Abstract
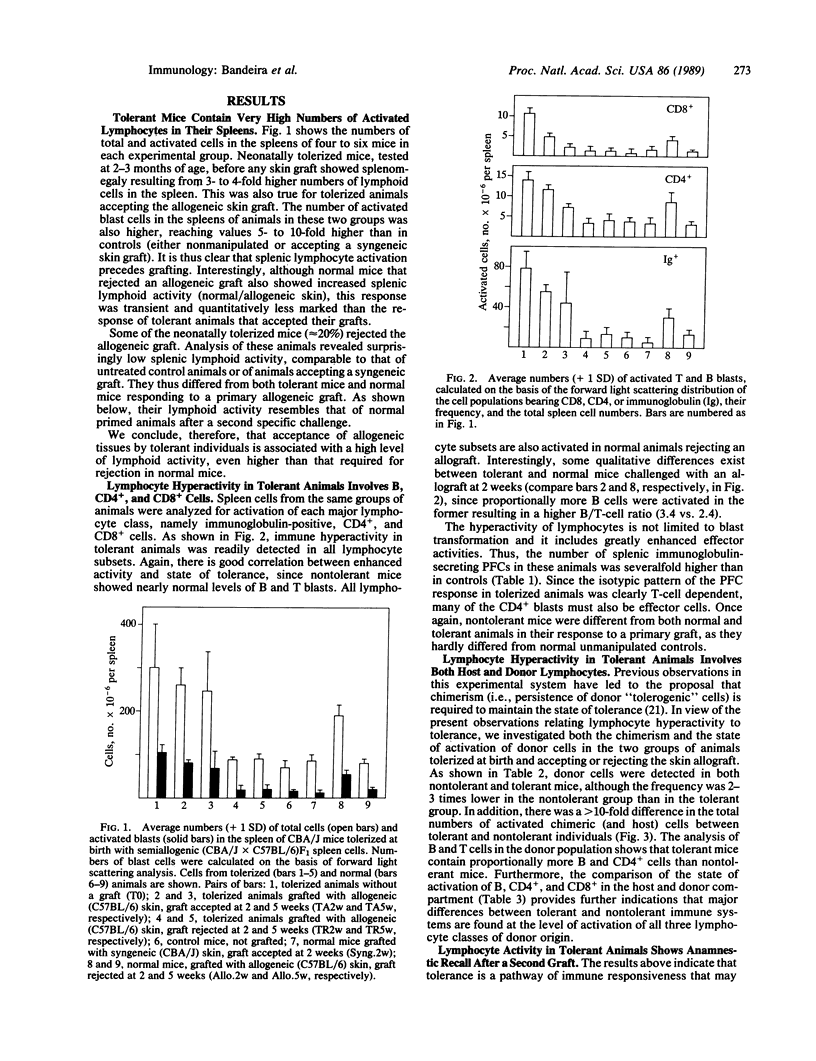
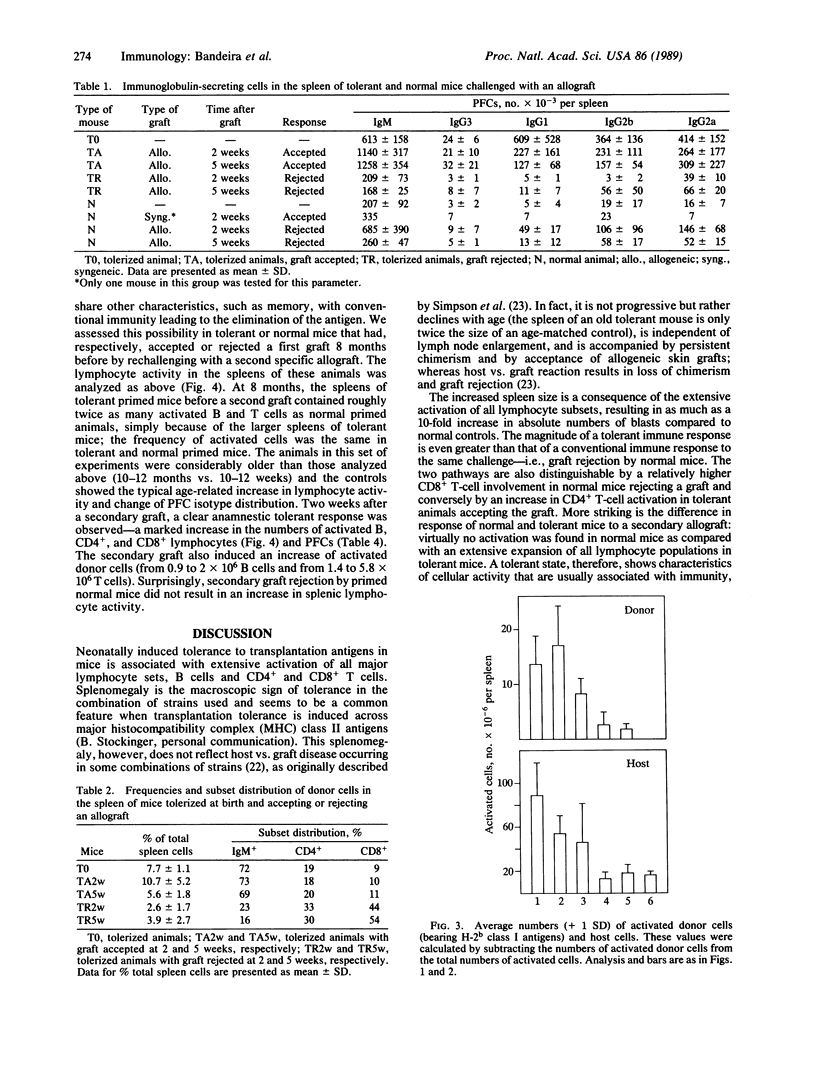
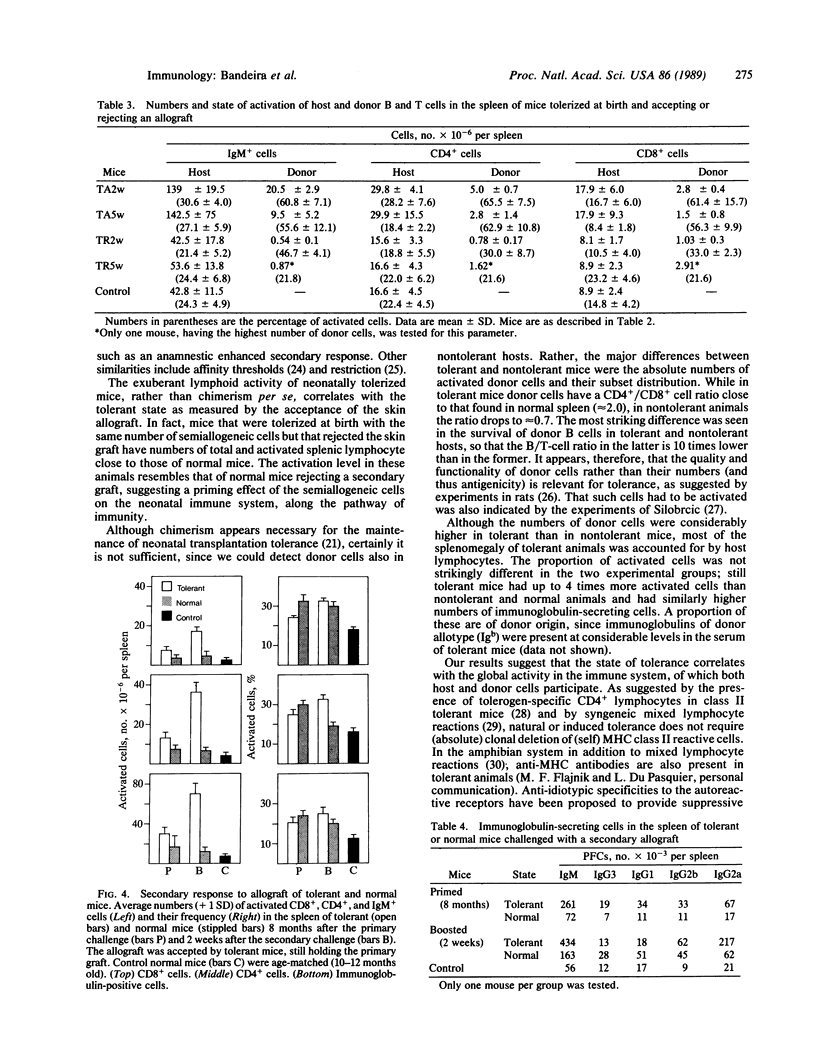
Mice tolerized (treated to make them tolerant) at birth to transplantation antigens by injection of semiallogeneic cells contain very high numbers of activated T and B lymphocytes in their spleen. Lymphoid hyperactivity correlates with the tolerant state: it is present only in animals accepting skin allografts. Tolerized mice that reject the allogeneic skin graft have approximately the same numbers of total and activated lymphocytes as normal mice. The high level of lymphocyte activation in tolerant mice persists for up to 1 year of age, although it declines with age, and is markedly increased by a secondary allograft. The magnitudes of both primary and secondary tolerant responses are significantly higher than the immunological response of a normal mouse rejecting the same type of allograft. These observations contradict concepts of clonal deletion or anergy as the basis of neonatally induced transplantation tolerance and may contribute additional approaches to experimentation and control of transplantation reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bazin H., Xhurdebise L. M., Burtonboy G., Lebacq A. M., De Clercq L., Cormont F. Rat monoclonal antibodies. I. Rapid purification from in vitro culture supernatants. J Immunol Methods. 1984 Feb 10;66(2):261–269. doi: 10.1016/0022-1759(84)90337-5. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Marquez C., Araujo P. M., Pereira P., Toribio M. L., Marcos M. A., Martinez C. A functional idiotypic network of T helper cells and antibodies, limited to the compartment of "naturally" activated lymphocytes in normal mice. Eur J Immunol. 1987 Jun;17(6):821–825. doi: 10.1002/eji.1830170614. [DOI] [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Dorsch S., Roser B. Suppressor cells in transplantation tolerance. I. Analysis of the suppressor status of neonatally and adoptively tolerized rats. Transplantation. 1982 May;33(5):518–524. [PubMed] [Google Scholar]
- Dorsch S., Roser B. Suppressor cells in transplantation tolerance. II. Identification and probable mode of action of chimeric suppressor T cells. Transplantation. 1982 May;33(5):525–529. [PubMed] [Google Scholar]
- Dorsch S., Roser B. T cells mediate transplantation tolerance. Nature. 1975 Nov 20;258(5532):233–235. doi: 10.1038/258233a0. [DOI] [PubMed] [Google Scholar]
- Flajnik M. F., Du Pasquier L., Cohen N. Immune responses of thymus/lymphocyte embryonic chimeras: studies on tolerance and major histocompatibility complex restriction in Xenopus. Eur J Immunol. 1985 Jun;15(6):540–547. doi: 10.1002/eji.1830150603. [DOI] [PubMed] [Google Scholar]
- Forni L., de Petris S. Use of fluorescent antibodies in the study of lymphoid cell membrane molecules. Methods Enzymol. 1984;108:413–425. doi: 10.1016/s0076-6879(84)08108-8. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., Longo D. L., Green I., Schwartz R. H. Murine syngeneic mixed lymphocyte response. I. Target antigens are self Ia molecules. J Exp Med. 1981 Nov 1;154(5):1652–1670. doi: 10.1084/jem.154.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Heitzmann H., Richards F. M. Use of the avidin-biotin complex for specific staining of biological membranes in electron microscopy. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3537–3541. doi: 10.1073/pnas.71.9.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg D., Forsgren S., Ivars F., Coutinho A. Reactions among IgM antibodies derived from normal, neonatal mice. Eur J Immunol. 1984 May;14(5):435–441. doi: 10.1002/eji.1830140510. [DOI] [PubMed] [Google Scholar]
- Holmberg D., Freitas A. A., Portnoï D., Jacquemart F., Avrameas S., Coutinho A. Antibody repertoires of normal BALB/c mice: B lymphocyte populations defined by state of activation. Immunol Rev. 1986 Oct;93:147–169. doi: 10.1111/j.1600-065x.1986.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Vakil M. Functional idiotype networks during B-cell ontogeny. Ann Inst Pasteur Immunol. 1986 Jan-Feb;137C(1):77–82. doi: 10.1016/s0771-050x(86)80008-0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lubaroff D. M., Silvers W. K. Importance of chimerism in maintaining tolerance of skin allografts in mice. J Immunol. 1973 Jul;111(1):65–71. [PubMed] [Google Scholar]
- Luzuy S., Merino J., Engers H., Izui S., Lambert P. H. Autoimmunity after induction of neonatal tolerance to alloantigens: role of B cell chimerism and F1 donor B cell activation. J Immunol. 1986 Jun 15;136(12):4420–4426. [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Martinez C., Bernabé R. R., de la Hera A., Pereira P., Cazenave P. A., Coutinho A. Establishment of idiotypic helper T-cell repertoires early in life. Nature. 1985 Oct 24;317(6039):721–723. doi: 10.1038/317721a0. [DOI] [PubMed] [Google Scholar]
- Martinez C., Pereira P., Bernabé R., Bandeira A., Larsson E. L., Cazenave P. A., Coutinho A. Internal complementarities in the immune system: regulation of the expression of helper T-cell idiotypes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4520–4523. doi: 10.1073/pnas.81.14.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P., Zamoyska R., Waldmann H. Self tolerance is H-2-restricted. Nature. 1984 Apr 19;308(5961):738–741. doi: 10.1038/308738a0. [DOI] [PubMed] [Google Scholar]
- Mohler K. M., Streilein J. W. Tolerance to class II major histocompatibility complex molecules is maintained in the presence of endogenous, interleukin-2-producing, tolerogen-specific T lymphocytes. J Immunol. 1987 Oct 1;139(7):2211–2219. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Owen R. D. IMMUNOGENETIC CONSEQUENCES OF VASCULAR ANASTOMOSES BETWEEN BOVINE TWINS. Science. 1945 Oct 19;102(2651):400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Pereira P., Forni L., Larsson E. L., Cooper M., Heusser C., Coutinho A. Autonomous activation of B and T cells in antigen-free mice. Eur J Immunol. 1986 Jun;16(6):685–688. doi: 10.1002/eji.1830160616. [DOI] [PubMed] [Google Scholar]
- Pereira P., Larsson E. L., Forni L., Bandeira A., Coutinho A. Natural effector T lymphocytes in normal mice. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7691–7695. doi: 10.1073/pnas.82.22.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Portnoï D., Freitas A., Holmberg D., Bandeira A., Coutinho A. Immunocompetent autoreactive B lymphocytes are activated cycling cells in normal mice. J Exp Med. 1986 Jul 1;164(1):25–35. doi: 10.1084/jem.164.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silobrcić V. Life-long tolerance and chimerism in parental mice induced with F 1 hybrid cells. Eur J Immunol. 1971 Nov;1(5):313–315. doi: 10.1002/eji.1830010502. [DOI] [PubMed] [Google Scholar]
- Simpson E., O'Hopp S., Harrison M., Mosier D., Melief K., Cantor H. Immunological disease induced by injecting F1 lymphoid cells into certain parental strains. Immunology. 1974 Dec;27(6):989–1007. [PMC free article] [PubMed] [Google Scholar]
- Stockinger B. Cytotoxic T-cell precursors revealed in neonatally tolerant mice. Proc Natl Acad Sci U S A. 1984 Jan;81(1):220–223. doi: 10.1073/pnas.81.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J. W., Gruchalla R. S. Analysis of neonatally induced tolerance of H-2 alloantigens. I. Adoptive transfer indicates that tolerance of class I and class II antigens is maintained by distinct mechanisms. Immunogenetics. 1981;12(1-2):161–173. doi: 10.1007/BF01561659. [DOI] [PubMed] [Google Scholar]
- Tateno M., Kondo N., Itoh T., Yoshiki T. Autoimmune disease and malignant lymphoma associated with host-versus-graft disease in mice. Clin Exp Immunol. 1985 Dec;62(3):535–544. [PMC free article] [PubMed] [Google Scholar]



