Abstract
Acute pain is a common symptom experienced after spinal cord injury (SCI). The presence of this pain calls for treatment with analgesics, such as buprenorphine. However, there are concerns that the drug may exert other effects besides alleviation of pain. Among those reported are in vitro changes in gene expression, apoptosis, and necrosis. In this investigation, the effect of buprenorphine was assessed at the molecular, behavioral, electrophysiological, and histological levels after SCI. Rats were injured at the T10 thoracic level using the NYU impactor device. Half of the animals received buprenorphine (0.05 mg/kg) for 3 consecutive days immediately after SCI, and the other half were untreated. Microarray analysis (n = 5) was performed and analyzed using the Array Assist software. The genes under study were grouped in four categories according to function: regeneration, apoptosis, second messengers, and nociceptive related genes. Microarray analysis demonstrated no significant difference in gene expression between rats treated with buprenorphine and the control group at 2 and 4 days post-injury (DPI). Experiments performed to determine the effect of buprenorphine at the electrophysiological (tcMMEP), behavioral (BBB, grid walking and beam crossing), and histological (luxol staining) levels revealed no significant difference at 7 and 14 DPI in the return of nerve conduction, functional recovery, or white matter sparing between control and experimental groups (p > 0.05, n = 6). These results show that buprenorphine (0.05 mg/kg) can be used as part of the postoperative care to reduce pain after SCI without affecting behavioral, physiological, or anatomical parameters.
Key words: analgesic, behavior, microarrays, pain, spare tissue
Introduction
Spinal cord injury (SCI) is a condition with no known cure. Once a contusion to the spinal cord occurs, there are changes at the molecular and cellular levels, as well as systemically such as loss of locomotor activity, bowel control, respiratory difficulties, and erectile dysfunction, among other problems (Hulsebosch, 2002; Von Wild et al., 2002; Bloemen-Vrencken et al., 2005; Brown et al., 2006). SCI triggers a cascade of events that generates several types of pain.
The first is acute pain, caused primarily by physical damage to the body. The release of substances like ATP, histamine, potassium, leukotrienes, substance P, and prostaglandins by damaged cells, or by cells of the immune system, infiltrate the lesion site and promote the activation of nociceptive afferent fibers (Mills et. al., 2001; Hulsebosch, 2005). Two kinds of acute pain have been described: first a sharp pain followed by a diffuse, longer-lasting secondary pain (Fields, 1990). These forms of pain are carried by different axons, the former being the Aδ fibers, the latter being C-unmyelinated fibers. SCI may also cause central neuropathic pain (CNP), which arises a few weeks to months after the injury (Hulsebosch, 2002).
Pain requires treatment with analgesics. There is a wide variety of analgesics that can be administered to manage this pain, but many have unwanted side effects or present reduced effectiveness with time (Fernández-Dueñas et al., 2007; Ledeboer et al., 2007; Noble and Roques, 2007). In rodents, compounds like non-steroidal anti-inflammatory drugs (NSAID) and opioid-based agents may be used to treat acute pain. However, some of these agents require continuous administration that may be stressful to the animal. Others are not recommended in surgeries related to spinal cord injury because of their short half-life, addictive effects, and confounding side effects they may produce.
Morphine, for example, an agonist of the mu opioid receptor (μOR), is a key player involved in the anti-nociceptive system. The activation of the μOR leads to hyperpolarization of the neurons in the dorsal horn, reducing the nociceptive signaling and therefore affecting pain sensation (Wu et al., 1999; Wang et al., 2005). However, prolonged use of morphine may lead to tolerance and, subsequently, withdrawal symptoms (Fernández-Dueñas et al., 2007; Ledeboer et al., 2007; Noble and Roques, 2007). In trauma, rat model compounds, such as butorphanol, nalbuphine, meperidine, pentazocine, and codeine, can be used to alleviate acute pain, but buprenorphine is the analgesic recommended by veterinarians due to its potency and long-lasting duration (Kaiko et al., 1983; Gear et al., 1999; Katzung, 2001; Roughan and Flecknell, 2002). Moreover, subcutaneously administration of 0.05 mg/kg reduces pain sensitivity after postoperative surgeries (Curtin et al., 2009).
Buprenorphine, an analogue of morphine, is commercially available as Buprenex. Buprenorphine acts as a μOR agonist, but has less affinity for the receptor than does morphine. However, not much is known regarding the effect of this analgesic on other physiological processes. Most scientists do not administer any type of analgesic to rats after SCI (contusion, transection, or hemisection) because they are concerned about the secondary effects that the drug may have. Some in vitro studies suggest that buprenorphine may have neuroprotective effects on neuronal cells (Ozden and Isenmann, 2004), while others indicate that it may promote apoptosis (Kugawa and Aoki, 2004). These contradictory results, and the absence of detailed studies limit the use of buprenorphine as an analgesic in SCI animals with trauma. Therefore, the question addressed in this study is whether providing buprenorphine at 0.05 mg/kg for 3 days after trauma (Flecknell, 1991, 1996; Curtin et al., 2009) to an animal used in SCI experimental studies will affect specific molecular, anatomical, physiological and behavioral outcomes.
Materials and Methods
Spinal cord injury
All animal experiments were conducted with the approval of the University of Puerto Rico Animal Care and Use Committee and followed NIH guidelines. Adult (200–220 g) female Sprague-Dawley rats (Hilltop Lab Animals, Scottdale, PA) were anesthetized with intramuscular injections of ketamine (87.7 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA), xylazine (4.2 mg/kg; Fort Dodge Animal Health) and acepromazine (0.85 mg/kg; Vetus Animal Health, Rockville Center, NY). The doses of this dissociative anesthetic and analgesic have been used in our laboratory routinely (Figueroa et al., 2006) and its half-life is only a few hours. Following the pinch toe and corneal reflex tests, these animals were deeply anesthetized (Wixson et al., 1987; Wixson and Smiler, 1997). An incision was made on the back of the animal, and laminectomy at the thoracic T10 level was performed to expose the spinal cord. A moderate contusion with the New York University (NYU) impactor device (Gruner, 1992) was done at the T10 level of the spinal cord by adjusting the impactor (10 g) to a height of 12.5 mm, as previously described (Miranda et al., 1999; Willson et al., 2002; Irrizary-Ramirez et al., 2005). Control sham rats received only a laminectomy without damaging the dura matter.
After SCI, the muscles were sutured in layers and the animals were placed in individual cages. At this point, half the rats received 0.05 mg/kg of buprenorphine (Buprenex, Ben Venue Laboratories, Bedford, OH) every 12 h for 3 consecutive days. This dose and frequency has been recommended to reduce pain in rats during procedures that produce moderate to marked pain (Flecknell 1991, 1996; Curtin et al., 2009). Each rat was placed individually (after a complete recovery from the anesthesia) into a clean cage with paper towel and round paper tubes to reduce stress since they tend to chew. All animals were treated with the antibiotic cefazolin (25 mg/kg; West-Ward Pharmaceutical, Eatontown, NJ) for 7 days after the surgery, and their bladder was expressed until spontaneous voiding returned, usually after 7 days.
Euthanasia and treatment of tissue
The animals used for the RNA and Western blot studies were euthanized as described by Irizarry and colleagues (2005). Briefly, the animals were anesthetized by intraperitoneal administration of pentobarbital (40–50 mg/kg) and transcardially perfused with 350 mL of chilled 0.01 M phosphate-buffered saline (PBS [pH 7.4], Dulbecco's phosphate-buffered saline, Sigma-Aldrich, St. Louis, MO) to remove the blood from the spinal cord. The T10 lesion epicenter (5–10 mm) was dissected, as well as rostral and caudal segments of equal size.
The animals used for the histological analysis (luxol staining) followed the same procedure described above, but after the perfusion with cold PBS, the animals were perfused with 4% PFA (paraformaldehyde, Fluka Chemika, Buchs, Switzerland) in 0.1 M PBS (pH 7.4) (Figueroa et al., 2006). Spinal cords were removed and post-fixed in 4% PFA/PBS at 4°C for 3 h, then incubated in 30% sucrose/PBS and allowed to submerge at 4°C overnight. Approximately, 1.5 cm of spinal cord containing the lesion epicenter was submerged in Tissue-Tek O.C.T. embedding media (Miles, Elkhart, IN). Transverse sections of 20 μm were sectioned in a Leica Cryocut 1800 Cryostat (Nussloch, Germany) and mounted on superfrost microscope slides (Fisher Scientific, Pittsburgh, PA).
Animal groups
For this study, 40 rats were used. Half served as sham controls; the other 20 animals were injured. Half of the latter (10) were sacrificed at 2 days post-injury (DPI) and the other half (10) at 4 DPI. Within each group, half (5) was treated with buprenorphine (0.05 mg/kg) subcutaneously every 12 h for 3 consecutive days, as indicated by the Formulary for Laboratory Animals (Hawk and Leary, 1999) and as previously recommended (Roughan and Flecknell, 2002; Curtin et al., 2009). The other half (5) was treated with saline. The same experimental design was used with the sham control.
RNA extraction
RNA extraction was performed using Tri-Reagent (Trizol, Sigma-Aldrich, St. Louis, MO) followed by a clean-up treatment with columns (RNeasy Protect Mini Kit, Qiagen, Gaithersburg, MD) to remove any genomic DNA contamination. Afterward, the RNA integrity was confirmed in a 2.0% agarose gel (Certified Molecular Biology Agarose, Bio-Rad Laboratories, Hercules, CA), and the RNA concentration determined with spectrophotometer by absorbance at 260 nm (Eppendorf BioPhotomerter, Westbury, NY).
Microarray analysis
Microarray experiments were performed at the Bionomics Research and Technology Center (BRTC, Piscataway, NJ) using the Affymetrix rat 8k gene chip (Affymetrix, Santa Clara, CA). cDNA was synthesized and PCR performed with NuGEN Ovation Biotin Amplification and Labeling system (NuGEN Technologies, San Carlos, CA). The PCR products were hybridized to 8K gene chips. The strategy was to compare gene profiles of the control group (injured rats without buprenorphine treatment) with the experimental group (injured rats treated with buprenorphine). The microarrays were evaluated using Array Assist software (Stratagene, La Jolla, CA).
The arrays were analyzed by clustering differences between the treatments and searching for significant fold changes. A fold change of 2.0 was considered significant (above 2.0 [upregulation] or below 2.0 [downregulation]). Furthermore, evaluated genes were categorized by their biological function: regeneration, apoptosis, second messengers, and nociception. Under the regeneration category, the genes studied were growth associated protein-43 (GAP-43) and glial fibrillary acidic protein (GFAP). The former gene is related to neurite outgrowth, whereas the latter is a cellular marker associated with axonal outgrowth inhibition. Genes associated to the second messengers were protein kinase A (PKA) regulatory unit, calcium-calmodulin kinase kinase (CamKK), protein kinase C gamma (PKCγ), and mitotic activated protein 4 kinase kinase kinase (MAP4K3). The apoptotic genes under study were Bcl-2 associated genes and caspase-3. Finally, the categories of molecules associated to nociception were kappa opioid receptor (κOR), tachrine receptor, propiomelanocorticotropin (POMC), and a subunit of the NMDA receptor.
Western blot
Pentobarbital (40–50 mg/kg) was administered, as before, to anesthetize the rats, and transcardial perfusion with 0.01 M PBS was performed at 2, 4, 7, and 14 days post-injury. The spinal cord segment containing the lesion epicenter (5 mm) was dissected, and protein extraction was performed as described by Lai and colleagues (2001). Briefly, dissected tissue was homogenized in ice-cold Tris lysis buffer (20 mM Tris, 150 mM NaCl, 5 mM NaF, 1 mM EDTA, 1 mM EGTA) that contains 2 μg/mL antipain, 10 μg/mL aprotinin, 5 mM benzamidine, 1 mM DTT, 10 μg/mL leupeptin, 1 mM sodium orthovanadate, 1 mM PMSF, and 10 μg/mL trypsin inhibitors. The homogenate was centrifuged at 14,000 g for 90 min, and the pellet was resuspended in lysis buffer with 1% Nonident P-40 for 45 min at 4°C. Following a brief 10 min centrifugation at 14,000 g, the protein concentration of the supernatant was determined using the Bio-Rad Protein Assay protocol, as suggested by the manufacturer's instructions. Then the extracted proteins were analyzed in a 10% polyacrylamide-SDS gel. The proteins were electroblotted to a nitrocellulose membrane and stained with 0.1% Ponceaus S (made in 1% glacial acetic acid) to verify transfer and reveal molecular weight markers. The nitrocellulose membrane was blocked with Blotto (3% non-fat dry milk, 20 mM Tris-NaCl [pH 7.5], 10% Tween-20) for 2 h at room temperature. The membrane was probed with the monoclonal anti-GFAP antibody (1:400; Pharmigen, BD Biosciences, San Jose, CA) in blocking solution for 1 h at 37°C. Then the membrane was washed and incubated with HRP-conjugated rabbit anti-mouse IgG (1:600; Sigma-Aldrich) for 1 h at room temperature. HRP signal was enhanced with SuperSignal West Dura extended version (Pierce, Rockford, IL) for 1 min, according to the manufacturer's instructions, before exposure and development. As a loading control, the housekeeping gene, actin, was used and its immunoreactivity determined with mouse anti-actin (1:1000; Sigma-Aldrich) and rabbit anti-mouse (1:2000; Sigma-Aldrich). The development and analysis of the nitrocellulose membranes were performed in the Versadoc imaging system (Bio-Rad) and Quantity One Software (Bio-Rad).
Behavioral assessment after spinal cord injury
The animals were injured; half were sacrificed at 7 DPI, the other half at 14 DPI. Half of the animals within each group were treated with Buprenex, as stated previously (Hawk and Leary, 1999; Curtin et al., 2009). The other half was treated with saline for the same 3 days. The same experimental design was also performed on 24 sham rats. These animals were used for the behavioral, electrophysiological, and anatomical studies.
Rat locomotor recovery was assessed using BBB scores (Basso et al., 1995). Briefly, rats were observed in an open field and evaluated within a period of 4 min. The score ranges from a scale of 1 to 21. A score of 1 means that the rat has no movement in the hindlimbs. A score of 21 means that the rat has perfect coordination, including weight support, hindlimb coordination, and toe clearance among other things. High scores of perfect coordination were always observed in our sham rats.
Additional behavioral assays were performed, such as grid walking and the beam crossing tests, to analyze the severity of the functional deficit (Merkler et al., 2001; Cruz-Orengo et al., 2006). Briefly, in the grid-walking test, the animals were placed on a horizontal ladder (3 feet long), with bar distance alternated randomly to prevent habituation of the animal (the distance between the bars varied between 1 and 2 inches). The animals were evaluated as they crossed from one end of the ladder to the other, and the number of errors was determined. An error in this test means that at least one of the hindlimbs was between the bars, leading to falling of the posterior part of the animal or dragging of the hindlimbs. Dragging of the hindlimbs means that the animals passed the hindlimb over the bars (dorsal side facing down) but with no weight support in the posterior area.
The beam-crossing test has been used to test the ability of a rat to balance and cross a 1 meter bar (square, 2 × 2 cm; round, 2-cm diameter) at 15–18 inches from the ground (Merkler et al., 2001). The score given in this test can be up to 4 points (2 points for the square test and 2 points for the round bar test). Crossing half the bar equals 0.5 points, crossing the whole bar represents 1.0 point; if the animal uses one hindlimb to cross the entire bar then another 0.5 points is added to the former 1.0 point. If the animal crosses the entire bar using both hindlimbs, then the score achieved by the animal would be 2. Successful completion of both tests equates to a final score of 4. These three behavioral assays were performed before the contusion, to set a baseline, and at 7 and 14 DPI.
Electrophysiological evaluation after SCI
The effect of buprenorphine on the return of nerve conduction was monitored with transcranial magnetic motor evoked potentials (tcMMEP) at 0, 7, and 14 DPI. The Magstim 2002 stimulator (Magstim Company, Spring Gardens, United Kingdom) generates short magnetic pulses (70 μs) through a 50-mm diameter handheld magnetic transducer placed over the skull. Electromyograph (EMG) responses were recorded from subdermal EMG needle electrodes inserted into the gastrocnemius. The animals were injected intramuscularly with ketamine (40 mg/kg; Fort Dodge Animal Health) and acepromazine (0.85 mg/kg; Vetus Animal Health) (Figueroa et al., 2006; Cruz-Orengo et al., 2007). In this study, amplitude measurements above 0.15 mV and latencies shorter than 25 ms were considered physiological responses. The tcMMEP responses were measured at 70%, 85%, and 100% stimulation intensity to validate the obtained responses. Amplification of the data was done by using Magstim Neurosign 100. Finally, the signal was converted and analyzed using the Digidata 1322A (Molecular Devices, Sunnyvale, CA) and Axoscope 8.2 software, respectively (Scientifica, Uckfield, United Kingdom).
White matter sparing tissue assessment
Five sections, with the lesion epicenter and regions rostral or caudal (1 cm) to it per animal, were treated with luxol (Luxol Fast Blue, Alfa Aesar, Heysham, United Kingdom) and counterstained with cresyl violet (Sigma-Aldrich), as previously reported by Figueroa and colleagues (2006). Slides were observed in a digital microscope (Fisher Scientific), and photomicrographs were taken using Motic, version 1.2 software. The stained spinal cord sections were morphometrically analyzed (MCID, Imaging Research, Ontario, Canada) to determine the extent of white matter spared. Briefly, the outer border of the spinal cord sectioning was identified to delineate the site of the spinal cord. Then, stained with luxol, the amount of white matter spare tissue and the lesion cavity was delineated. Both parameters were used to calculate the area of white matter spared tissue using density per unit area (density/area).
Statistical analysis
Data were analyzed by using either ANOVA followed by Bonferroni multiple-comparison test or unpaired two-tailed t tests. Results are expressed as mean ± standard error means (SEM). Differences were considered significant when p < 0.05. All the statistical tests were performed with the GraphPad Instat software, version 3.06 (La Jolla, CA) and Prism 5 (La Jolla, CA).
Results
Microarrays
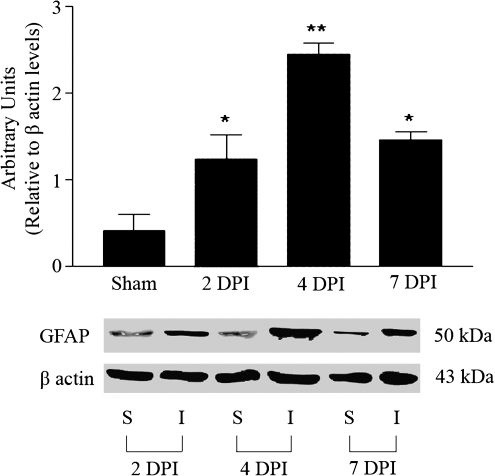
Injury to the spinal cord upregulates a set of genes at the lesion epicenter (Carmel et. al., 2001) when compared to sham animals. Specifically an increase in glial fibrillary acidic protein (GFAP) has been reported (Wu et al., 2005). In our laboratory, we also observed GFAP upregulation at the protein level (Fig. 1). Western blot analysis showed that compression to the spinal cord induced a 1.237 ± 0.2853 fold change of GFAP at 2 DPI that remained elevated until 7 DPI, without any significant change in sham animals treated with buprenorphine. Moreover, β-actin levels remained unchanged after SCI, confirming the specificity of the response and the absence of an effect by buprenorphine. However, injured rats treated with buprenorphine did not show significant fold change in the microarray analysis for any gene selected when compared to injured animals treated with saline at 2 and 4 DPI.
FIG. 1.
Western blot analysis of glial fibrillary acidic protein (GFAP) expression in injured rats treated with buprenorphine. An increase can be observed in GFAP protein expression in the spinal cord injured animals at 2, 4, and 7 DPI when compared to the sham animals, without a change in β-actin level. Densitometry analysis demonstrated a significant change in GFAP levels relative to β-actin expression after SCI (ANOVA followed by Bonferroni multiple-comparison test, p < 0.05, n = 3). The values presented are the mean ± SEM (n = 3).
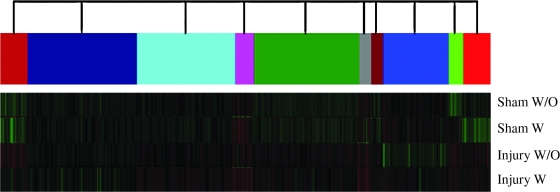
The expression profile of genes related to regeneration, apoptosis, second messengers, and nociception did not change after treatment with buprenorphine (p > 0.05, n = 5). Fold change analysis of the genes GAP-43, GFAP, Caspase3, Bcl2L1, Bcl2L10, PKA regulatory unit, CamKK, PKCγ, MAP4K3, POMC, tachrine receptor, NMDA subunit, and kappa opioid receptor were performed with microarray analysis and are summarized in Table 1. The selected genes did not show any significant change when compared to treated animals with buprenorphine at 2 and 4 DPI. The same analysis was performed at different levels of the spinal cord (1 cm rostral and caudal to the lesion epicenter), and no significant changes were observed (data not shown). Cluster analysis of genes from the spinal cord demonstrated that buprenorphine did not exert a significant change in the expression profile of genes in the sham or injured spinal cords (Fig. 2). However, the pattern of gene expression after SCI is different from that of the sham treated animals.
Table 1.
Fold Change of Selected Genes after Microarray Analysis in Animals Treated and Untreated with Buprenorphine
| Gene | 2 days post-injury | 4 days post-injury |
|---|---|---|
| GAP-43 | 0.443 ± 0.7364 | −0.35 ± 0.6852 |
| GFAP | −1.84 ± 0.2307 | −1.42 ± 1.267 |
| Caspase 3 | 1.015 ± 0.1748 | 0.368 ± 0.6735 |
| Bcl2L1 | −0.47 ± 0.8053 | −0.463 ± 0.8882 |
| Bcl2L10 | −0.3667 ± 0.8735 | −0.2 ± 0.8250 |
| PKA regulatory unit | −0.5124 ± 0.8198 | −0.5322 ± 0.8993 |
| CamKK | −0.6 ± 1.016 | 1.32 ± 0.1497 |
| PKCγ | 0.4967 ± 0.8198 | −0.4333 ± 0.8123 |
| MAP4K3 | −1.383 ± 0.1648 | 0.47 ± 0.8487 |
| POMC | −0.09 ± 0.9351 | 0.3867 ± 0.8687 |
| Tachrine receptor | 0.86 ± 1.228 | 0.033 ± 1.032 |
| NMDA subunit | −0.5267 ± 0.7795 | 1.2767 ± 1.587 |
| Kappa opioid receptor | −0.53 ± 0.7845 | 0.33 ± 0.7406 |
FIG. 2.
Representative dendogram of the genes analyzed in animals untreated and treated with buprenorphine evaluated at 4 DPI. Sham animals showed very similar patterns of expression between them but not when compared to the injured animals in any of the conditions (treated with buprenorphine). Injured animals showed no significant change in the pattern of global gene expression in two conditions. The color code indicates groups of genes that are expressed with a similar profile, with or without buprenorphine.
Behavioral analysis
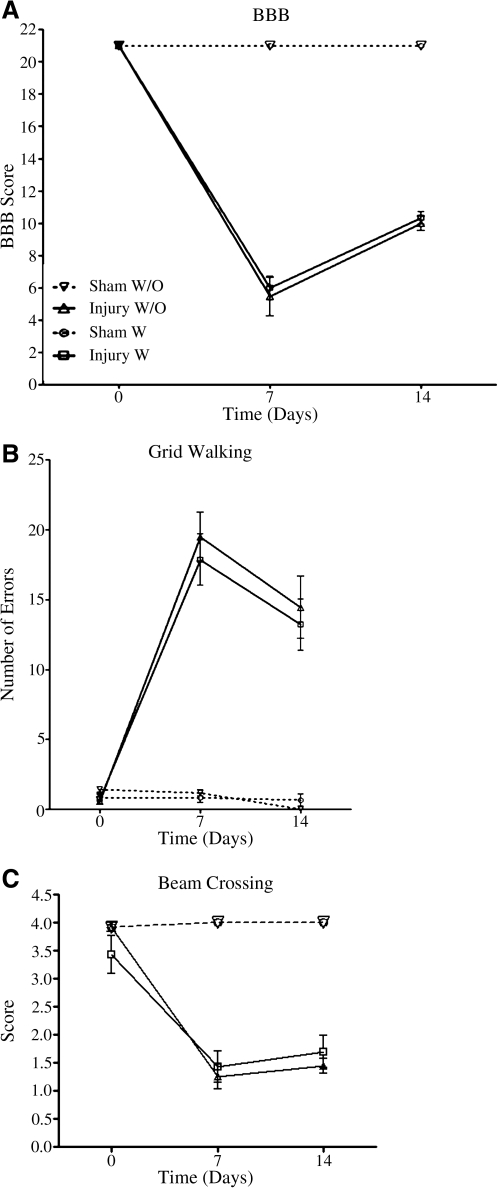
The effect of buprenorphine on functional recovery was determined with three behavioral assays: BBB, grid walking and beam crossing. Rats subjected to spinal cord injury that received saline showed no significant difference (p > 0.05) in BBB scores at 7 or 14 DPI compared to the injured animals treated with the buprenorphine (Fig. 3A). Injured animals treated with buprenorphine showed BBB scores (5.977 ±0.7234, n = 11) similar to the control animals (5.458 ± 1.194, n = 6) at 7 DPI. A similar pattern was observed at 14 DPI for these two groups. The group treated with buprenorphine present BBB scores of 10.344 ± 0.375 (n = 9) that are similar to those of the control group (10.00 ± 0.4583, n = 9). Sham animals left untreated with buprenorphine had scores of 21.00 ±0.00 (n = 6) at 7 days post-laminectomy (DPL) and this score remained equal at 14 DPI (n = 3). Buprenorphine treatment did not affect the score of 21 in the animals at 7 (21.00 ± 0.00, n = 6) or 14 days after the surgery (21.00 ± 0.00, n = 6), with the exception of the sham groups versus the injury groups. ANOVA analysis for the injury group followed by the Bonferroni post hoc test showed no significant difference (F(3,30) = 13.271; p > 0.05, n = 11) among any of the groups at any of the time points. However, significant differences were observed when sham animals (treated or untreated) were compared with injured rats (treated or untreated) at both time points (p < 0.001).
FIG. 3.
Behavioral tests conducted at 7 and 14 DPI to analyze the effect of buprenorphine on functional locomotor recovery. BBB scores were used to evaluate the locomotor recovery of the animals, and there was no significant difference between the injured animals treated and untreated with buprenorphine at 7 or 14 DPI, the same was observed for the sham animals (A). Grid-walking analysis demonstrated that injured animals made more errors than sham animals. However there was no significant difference in the number of errors performed by the rats when the two groups (injured with buprenorphine versus saline treated, and sham with buprenorphine versus saline treated) were compared at 7 and 14 DPI (B). Beam crossing was also performed on the same animals and the same results were observed; there was no significant difference observed when both groups were compared at 7 and 14 DPI (C). None of the comparisons were significant (p > 0.05), using two-way ANOVA followed by Bonferroni multiple-comparison test, with the exception of sham animals compared to injured rats. The values presented are the mean ± SEM.
Animals that were injured and treated with buprenorphine showed a similar number of errors (17.857 ± 1.822, n = 14) at 7 DPI in the grid-walking test compared to that of lesioned rats that were administered saline (19.5 ± 1.766, n = 12) (Fig. 3B). Moreover, at 14 DPI the injured animals treated with buprenorphine presented a mean error value of 13.20 ±1.826 (n = 10), and value for the saline treated animals was 14.444 ± 2.215 (n = 9). Sham animals treated with saline showed none to few errors in the grid test (0.667 ± 0.211, n = 6) at 7 DPL, and similar values were obtained for sham animals treated with buprenorphine (0.833 ± 0.357, n = 6). At 14 DPL, sham untreated animals had no errors (n = 3), while buprenorphine treated animals showed values of 0.667 ±0.441 (n = 3). Although significant changes were observed between sham and injured animals, statistical analysis confirmed that differences between any of the groups (injured with buprenorphine versus injured with saline; or sham with buprenorphine versus sham with saline) were not significant (F(3,41) = 2.296, p = 0.0919, ANOVA followed by Bonferroni multiple-comparison test).
The scores for the beam-crossing test were not significantly different between the studied groups (Fig. 3C). Treated injured animals obtained scores of 1.429 ± 0.286 (n = 14) at 7 DPI and contused rats with saline obtained scores of 1.250 ± 0.218 (n = 12). No significant differences were observed at 14 DPI between the untreated injured animals (1.444 ± 0.130, n = 9) and the buprenorphine treated rats (1.700 ± 0.291, n = 10). Sham animals treated with saline (4.00 ± 0.00, n = 5) and the group treated with buprenorphine (4.00 ± 0.00, n = 5) showed identical values at 7 DPI. At 14 DPI the same pattern was observed with maximum values of 4.00 ± 0.00 (n = 3) for the untreated group and 4.00 ± 0.00 (n = 3) for the treated group. No significant differences were observed between the groups analyzed in this test (F(3,41) = 0.5058, p = 0.6804, ANOVA followed by Bonferroni multiple-comparison test). However, significant behavioral differences between sham and injured animals were observed (p < 0.05).
Transcranial magnetic motor evoked potentials
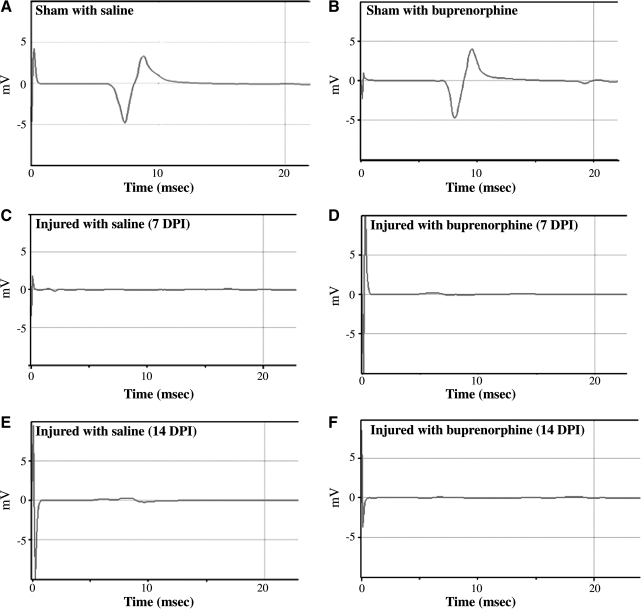
Transcranial magnetic motor evoked potential (tcMMEP) responses were recorded from the gastrocnemius muscles. Figure 4 shows representative traces from sham and injured animals treated with saline or buprenorphine. Sham operated rats treated with saline (Fig. 4A) presented a typical response with a latency of approximately 8 ms that was no different from sham animals treated with buprenorphine (Fig. 4B). The absence of tcMMEP responses in contused rats at 7 DPI treated with saline (Fig. 4C) or buprenorphine (Fig. 4D) was observed. Moreover, at 14 DPI the animals treated with saline (Fig. 4E) or buprenorphine (Fig. 4F) did not produce any tcMMEP responses. Similar observations of tcMMEP responses between sham and injured animals were observed by Figueroa and colleagues (2006). The latencies obtained at 7 days after laminectomy were 7.91 ms ± 0.2090 (n = 9) for sham animals treated with saline and 8.07 ms ± 0.2141 (n = 9) for animals treated with buprenorphine (data not shown). For animals treated with saline evaluated at 14 days post-surgery, the latencies were 7.65 ms ± 0.2614 (n = 6). In animals treated with buprenorphine, latencies periods were 7.71 ms ± 0.3755 (n = 6). No significant difference in the tcMMEP latencies was observed in any of the sham groups tested at either 7 or 14 days after surgery (F(3,26) = 0.5246, p = 0.6692, ANOVA followed by Bonferroni multiple-comparison test). TcMMEP response amplitudes were not reported because of the variability that arose due to the positioning of the electrodes each week (Linden et al., 1999).
FIG. 4.
Effect of buprenorphine on the return of nerve conduction. Electrophysiological studies were performed on animals at 7 and 14 DPI. Transcranial magnetic motor evoked potential (tcMMEP) tracings of sham animal not treated with buprenorphine (A) and treated with buprenorphine (B) at 7 days after the surgery showed normal responses after stimulation. Injured animals not treated with buprenorphine (C) and treated with buprenorphine (D) showed no response after stimulus as expected at 7 DPI and 14 DPI (E and F).
Anatomical studies
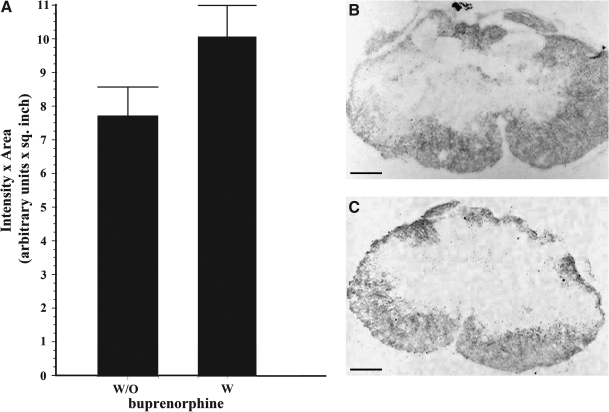
The effect of the analgesic in the white matter sparing tissue was determined in injured animals treated with saline and in contused rats treated with buprenorphine. Untreated injured animals had an area of white matter spared tissue at the rostral level (∼1 cm to the lesion epicenter) of 8.44 ± 1.613 intensity (arbitrary units) per square inch, while the treated injured animals showed values of 7.14 ± 0.3255 at 7 DPI (data not shown). At the same time point, the caudal areas (∼1 cm to the injury epicenter) in untreated animals had values of 7.38 ± 1.940 versus 7.77 ± 1.980 of treated animals (data not shown). At 14 DPI the spare tissue density in the rostral areas of the untreated injured animals had measured values of 11.10 ± 0.6220 and the treated animals had values of 8.61 ±0.057 intensity (arbitrary units) per square inch (data not shown). Spared tissue at the lesion epicenter was observed to be reduced relative to the regions rostral or caudal to the lesion. However, treatment with buprenorphine did not significantly alter the amount of spared tissue (10.07 ± 0.92, n = 6) when compared to saline treated animals (7.73 ± 0.85 intensity [arbitrary units] per square inch, n = 6) according to the Student t test (p > 0.05; Fig. 5). The caudal areas of the animals at the same time point revealed values of 10.1 ± 0.3120 in the untreated animals and the treated animals revealed values of 10.31 ± 1.160 (data not shown). Analysis of injured rats treated with the drug demonstrated that the amount of white matter is not significantly different in injured untreated control rats at the lesion epicenter nor in regions rostral and caudal to the lesion. Sham-control animals presented an amount of white matter similar to the group of sham rats treated with buprenorphine at 7 and 14 days after the surgery (data not shown). None of the comparisons made between the groups were significantly different.
FIG. 5.
Effect of buprenorphine on white matter spare tissue at 14 DPI. Histological studies were performed using luxol staining and cresyl violet counterstaining. White matter spare tissue was evaluated at the lesion epicenter in injured animals treated and untreated with buprenorphine at 14 DPI. The area of spare tissue was morphometrically analyzed as density ×area (A). The values presented are the mean ± SEM. Luxol and cresyl violet stained representative segment of a lesion epicenter of an untreated (B) and a treated (C) injured animal at 14 DPI are shown. No significant difference was observed in any of the groups compared at any of the segments evaluated (Student t test, p > 0.05, n = 6). Scale bar, 100 μm.
Discussion
Buprenorphine is a well-known analgesic used to reduce pain in rats with moderate to marked pain (Flecknell, 1991, 1996; Curtin et al., 2009). However, studies administering buprenorphine to neural cells in vitro show a variety of results. Some results demonstrated that administration of this analgesic to retinal ganglional cells promotes neuroprotection (Ozden and Isenmann, 2004). Others demonstrated that buprenorphine administration to NG108-15 cells promotes apoptosis (Kugawa et al., 2004), and further studies showed that buprenorphine administration to NG108-15 cells promotes changes in gene expression profile of this cell line (Kugawa and Aoki, 2004). All of these conflicting results have been utilized by scientists to reject the use of analgesics to reduce acute pain in their animals after trauma to the spinal cord. Moreover, the effects of buprenorphine at the gene, physiological, behavioral, and anatomical levels are unknown.
In order to evaluate the effects of buprenorphine on gene expression, microarray analysis was performed. Cluster analysis of genes expressed after SCI were similar to those of Carmel and colleagues (2001), showing changes in gene expression after trauma (sham vs. injury). The results showed no significant difference in the gene profile between animals treated with buprenorphine and those without. These evaluations were performed at 2 and 4 DPI on injured and sham rats. This means that buprenorphine can be administered to a rat for 3 consecutive days in the doses of 0.05 mg/kg subcutaneously every 12 h without significantly affecting the general profile of gene expression. We selected several genes associated with regeneration, apoptosis, second messengers, and nociception. No changes were observed in the levels of GAP-43 expression, implying that buprenorphine does not affect genes associated with neurite outgrowth. A similar observation was obtained in the expression pattern of GFAP, one of the most characterized genes associated with reactive astrocytes and the inhibition of axonal regeneration. Expression of GFAP after SCI in animals treated with the analgesic presented a similar profile to that reported by Wu and colleagues (2005), confirming that buprenorphine did not alter the pattern or specificity of the response to trauma. In addition, genes that are known to play a key role in apoptotic and anti-apoptotic events were not altered by buprenorphine after SCI, suggesting that the analgesic is less likely to alter this cellular cascade at the gene level. This result is contradictory to that observed by Kugawa and colleagues (2004), and may be due to differences in the experimental models used (in vitro vs. in vivo). Similar results were obtained with genes related to second messenger systems like cAMP-PKA, IP3-PKC, and Ca-calmodulin. Therefore, buprenorphine administration after SCI does not affect the expression levels of proteins related to several second messenger cascades. This suggests that the treatment of rats with an analgesic does not seem to alter events that rely on the proteins studied. Moreover, the use of an analgesic to reduce the acute pain generated by contusion to the cord does not alter specific genes that are related directly or indirectly with the mechanism of action of buprenorphine. Therefore, the experimental outcomes obtained from the animals that received buprenorphine (at 0.05 mg/kg for 3 consecutive days after SCI) demonstrated that the administration of this analgesic did not produce significant changes in several genes related to cell death, axonal regeneration, signal transduction, and/or pain. These results suggest that investigators working in the field of SCI may use buprenorphine to reduce the acute pain generated by the surgery without significant changes in the expression of the mentioned genes. We could not discard the possibility of subtle changes not apparent in gene arrays or post-translational changes after drug treatment. However, if these cellular events are taking place, they are not significant because no differences were observed at the behavioral, physiological, or anatomical level.
The effect of buprenorphine on functional locomotor recovery was assessed by using BBB scores and grid-walking and beam-crossing tests. The behavioral assays of the animals that were treated with buprenorphine were not significantly different from those that were treated with saline. These results show that buprenorphine does not have a positive or negative effect on the locomotor recovery in the animals. The results suggest that buprenorphine may be administered after SCI without the concern of behavioral alterations.
Electrophysiological studies provide a more precise and objective analyses of functional recovery. The return of nerve conduction by tcMMEPs is more sensitive in measuring axonal outgrowth or reorganization than behavioral tests. This assay does not require utilization of several muscles or coordination of those muscles for any type of locomotor activity. Therefore, some axonal regeneration or sprouting can be monitored by this physiological strategy, which might be missed with the behavioral experiments. The tcMMEP was performed in treated animals; no responses were observed in injured animals treated or untreated with buprenorphine. The responses observed were similar to the traces reported by Cruz-Orengo et al. (2007) and Figueroa et al. (2006). This data suggests that the analgesic is not affecting the return of nerve conduction in sham or injured animals. Sham animals that were evaluated in this test showed responses to the stimuli that were not significantly different between the two groups (treated and untreated with the drug). This proves that buprenorphine does not alter the electrophysiological responses that the supraspinal and spinal neurons may produce when stimulated by a transcranial magnetic motor evoked potential. Therefore, experiments that involve testing the electrophysiological components of the animal with a contusion to the spinal cord could be treated with buprenorphine as part of the postoperative care.
Staining of the spinal cord with luxol and a counterstaining with cresyl violet allowed the assessment of the spared tissue in the spinal cord after trauma (Sribnick et al., 2005). The white matter spared tissue and the gray matter (data not shown) were not significantly different in the animals treated with buprenorphine than in those treated with saline. This demonstrates that buprenorphine does not have any effect on the amount of tissue that forms the spinal cord. The observation that buprenorphine did not affect white matter spared tissue in sham and injured animals and did not change functional locomotor behavior or tcMMEP latencies suggests that this analgesic does not have an effect on the lesion volumes or the cellular events that alter the spinal cord tissue after trauma.
Buprenorphine, at the dose of 0.05 mg/kg and a frequency of every 12 h for 3 consecutive days as stated here and as recommended in the Formulary for Laboratory Animals (Terrence, 2005), should be used as a standard postoperative analgesic in animal models of SCI. Administered at this dose and frequency, this drug will reduce the acute pain (Flecknell, 1991, 1996; Curtin et al., 2009) generated by trauma and surgery (skin incision, muscle disruption, blood vessel damage) without affecting gene expression, behavioral outcomes, electrophysiological responses, or anatomical structures. Although no differences were observed with the outcomes selected in this project (at the optimal dose of buprenorphine, as recommended by Curtin et al. [2009]), we cannot eliminate the possibility that this analgesic may have an effect on other outcomes. The advantage of buprenorphine is that it has a long lasting effect (12 h), which reduces the handling of animals. Other analgesics, such as meperidine, nalbuphine, pentazocine, or butorphanol, need to be administered every 3–4 h. The continuous handling of the rats during injection may increase stress to the animal. Future experiments should be designed to assess the effect of buprenorphine in reducing chronic neuropathic pain generated weeks after injury. Confounding effects of this analgesic when combined with other drugs is also an area that warrants further investigation.
Acknowledgments
Special thanks to the Animal Research Center and the veterinarian Vanessa Rodríguez for her continuous support and recommendations for this project; and to Dr. Annabell Segarra, Dr. Alan Preston, Héctor Franco, and Iris K. Salgado for their comments on the revision of this manuscript. Also our special thanks to Dr. Andrew I. Brooks and Dr. Qi Wang and their staff from the Bionomics Research and Technology Center (BRTC) for support with the microarray studies. In addition, our gratitude to the Experimental Surgery Facility at the UPR Medical Sciences Campus, the MBRS/SCORE Molecular Facilities, and the RCMI Image Center (G12RR03051). This work was supported by the MBRS-RISE (R25-GM061838), MBRS/SCORE (S06-GM008224), NIH/NINDS (39405), and the M-RISP (532851) programs.
Author Disclosure Statement
No competing financial interests exist.
References
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bloemen-Vrencken J.H. Post M.W. Hendriks J.M. De Reus E.C. De Witte L.P. Health problems of persons with spinal cord injury living in the Netherlands. Disabil. Rehabil. 2005;27:1381–1389. doi: 10.1080/09638280500164685. [DOI] [PubMed] [Google Scholar]
- Brown R. DiMarco A.F. Hoit J.D. Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir. Care. 2006;51:853–868. [PMC free article] [PubMed] [Google Scholar]
- Carmel J.B. Galente A. Soteropoulos P. Tolias P. Recce M. Young W. Hart R.P. Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiol. Genomics. 2001;7:201–213. doi: 10.1152/physiolgenomics.00074.2001. [DOI] [PubMed] [Google Scholar]
- Cruz-Orengo L. Figueroa J.D. Torrado A. Puig A. Whittemore S.R. Miranda J.D. Reduction of EphA4 receptor expression after spinal cord injury does not induce axonal regeneration or return of tcMMEP response. Neurosci. Lett. 2007;418:49–54. doi: 10.1016/j.neulet.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orengo L. Figueroa J.D. Velazquez I. Blocking EphA4 upregulation after spinal cord injury results in enhanced chronic pain. Exp. Neurol. 2006;202:421–433. doi: 10.1016/j.expneurol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Curtin L.I. Grakowsky J.A. Suarez M. Thompson A.C. Dipirro J.M. Martin L.B. Kristal M.B. Evaluation of buprenorphine in a postoperative pain model in rats. Comp. Med. 2009;59:60–71. [PMC free article] [PubMed] [Google Scholar]
- Fernández-Dueñas V. Pol O. García-Nogales P. Hernández L. Planas E. Puig M.M. Tolerance to the antinociceptive and antiexudative effects of morphine in a murine model of peripheral inflammation. J. Pharmacol. Exp. Ther. 2007;322:360–368. doi: 10.1124/jpet.106.118901. [DOI] [PubMed] [Google Scholar]
- Fields H.L. Pain Syndromes in Neurology. Butterworths; London: 1990. [Google Scholar]
- Figueroa J.D. Benton R.L. Velazquez , et al. Inhibition of EphA7 up-regulation after spinal cord injury reduces apoptosis and promotes locomotor recovery. J. Neurosci. Res. 2006;84:1438–1451. doi: 10.1002/jnr.21048. [DOI] [PubMed] [Google Scholar]
- Flecknell P.A. Post-operative analgesia in rabbits and rodents. Lab. Anim. 1991;20(9):34–37. [Google Scholar]
- Flecknell P.A. Laboratory Animal Anesthesia; An Introduction for Research Workers and Technicians. 2nd edition. Academic Press; New York: 1996. pp. 143–153. [Google Scholar]
- Gear R.W. Miaskowski C. Gordon N.C. Paul S.M. Heller P.H. Levine J.D. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83(2):339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- Gruner J.A. A monitored contusion model of spinal cord in the rat. J. Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Hawk T. Leary S.L. Morris T.H. Formulary for Laboratory Animals. 3rd edition. Wiley-Blackwell; New York: 2005. [Google Scholar]
- Hulsebosch C.E. Recent advances in pathophysiology and treatment of spinal cord injury. Am. J. Physiol. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Hulsebosch C.E. From discovery to clinical trials: treatment strategies for central neuropathic pain after spinal cord injury. Curr. Pharm. Design. 2005;11:1411–1420. doi: 10.2174/1381612053507864. [DOI] [PubMed] [Google Scholar]
- Irrizary-Ramirez Z.M. Willson C.A. Cruz-Orengo L., et al. Upregulation EphA3 receptor after spinal cord injury. J. Neurotrauma. 2005;22:9276–9288. doi: 10.1089/neu.2005.22.929. [DOI] [PubMed] [Google Scholar]
- Kaiko R.F. Foley K.M. Grabinski P.Y. Heidrich G. Rogers A.G. Inturrisi C.E. Reidenberg M.M. Central nervous system excitatory effects of meperidine in cancer patients. Ann. Neurol. 1983;13(2):180–185. doi: 10.1002/ana.410130213. [DOI] [PubMed] [Google Scholar]
- Katzung B.G. Opioid analgesics and antagonists. In: Foltin J., editor; Ransom J., editor; Nogueira I., editor; Davis K., editor. Basic and Clinical Pharmacology. McGraw-Hill; New York: 2001. pp. 525–527. [Google Scholar]
- Kugawa F. Aoki M. Expression of the polyubiquitin gene early in the buprenorphine hydrochloride-induced apoptosis of NG108-15 cells. DNA Seq. 2004;15:237–245. doi: 10.1080/10425170400006372. [DOI] [PubMed] [Google Scholar]
- Kugawa F. Nakamura M. Ueno A. Aoki M. Over-expressed Bcl-2 cannot suppress apoptosis via the mitochondria in buprenorphine hydrochloride-treated NG108-15 cells. Biol. Pharm. Bull. 2004;27:1340–1347. doi: 10.1248/bpb.27.1340. [DOI] [PubMed] [Google Scholar]
- Lai K.O. Ip F.C. Cheung J. Fu A.K. Ip N.Y. Expression of Eph receptors in the skeletal muscle and their localization at the neuromuscular junction. Mol. Cell Neurosci. 2001;17:1034–1047. doi: 10.1006/mcne.2001.0997. [DOI] [PubMed] [Google Scholar]
- Ledeboer A. Hutchinson M.R. Watkins L.R. Johnson K.W. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin. Invest. Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- Linden R.D. Zhang Y.P. Burke D.A. Hunt M.A. Harpring J.E. Shields C.B. Magnetic motor evoked potential monitoring in the rat. J. Neurosurg. 1999;91:205–210. doi: 10.3171/spi.1999.91.2.0205. [DOI] [PubMed] [Google Scholar]
- Merkler D. Metz G.A. Raineteau O. Dietz V. Schwab M.E. Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J. Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C.D. Grady J.J. Hulsebosch C.E. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J. Neurotrauma. 2001;18:1091–1105. doi: 10.1089/08977150152693773. [DOI] [PubMed] [Google Scholar]
- Miranda J.D. White L.A. Willson C.A. Marcillo A. Jagid J. Whittemore S.R. Introduction of EphB3 RPTK after spinal cord injury. Exp. Neurol. 1999;156:218–222. doi: 10.1006/exnr.1998.7012. [DOI] [PubMed] [Google Scholar]
- Niclou S.P. Ehlert E.M. Verhaagen J. Chemorepellent axon guidance molecules in spinal cord injury. J. Neurotrauma. 2006;23:409–421. doi: 10.1089/neu.2006.23.409. [DOI] [PubMed] [Google Scholar]
- Noble F. Roques B.P. Protection of endogenous enkephalin catabolism as natural approach to novel analgesic and antidepressant drugs. Expert Opin. Ther. Targets. 2007;11:145–159. doi: 10.1517/14728222.11.2.145. [DOI] [PubMed] [Google Scholar]
- Ozden S. Isenmann S. Neuroprotective properties of different anesthetics on axotomized rat retinal ganglion cells in vivo. J. Neurotrauma. 2004;21:73–82. doi: 10.1089/089771504772695968. [DOI] [PubMed] [Google Scholar]
- Roughan J.V. Flecknell P.A. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab. Anim. 2002;36:322–343. doi: 10.1258/002367702320162423. [DOI] [PubMed] [Google Scholar]
- Sribnick E.A. Wingrave J.M. Matzelle D.D. Wilford G.G. Ray S.K. Banik N.L. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J. Neurosci. Res. 2005;15:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Von Wild K. Rabischong P. Brunelli G. Benichou M. Krishnan K. Computer added locomotion by implanted electrical stimulation in paraplegic patients (SUAW) Acta Neurochir. Suppl. 2002;79:99–104. doi: 10.1007/978-3-7091-6105-0_22. [DOI] [PubMed] [Google Scholar]
- Wang H.Y. Friedman E. Olmstead M.C. Burns L.H. Ultra-low dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience. 2005;135:247–261. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Willson C.A. Irrizary-Ramirez M. Gaskin H.E. Cruz-Orengo L. Figueroa J.D. Whittermore S.R. Miranda J.D. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplant. 2002;11:279–290. [PubMed] [Google Scholar]
- Wixson S.K. Smiler K.L. Anesthesia and analgesia in rodents. In: Kohn D.F., editor; Wixson S.K., editor; White W.J., editor; Benson G.J., editor. Anesthesia and Analgesia in Laboratory Animals: American College of Laboratory Animal Medicine Series. Academic Press; New York: 1997. pp. 165–203. [Google Scholar]
- Wixson S.K. White W.J. Hughes H.C., Jr. Lang C.M. Marshall W.K. A comparison of pentobarbital, fentacyl-droperdiol, ketamine-xylazine and ketamine-diazepam anesthesia in adult male rats. Lab. Anim. Sci. 1987;37(6):726–730. [PubMed] [Google Scholar]
- Wu S.Y. Dun S.L. Wright M.T. Chang J.K. Dun N.J. Endomorphin like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons in vitro. Neuroscience. 1999;89:317–321. doi: 10.1016/s0306-4522(98)00570-3. [DOI] [PubMed] [Google Scholar]
- Wu X. Yoo S. Wrathall J.R. Real-time quantitative PCR analysis of temporal-spatial alterations in gene expression after spinal cord contusion. J. Neurochem. 2005;93:943–952. doi: 10.1111/j.1471-4159.2005.03078.x. [DOI] [PubMed] [Google Scholar]