated by Evans-Blue perfusion and the subsequent measurement of absorbance (Kleindienst et al., 2006). We found that even low-dose DMSO opened the barrier in the ischemic more than in the non-ischemic hemisphere (p < 0.037). PMA did not have any additional effect on the BBB. Thus there is a slight vasogenic edema effect that may be attributable to either reperfusion injury or to the DMSO vehicle.
While PMA was most effective when given 60 min before MCA occlusion, its effect was attenuated when the PMA infusion was postponed. Although PKC activation has been found to decrease AQP4 mRNA in cultured astrocytes (Yamamoto et al., 2001), prolonged treatment eliminated the subsequent decrease in AQP4 mRNA (Nakahama et al., 1999). Since PMA activates PKC by binding PKC to the cell membrane with a very high affinity, there may be a relative depletion of PKC with administration of PMA for a extended time period (Mosior and Newton, 1995). This interaction may result in a reduction in PMA's effect on brain edema at later time points. Furthermore, since AQP4 is involved in the bi-directional water transport across cell membranes, postponing PMA treatment for 1 h after the onset of ischemia may have resulted in a failure to reduce brain edema development, and may have delayed or prevented water clearance.
We found that treatment with the PKC activator PMA reverses ischemia-induced AQP4 upregulation and attenuates brain edema formation following MCA occlusion when given before or early after the onset of ischemia. Since the downregulation of AQP4 following ischemia and PMA treatment occurred concomitantly with reductions in brain edema, these effects appear to be related. Although the exact biochemical pathways of AQP4 regulation are not yet known, experimental evidence suggests the involvement of PKC, and the results detailed here indicate that AQP4 expression is altered by the PKC activator PMA. However, further studies are needed to elucidate the precise mechanisms behind AQP4 regulation and its effects on the brain edema seen post-ischemia.
Conclusions
Different mechanisms have been proposed to explain the origins of the brain edema that occurs during the early stages post-ischemia, but this issue remains unresolved (Chen and Swanson, 2003; Kimelberg et al., 1995). To the best of our knowledge, we demonstrate here for the first time that the intravenous infusion of PMA reduces brain water content and the resulting shift of electrolytes in a rat model of MCA occlusion. Since the attenuation of brain edema development by PMA is accompanied by a normalization of ischemia-induced AQP4 upregulation, we provide evidence that the effects of PMA on brain edema are mediated by AQP4, possibly via PKC-dependent receptor signaling.
Acknowledgment
This research was supported by grant NS 12587 and grant NS 19235 from the National Institutes of Health to A.M.
Author Disclosure Statement
No competing financial interests exist.
References
- Belayev L. Alonso O.F. Busto R. Zhao W. Ginsberg M.D. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. ; discussion 1623. [DOI] [PubMed] [Google Scholar]
- Chen Y. Swanson R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Marshall L.F. Klauber M.R. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen R.M. de Graaf R.A. Tulleken K.A. Nicolay K. Changes in the diffusion of water and intracellular metabolites after excitotoxic injury and global ischemia in neonatal rat brain. J. Cereb Blood Flow Metab. 1999;19:341–349. doi: 10.1097/00004647-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Fu X. Li Q. Feng Z. Mu D. The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia. 2007;55:935–941. doi: 10.1002/glia.20515. [DOI] [PubMed] [Google Scholar]
- Gotoh O. Asano T. Koide T. Takakura K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke. 1985;16:101–109. doi: 10.1161/01.str.16.1.101. [DOI] [PubMed] [Google Scholar]
- Han Z. Wax M.B. Patil R.V. Regulation of aquaporin-4 water channels by phorbol ester-dependent protein phosphorylation. J. Biol. Chem. 1998;273:6001–6004. doi: 10.1074/jbc.273.11.6001. [DOI] [PubMed] [Google Scholar]
- Han Z.T. Tong Y.K. He L.M. Zhang Y. Sun J.Z. Wang T.Y. Zhang H. Cui Y.L. Newmark H.L. Conney A.H. Chang R.L. 12-O-Tetradecanoylphorbol-13-acetate (TPA)-induced increase in depressed white blood cell counts in patients treated with cytotoxic cancer chemotherapeutic drugs. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5362–5365. doi: 10.1073/pnas.95.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatashita S. Hoff J.T. Salamat S.M. Ischemic brain edema and the osmotic gradient between blood and brain. J. Cereb. Blood Flow Metab. 1988;8:552–559. doi: 10.1038/jcbfm.1988.96. [DOI] [PubMed] [Google Scholar]
- Hourez R. Azdad K. Vanwalleghem G. Roussel C. Gall D. Schiffmann S.N. Activation of protein kinase C and inositol 1,4,5-triphosphate receptors antagonistically modulate voltage-gated sodium channels in striatal neurons. Brain Res. 2005;1059:189–196. doi: 10.1016/j.brainres.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hu H. Yao H.T. Zhang W.P. Zhang L. Ding W. Zhang S.H. Chen Z. Wei E.Q. Increased expression of aquaporin-4 in human traumatic brain injury and brain tumors. J. Zhejiang Univ. Sci. B. 2005;6:33–37. doi: 10.1631/jzus.2005.B0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussaini I.M. Karns L.R. Vinton G. Carpenter J.E. Redpath G.T. Sando J.J. VandenBerg S.R. Phorbol 12-myristate 13-acetate induces protein kinase ceta-specific proliferative response in astrocytic tumor cells. J. Biol. Chem. 2000;275:22348–22354. doi: 10.1074/jbc.M003203200. [DOI] [PubMed] [Google Scholar]
- Iannotti F. Hoff J. Ischemic brain edema with and without reperfusion: an experimental study in gerbils. Stroke. 1983;14:562–567. doi: 10.1161/01.str.14.4.562. [DOI] [PubMed] [Google Scholar]
- Ke C. Poon W.S. Ng H.K. Pang J.C. Chan Y. Heterogeneous responses of aquaporin-4 in oedema formation in a replicated severe traumatic brain injury model in rats. Neurosci. Lett. 2001;301:21–24. doi: 10.1016/s0304-3940(01)01589-0. [DOI] [PubMed] [Google Scholar]
- Kiening K.L. van Landeghem F.K. Schreiber S. Thomale U.W. von Deimling A. Unterberg A.W. Stover J.F. Decreased hemispheric aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci. Lett. 2002;324:105–108. doi: 10.1016/s0304-3940(02)00180-5. [DOI] [PubMed] [Google Scholar]
- Kimelberg H.K. Rutledge E. Goderie S. Charniga C. Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J. Cereb. Blood Flow Metab. 1995;15:409–416. doi: 10.1038/jcbfm.1995.51. [DOI] [PubMed] [Google Scholar]
- Kleindienst A. Dunbar J.G. Glisson R. Okuno K. Marmarou A. Effect of dimethyl sulfoxide on blood-brain barrier integrity following middle cerebral artery occlusion in the rat. Acta Neurochir. Suppl. 2006;96:258–262. doi: 10.1007/3-211-30714-1_55. [DOI] [PubMed] [Google Scholar]
- Lafuze J.E. Baker M.D. Oakes A.L. Baehner R.L. Comparison of in vivo effects of intravenous infusion of N-formyl-methionyl-leucyl-phenylalanine and phorbol myristate acetate in rabbits. Inflammation. 1987;11:481–488. doi: 10.1007/BF00915990. [DOI] [PubMed] [Google Scholar]
- Loo D.D. Zeuthen T. Chandy G. Wright E.M. Cotransport of water by the Na + /glucose cotransporter. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubinoux I. Volk A. Borredon J. Guirimand S. Tiffon B. Seylaz J. Meric P. Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke. 1997;28:419–426. doi: 10.1161/01.str.28.2.419. discussion 426–417. [DOI] [PubMed] [Google Scholar]
- Lu H. Sun S.Q. A correlative study between AQP4 expression and the manifestation of DWI after the acute ischemic brain edema in rats. Chin. Med. J. (Engl.) 2003;116:1063–1069. [PubMed] [Google Scholar]
- Madrid R. Le Maout S. Barrault M.B. Janvier K. Benichou S. Merot J. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J. 2001;20:7008–7021. doi: 10.1093/emboj/20.24.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe S. Lin Y.C. Takaoka M. Yamoto T. Yanai T. Yamashita K. Tarumi C. Matsunuma N. Masuda H. Species difference in susceptibility to phorbol myristate acetate-induced leukopenia and lung injury: rat vs. dog. J. Toxicol. Sci. 1992;17:211–223. doi: 10.2131/jts.17.211. [DOI] [PubMed] [Google Scholar]
- Manley G.T. Fujimura M. Ma T. Noshita N. Filiz F. Bollen A.W. Chan P. Verkman A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Marmarou A. Fatouros P.P. Barzo P. Portella G. Yoshihara M. Tsuji O. Yamamoto T. Laine F. Signoretti S. Ward J.D. Bullock M.R. Young H.F. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J. Neurosurg. 2000;93:183–193. doi: 10.3171/jns.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- Meng S. Qiao M. Lin L. Del Bigio M.R. Tomanek B. Tuor U.I. Correspondence of AQP4 expression and hypoxic-ischaemic brain oedema monitored by magnetic resonance imaging in the immature and juvenile rat. Eur. J. Neurosci. 2004;19:2261–2269. doi: 10.1111/j.0953-816X.2004.03315.x. [DOI] [PubMed] [Google Scholar]
- Mosior M. Newton A.C. Mechanism of interaction of protein kinase C with phorbol esters. Reversibility and nature of membrane association. J. Biol. Chem. 1995;270:25526–25533. doi: 10.1074/jbc.270.43.25526. [DOI] [PubMed] [Google Scholar]
- Nakahama K. Nagano M. Fujioka A. Shinoda K. Sasaki H. Effect of TPA on aquaporin 4 mRNA expression in cultured rat astrocytes. Glia. 1999;25:240–246. [PubMed] [Google Scholar]
- Neely J.D. Christensen B.M. Nielsen S. Agre P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry. 1999;38:11156–11163. doi: 10.1021/bi990941s. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T. Kastrup A. de Crespigny A. Yenari M.A. Ringer T. Sun G.H. Moseley M.E. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31:1965–1972. doi: 10.1161/01.str.31.8.1965. discussion 1972–1963. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J.T. Cousart S. Lineberger A.S. Bond E. Bass D.A. DeChatelet L.R. Leake E.S. McCallm C.E. Phorbol myristate acetate: in vivo effects upon neutrophils, platelets, and lung. Am. J. Pathol. 1980;101:79–92. [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Mde C. Hirt L. Bogousslavsky J. Regli L. Badaut J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J. Neurosci. Res. 2006;83:1231–1240. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- Sato S. Umenishi F. Inamasu G. Sato M. Ishikawa M. Nishizawa M. Oizumi T. Expression of water channel mRNA following cerebral ischemia. Acta Neurochir. Suppl. 2000;76:239–241. doi: 10.1007/978-3-7091-6346-7_48. [DOI] [PubMed] [Google Scholar]
- Strair R.K. Schaar D. Goodell L. Aisner J. Chin K.V. Eid J. Senzon R. Cui X.X. Han Z.T. Knox B. Rabson A.B. Chang R. Conney A. Administration of a phorbol ester to patients with hematological malignancies: preliminary results from a phase I clinical trial of 12-O-tetradecanoylphorbol-13-acetate. Clin. Cancer Res. 2002;8:2512–2518. [PubMed] [Google Scholar]
- Sun M.C. Honey C.R. Berk C. Wong N.L. Tsui J.K. Regulation of aquaporin-4 in a traumatic brain injury model in rats. J. Neurosurg. 2003;98:565–569. doi: 10.3171/jns.2003.98.3.0565. [DOI] [PubMed] [Google Scholar]
- Taniguchi M. Yamashita T. Kumura E. Tamatani M. Kobayashi A. Yokawa T. Maruno M. Kato A. Ohnishi T. Kohmura E. Tohyama M. Yoshimine T. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Brain Res. Mol. Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Taylor R.G. McCall C.E. Thrall R.S. Woodruff R.D. O'Flaherty J.T. Histopathologic features of phorbol myristate acetate-induced lung injury. Lab. Invest. 1985;52:61–70. [PubMed] [Google Scholar]
- Venero J.L. Vizuete M.L. Ilundain A.A. Machado A. Echevarria M. Cano J. Detailed localization of aquaporin-4 messenger RNA in the CNS: preferential expression in periventricular organs. Neuroscience. 1999;94:239–250. doi: 10.1016/s0306-4522(99)00182-7. [DOI] [PubMed] [Google Scholar]
- Vizuete M.L. Venero J.L. Vargas C. Ilundain A.A. Echevarria M. Machado A. Cano J. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol. Dis. 1999;6:245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- Won J.S. Song D.K. Kim Y.H. Huh S.O. Suh H.W. The stimulation of rat astrocytes with phorbol-12-myristate-13-acetate increases the proenkephalin mRNA: involvement of proto-oncogenes. Brain Res. Mol. Brain Res. 1998;54:288–297. doi: 10.1016/s0169-328x(97)00344-6. [DOI] [PubMed] [Google Scholar]
- Wright E.M. Loo D.D. Coupling between Na+, sugar, and water transport across the intestine. Ann. N.Y. Acad. Sci. 2000;915:54–66. doi: 10.1111/j.1749-6632.2000.tb05223.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto N. Sobue K. Miyachi T. Inagaki M. Miura Y. Katsuya H. Asai K. Differential regulation of aquaporin expression in astrocytes by protein kinase C. Brain Res. Mol. Brain Res. 2001;95:110–116. doi: 10.1016/s0169-328x(01)00254-6. [DOI] [PubMed] [Google Scholar]
- Yang G.Y. Betz A.L. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–1664. doi: 10.1161/01.str.25.8.1658. discussion 1664–1655. [DOI] [PubMed] [Google Scholar]