Abstract
Outcome after traumatic brain injury (TBI) is worsened by hemorrhagic shock (HS), but the optimal resuscitation approach is unclear. In particular, treatment of TBI patients with colloids remains controversial. We hypothesized that resuscitation with the colloids polynitroxylated albumin (PNA) or Hextend (HEX) is equal or superior to resuscitation with the crystalloids hypertonic (3%) saline (HTS) or lactated Ringer's solution (LR) after TBI plus HS in mice. C57/BL6 mice (n = 30) underwent controlled cortical impact (CCI) and 90 min of volume-controlled HS (2 mL/100 g). The mice were randomized to resuscitation with LR, HEX, HTS, or PNA, followed by 30 min of test fluid administration targeting a mean arterial pressure (MAP) of >50 mm Hg. Shed blood was re-infused to target a MAP >70 mm Hg. At 7 days post-insult, hippocampal neuron counts were assessed in hematoxylin and eosin–stained sections to quantify neuronal damage. Prehospital MAP was higher, and prehospital and total fluid requirements were lower in the PNA and HEX groups (p < 0.05 versus HTS or LR). Also, 7-day survival was highest in the PNA group, but was not significantly different than the other groups. Ipsilateral hippocampal CA1 and CA3 neuron loss did not differ between groups. We conclude that the colloids PNA and HEX exhibited more favorable effects on acute resuscitation parameters than HTS or LR, and did not increase hippocampal neuronal death in this model.
Key words: colloid, head injury, nitroxide, oxidative stress, secondary insult
Introduction
Secondary insults after traumatic brain injury (TBI) increase morbidity and mortality, and the combination of TBI plus hemorrhagic shock (HS) is particularly deleterious. Miller and Becker (1982) first reported that hypotension (systolic blood pressure <90 mm Hg) worsened outcome after TBI. Chesnut and associates (1993) reported a correlation between hypotension and hypoxemia and increased morbidity/mortality after TBI in humans, with hypotension being the most critical parameter. These observations have been confirmed in experimental studies, in which secondary insults also worsened brain injury. Controlled cortical impact (CCI) with superimposed ischemia reduced cerebral blood flow (CBF) (Giri et al., 2000) and increased hippocampal neuronal loss (Cherian et al., 1996) versus ischemia alone. Jenkins and colleagues (1989) noted increased CA1 neuronal death by combining hemorrhagic hypotension with TBI in rats, and Matsushita and co-workers (2001) reported an increase in contusion area by hemorrhagic shock after fluid percussion injury in rats. Thus clinical and experimental evidence supports an association between secondary insults and increased morbidity and mortality after TBI.
The optimal fluid for resuscitation of TBI plus HS remains unclear. Characteristics of the ideal resuscitation fluid include ease of transport and administration in the pre-hospital setting, small infusion volumes to minimize cerebral edema, prevention of acute causes of mortality, and attenuation of secondary injury. Traditional acute resuscitation solutions for TBI plus HS include lactated Ringer's solution (LR) or Hextend® (HEX; Hospira, Lake Forest, IL). More novel resuscitation solutions under evaluation include hypertonic (3%) saline (HTS) and polynitroxylated albumin (PNA; SynZyme Technologies, Irvine, CA), among others. Isotonic crystalloids, particularly LR, are used for resuscitation in civilian trauma, but often require large volumes to maintain the desired blood pressure. HEX is the preferred fluid for resuscitation in combat casualty care. In several animal models, resuscitation with HEX required less volume and improved cerebrovascular function versus resuscitation with crystalloid (Crookes et al., 2004; Kelly et al., 2003; King et al., 2004). With regard to more novel solutions, there is extensive pre-clinical and clinical experience evaluating the use of HTS for resuscitation. Prough and associates (1991) and others have shown that use of HTS in experimental hemorrhagic hypotension restores hemodynamics and improves microcirculation. In animal models of TBI, HTS also improved CBF and lowered intracranial pressure (ICP) versus LR (Walsh et al., 1991; Shackford et al., 1992). Finally, PNA is a novel compound composed of 55 nitroxide moieties covalently linked to albumin that is administered as a 10% solution. Its stable nitroxyl radicals mimic superoxide dismutase (SOD) and catalase, and detoxify reactive oxygen species (Li et al,. 2002). PNA improved survival in a rat HS model and reduced lesion size in experimental stroke (Kentner et al., 2002; Beaulieu et al., 1998). It has not, however, been evaluated in combined TBI plus HS.
Recently, controversy about the optimal resuscitation fluid in TBI was raised by the SAFE study (Myburgh et al., 2007), which included a post-hoc analysis of the use of albumin versus saline in TBI victims. It suggested that the use of albumin in patients with TBI increased mortality versus saline, but no mechanism for the increased mortality seen with colloid use was presented. A recent report by Baker and colleagues (2008) in experimental TBI in rats challenged this finding, and showed enhanced electrophysiological recovery with albumin versus saline resuscitation, although the neuropathology was not assessed.
We recently developed a clinically relevant mouse model of TBI plus HS that allows us to evaluate acute hemodynamics, 7-day survival, and long-term neuropathology (Dennis et al., 2009). At the injury level used, CA1 neuronal death was seen only in combined CCI plus HS, but not in CCI or HS alone. We now use this model to evaluate the resuscitation of TBI plus HS using several traditional and novel fluids. We hypothesized that resuscitation with the colloids PNA or HEX would require smaller volumes than the crystalloids HTS or LR to reach resuscitation goals and produce higher mean arterial pressures (MAPs) in the resuscitation phase. We also hypothesized that the colloids PNA or HEX versus the crystalloids HTS or LR would not worsen 7-day survival or hippocampal neuronal death.
Methods
The Institutional Animal Care and Use Committee of the University of Pittsburgh School of Medicine approved this study. Male C57/BL6 mice (Jackson Laboratories, Bar Harbor, ME), 12–15 weeks of age and weighing 22–29 grams, were housed under controlled environmental conditions and allowed ad libitum food and water until the study began.
Anesthesia was induced via nose cone with 4% isoflurane in oxygen, and maintained with 1% isoflurane in a 2:1 N2O/oxygen mixture. Under sterile conditions, central femoral venous and arterial catheters were placed using modified PE-50 tubing. The mouse was placed in a stereotaxic frame, a 5-mm craniotomy was performed over the left parietal cortex using a dental drill, and the bone flap was removed. A brain temperature micro-probe (Physitemp, Clifton, NJ) was then inserted through the burr hole. Body temperature was also monitored by rectal probe. Immediately after craniotomy, the inhalational anesthesia was changed to 1% isoflurane and room air for 10 min before CCI and onset of HS. A mild to moderate CCI was performed with a pneumatic impactor (Bimba, Monee, IL) as previously reported with modifications. A 3-mm flat-tip impounder was deployed at a velocity of 5 m/sec and a depth of 1 mm. Brain temperature was maintained at 37° ± 0.5°C throughout the experiment. To achieve a clinically relevant level of HS, 2 mL of blood/100 g of body weight was removed via the venous catheter. This hemorrhage volume resulted in a decrease in MAP to 35–40 mm Hg. The mice remained in the HS phase for 90 min, mimicking the time between injury and the first field provision of medical attention. After the HS phase, the mice were randomized to one of four treatment groups (n = 8 for each group), including resuscitation with (1) LR, (2) HEX, (3) HTS, or (4) PNA.
After completing the HS phase, the mice entered the pre-hospital phase, corresponding to arrival of medical personnel and initiation of fluid resuscitation. This phase lasted 30 min. The mice were given boluses of test fluid to achieve a MAP ≥50 mm Hg (totaling between 1.0 and 1.5 mL). Subsequently, 0.1-mL aliquots of test fluid were administered for every minute the MAP remained less than the pre-hospital MAP target of 50 mm Hg. To simulate arrival at a definitive care setting, the mice entered the in-hospital phase. During this 30-min period, shed blood was rapidly re-infused, and a goal MAP of ≥70 mm Hg was maintained by the administration of additional 0.1-mL aliquots of test fluid for every minute that the MAP remained less than the in-hospital target of 70 mm Hg. During this phase inhalational anesthesia was also changed from 1% isoflurane in room air to 1% isoflurane in oxygen, which was maintained for the duration of the study. At completion of the in-hospital phase, the catheters were removed, anesthesia was discontinued, and the mice were returned to their cages. They were allowed free access to food and water, and observed for up to 7 days.
Mice were excluded from analysis by criteria defined before breaking randomization if they died during the HS phase, or if they did not reach the pre-hospital phase target MAP of ≥50 mm Hg with the initial boluses.
Brain temperature was monitored with a temperature probe placed in the right parietal cortex, and was maintained at 37° ± 0.5°C throughout the experiment. MAP was continuously monitored via a catheter placed in the femoral artery, and was recorded at baseline, after CCI, and every 5 min during all three phases of the study. Baseline heart rate was continuously monitored and recorded at baseline and once during each phase. Arterial blood gas, blood lactate, and glucose levels were obtained at baseline, after 30 min of shock, and at the end of the in-hospital phase.
At 7 days after the experiment, surviving mice were re-anesthetized with 4% isoflurane and killed by ice-cold saline transcardial perfusion, followed by 10% buffered formalin phosphate perfusion and fixation of brains with subsequent embedding in paraffin at 2 weeks. Multiple 5-μm sections, 125 μm apart, from the bregma −1.82 to −2.06 were prepared from each brain, and stained with hematoxylin and eosin (H&E; Thermo Scientific, Pittsburgh, PA). Hippocampal neuronal damage was quantified with 7-day cell counts in the H&E-stained sections by a blinded evaluator (J.E.) using a Nikon Eclipse E600 microscope (Melville, NY), and Image J software (http://rsb.info.nih.gov/ij/).
Statistical analysis
Physiologic measurements and neuron counts were compared between treatment groups using one-way analysis of variance (ANOVA), and post-hoc tests with appropriate correction for multiple comparisons. All data are provided as mean ± SEM. Seven-day survival was compared between treatment groups using Fisher's exact test. Significance was determined by a p value ≤ 0.05.
Results
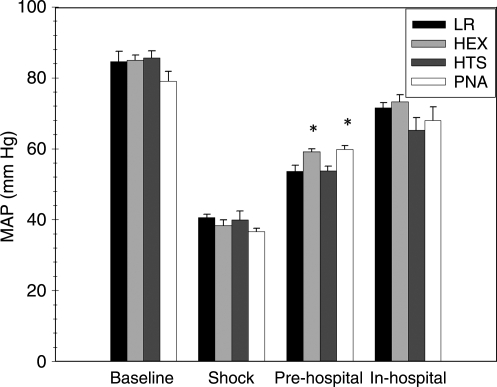
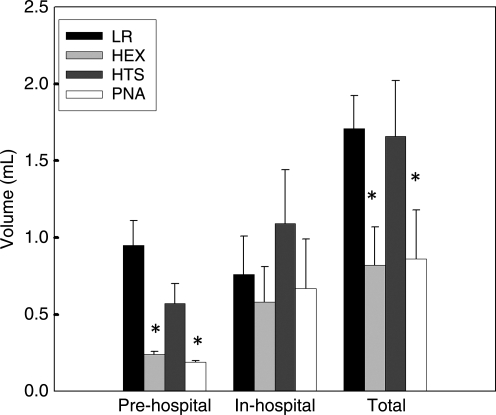
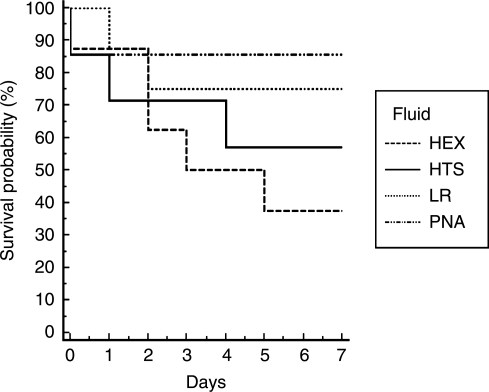
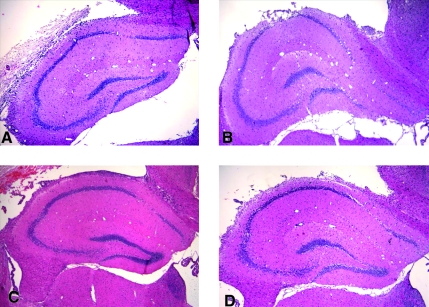
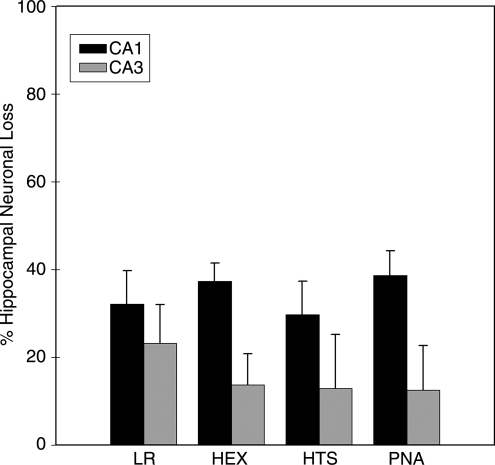
MAP did not differ significantly between groups at the end of the shock phase (p = 0.31) (Fig. 1). In contrast, the PNA and HEX groups achieved higher MAP in the pre-hospital phase than the LR or HTS groups (p < 0.05). MAP did not differ significantly between groups during the in-hospital phase (p = 0.20). The PNA and HEX groups required substantially less fluid to achieve resuscitation goals in the pre-hospital phase (p < 0.05) (Fig. 2). There was no difference between groups in volume required to achieve resuscitation goals in the in-hospital phase (p = 0.63). The PNA and HEX groups also required significantly smaller total fluid volumes to achieve resuscitation goals than the LR or HTS group (p < 0.05). At the end of the shock phase, arterial lactate levels in the LR, HEX, HTS, and PNA groups were 3.48 ± 1.29, 2.78 ± 0.47, 2.89 ± 0.84, and 3.40 ± 0.80 mmol/L, respectively, and did not differ significantly (p = 0.35). Arterial lactate levels at the end of the in-hospital phase also did not differ between groups (LR 2.00 ± 0.93 mmol/L, HEX 1.96 ± 0.78 mmol/L, HTS 2.99 ± 2.04 mmol/L, and PNA 1.50 ± 0.24 mmol/L; p = 0.15). Seven-day survival did not differ between groups (p = 0.33) (Fig. 3). H&E-stained sections of the hippocampus were also evaluated (Fig. 4). A pattern of neuronal death in the hippocampus was evident, predominantly in the CA1 subfield, mirroring the work of Dennis and associates (2009). Evaluation of ipsilateral CA1 neuron loss (as a percentage of the contralateral hemisphere) revealed that a ∼35% loss of CA1 neurons was seen at the injury level selected, which did not differ significantly between groups (p = 0.81) (Fig. 5). Similarly, ipsilateral CA3 neuron loss (as a percentage of the contralateral hemisphere), although much more modest than that seen in CA1, did not differ between groups (p = 0.86).
FIG. 1.
Mean arterial pressure (MAP) for the experimental groups during each phase of the model (*p < 0.05 for the PNA and HEX groups versus the LR and HTS groups). Data are mean and SEM (n = 8 for the LR and HEX groups; n = 7 for the HTS and PNA groups; LR, lactated Ringer's solution; HEX, Hextend; HTS, hypertonic saline; PNA, polynitroxylated albumin).
FIG. 2.
Fluid requirements for the experimental groups during each phase of the model (*p < 0.05 for the PNA and HEX groups versus the LR and HTS groups). Data are mean and SEM (n = 8 for the LR and HEX groups; n = 7 for the HTS and PNA groups; LR, lactated Ringer's solution; HEX, Hextend; HTS, hypertonic saline; PNA, polynitroxylated albumin).
FIG. 3.
Kaplan-Meier survival curve for 7-day survival probability for the four study groups.
FIG. 4.
Representative 40 × H&E microphotographs depicting the ipsilateral hippocampus in the four study groups. (A) LR. (B) HEX. (C) HTS. (D) PNA. Pyramidal neuron loss is evident within the medial region of CA1 in all groups (LR, lactated Ringer's solution; HEX, Hextend; HTS, hypertonic saline; PNA, polynitroxylated albumin).
FIG. 5.
Average amount of ipsilateral hippocampal neuron loss (as percentage of the contralateral hippocampal neuron count) in four study groups. Data are mean and SEM of all mice surviving to day 7 (n = 6 for the LR and PNA groups, n = 3 for the HEX group, and n = 4 for the HTS group). There was no significant difference between the four groups (LR, lactated Ringer's solution; HEX, Hextend; HTS, hypertonic saline; PNA, polynitroxylated albumin).
Discussion
Our findings show that resuscitation with the colloids PNA or HEX, in our mouse model of CCI plus HS, required less fluid volume to reach the target MAPs, and achieved and maintained higher MAPs in the pre-hospital phase, caused no adverse effects on recovery of lactate levels, and had comparable 7-day survival rates. Resuscitation with PNA or HEX also did not worsen hippocampal neuron survival versus HTS or LR. These findings are noteworthy, as the merits of resuscitation with colloids are the subject of intense debate.
Current fluid resuscitation strategies for TBI patients advise infusion of isotonic crystalloid solutions to normalize blood pressure (to maintain systolic BP > 90 mm Hg) (Badjatia et al., 2008). This recommendation has been challenged by numerous researchers who investigated the use of colloids, hypertonic fluids, vasopressors, and blood substitutes for post-TBI resuscitation. Recently, the authors of the SAFE study reported increased mortality of TBI patients treated with albumin (Finfer et al., 2004; Myburgh et al., 2007). Although no mechanism was offered to account for these findings, others have proposed the development of a dilution coagulopathy, which in the context of severe TBI worsens outcome (Billota and Rosa, 2007; Schirmer-Mikalsen et al., 2007). This subset of patients received more frequent blood transfusions early in their ICU course, but no information on the incidence of bleeding complications after enrollment in the SAFE study was provided. We did not observe excessive bleeding in our study animals. Alternatively, it is possible colloids move across the damaged blood–brain barrier, and remain trapped in brain tissue once the barrier is repaired. After degradation to protein components, an osmotic gradient could be created, promoting edema formation and worsening brain injury (Kawamata et al., 2007). We did not evaluate brain edema in our study, but we found that resuscitation with PNA or HEX offered considerable benefit in terms of volume requirement and hemodynamic status, and did not worsen hippocampal neuronal death versus LR. The SAFE study also did not evaluate the performance of albumin in the early, acute resuscitation of TBI plus HS, making it difficult to draw comparisons with our findings.
PNA is a colloid with beneficial effects across numerous experimental paradigms. It has been shown to reduce infarct size in experimental stroke in rats (Beaulieu et al., 1998; Sugawara et al., 2001), attenuate damage in experimental myocardial ischemia in rats (Li et al., 2002) and in murine models of sickle cell crisis (Kaul et al., 2006), and highly germane to our work, it reduces mortality in experimental HS in rats (Kentner et al., 2002). These beneficial effects may result from the potent intravascular antioxidant and nitric oxide–sparing effects conferred by the covalently linked nitroxide moieties in PNA. Nitroxides have potent SOD and catalase mimetic effects, and the free nitroxide tempol is neuroprotective in experimental TBI in rats (Trembovler et al., 1999; Leker et al., 2002; Deng-Bryant et al., 2008). Beneficial effects of PNA could also be mediated by its rheologic properties (Russell et al., 1998). Any or all of these effects could contribute to the positive hemodynamic effects we observed in the PNA group. PNA and HEX could also share beneficial oncotic effects with albumin that could enhance CBF, or as a relatively small volume of the resuscitation fluid, limit edema (Tu et al., 1988a,b; Ohtaki et al., 1993). Also, the fact that mice resuscitated with PNA or HEX showed more normalized MAP levels despite significantly lower resuscitation volumes, strongly supports their putative oncotic effects.
The target MAP for resuscitation of TBI plus HS remains unclear. We chose a pre-hospital MAP target of ≥50 mm Hg and in-hospital MAP target of ≥70 mm Hg as a compromise between optimized cerebral perfusion pressure, exacerbation of bleeding that can occur in the setting of uncontrolled HS, and volume overload with pulmonary edema. Dennis and colleagues (2009) reported mortality associated with pulmonary edema in mice rapidly fluid-resuscitated to normotension after CCI plus HS. Despite modest MAP targets, the mice in the LR and HTS groups still received ≥60 mL/kg versus the 31–34 mL/kg given to mice in the HEX and PNA groups, which is a clinically meaningful difference. The potential contribution of aggressive fluid resuscitation to the degree of cerebral edema cannot be overlooked (Earle et al., 2007).
We analyzed neuronal survival in the hippocampus, anticipating its enhanced vulnerability to TBI plus HS. We did not find a significant difference in ipsilateral hippocampal CA1 and CA3 neuron counts between the groups. However, the colloids (PNA or HEX) did not worsen neuronal survival versus the crystalloids (HTS or LR). One might have expected deleterious effects based on the results of the SAFE study. The decision to perform neuron counts at 7 days post-insult may bias our results against any possible protective effect of PNA, as mice that died before 7 days may have had more extensive hippocampal neuron loss, and survival was numerically greatest in the PNA group.
Our study has several limitations. It would be useful to compare the effects of albumin versus PNA in our model, and we are currently examining albumin in a new protocol. However, PNA may represent a colloid quite different from albumin—with a different molecular weight, charge, and other properties. Albumin may thus not represent the perfect control. Second, our study was not powered to detect differences between treatment groups in 7-day survival. The PNA group had the highest numeric 7-day survival rate. Comparison of survival between groups yielded a p = 0.33 with a power of 0.5; increasing the number in each group to 15 would be needed to address this hypothesis with a power of 0.8. Third, lactate levels may not represent an optimal marker of tissue perfusion and HS in our model. Despite 90 min at a MAP ∼35 mm Hg (<50% of the baseline MAP), lactate levels did not increase significantly from baseline to the end of HS. Investigation of a more severe level of HS is needed. Fourth, we did not measure intracranial pressure in our mouse model. This is technically difficult in mice, and could worsen brain injury. However, we recognize that intracranial pressure is important in our understanding of changes in MAP and cerebral perfusion pressure, and it is part of our ongoing work. Fifth, we did not regulate fluid balance in the mice beyond the initial monitoring period. Free access to water could limit the duration of effects of colloids or HTS. Long-term intensive care would be required to address this issue. Finally, we did not study resuscitation in the setting of uncontrolled bleeding.
The combination of TBI plus HS is deleterious, and the ideal fluid for resuscitation of this insult has yet to be identified. We have reported that resuscitation with PNA and HEX can be accomplished with smaller volumes than with either LR or HTS, and that despite smaller volumes, higher MAPs are achieved. In addition, in contrast to the SAFE study, we did not observe adverse effects of colloids on mortality, nor did we find that colloids worsened neuronal death. Further study of resuscitation with colloids, including the antioxidant colloid PNA, with assessment of effects on both acute cerebral hemodynamics and functional outcome, is warranted in TBI plus HS.
Acknowledgments and Author Disclosure Statement
We would like to thank the U.S. Army for their support through grant PR054755 81-06-10247. We also thank the NIH for support, NS 38087 (P.M.K.) and NS 30318 (C.E.D.). Dr. Exo is supported by a T32 grant (HD 040686) from the National Institute of Child Health and Human Development. Drs. Ma and Hsia are officers at SynZyme Technologies, LLC. No other conflicting financial interests exist.
References
- Badjatia N. Carney N. Crocco T.J. Fallat M.E. Hennes H.M. Jagoda A.S. Jernigan S. Letarte P.B. Lerner E.B. Moriarty T.M. Pons P.T. Sasser S. Scalea T. Schleien C.L. Wright D.W. the Brain Trauma Foundation. Guidelines for prehospital management of traumatic brain injury, 2nd ed. Prehosp. Emerg. Care. 2008;12(Suppl. 1):S1–S52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- Baker A.J. Park E. Hare G.M.T. Liu E. Sikich N. Mazer D.C. Effects of resuscitation on neurologic physiology after cerebral trauma and hemorrhage. J. Trauma. 2008;64:348–357. doi: 10.1097/01.ta.0000245973.71929.db. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. Busch E. Röther J. de Crispegny A. Hsia C.J.C. Moseley M.E. Polynitroxyl albumin reduces infarct size in transient focal cerebral ischemia in the rat: Potential mechanism studied by magnetic resonance imaging. J. Cereb. Blood Flow Metab. 1998;18:1022–1031. doi: 10.1097/00004647-199809000-00012. [DOI] [PubMed] [Google Scholar]
- Billota F.Rosa G.2007Correspondence to journal N. Engl. J. Med. 357263518095408 [Google Scholar]
- Cherian L. Robertson C.S. Goodman J.C. Secondary insults increase injury after controlled cortical impact in rats. J. Neurotrauma. 1996;13:371–383. doi: 10.1089/neu.1996.13.371. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Gautille T. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Crookes B.A. Cohn S.M. Bonet H. Burton E.A. Nelson J. Majetschak M. Varon A.J. Linden J.M. Proctor K.G. Building a better fluid for emergency resuscitation of traumatic brain injury. J. Trauma. 2004;57:547–554. doi: 10.1097/01.ta.0000135162.85859.4c. [DOI] [PubMed] [Google Scholar]
- Deng-Bryant Y. Singh I.N. Carrico K.M. Hall E.D. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 2008;28:114–126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Dennis A.M. Haselkorn M.L. Vagni V.A. Garman R.H. Feldman K. Bayır H. Clark R.S.B. Jenkins L.W. Dixon C.E. Kochanek P.M. Hemorrhagic shock after experimental brain injury in mice: Effect on neuronal death. J. Neurotrauma. 2009;26:889–899. doi: 10.1089/neu.2008.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle S.A. de Moya M.A. Zuccarelli J.E. Norenberg M.D. Proctor K.G. Cerebrovascular resuscitation after polytrauma and fluid restriction. J. Am. Coll. Surg. 2007;204:261–275. doi: 10.1016/j.jamcollsurg.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Finfer S. Bellomo R. Boyce N. French J. Myburgh J. Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- Giri B.K. Krishnappa I.K. Bryan R.M., Jr. Robertson C. Regional cerebral blood flow after cortical impact injury complicated by a secondary insult in rats. Stroke. 2000;31:961–967. doi: 10.1161/01.str.31.4.961. [DOI] [PubMed] [Google Scholar]
- Jenkins L.W. Moszynski K. Lyeth B.G. Lewelt W. DeWitt D.S. Allen A. Dixon C.E. Povlishock J.T. Majewski T.J. Clifton G.L. Young H.F. Becker D.P. Hayes R.L. Increased vulnerability of mildly traumatized rat brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Res. 1989;477:211–224. doi: 10.1016/0006-8993(89)91409-1. [DOI] [PubMed] [Google Scholar]
- Kaul D.K. Liu X.D. Zhang X. Ma L. Hsia C.J. Nagel R. Inhibition of sickle red cell adhesion and vasoocclusion in transgenic sickle mice. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H167–H175. doi: 10.1152/ajpheart.01096.2005. [DOI] [PubMed] [Google Scholar]
- Kawamata T. Mori T. Sato S. Katayama Y. Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg. Focus. 2007;22:E5. doi: 10.3171/foc.2007.22.5.6. [DOI] [PubMed] [Google Scholar]
- Kelly M.E. Miller P.R. Greenhaw J.J. Fabian T.C. Proctor K.G. Novel resuscitation strategy for pulmonary contusion after severe chest trauma. J. Trauma. 2003;55:94–105. doi: 10.1097/01.TA.0000029042.37577.A6. [DOI] [PubMed] [Google Scholar]
- Kentner R. Safar P. Behringer W. Wu X. Kagan V.E. Tyurina Y.Y. Henchir J. Ma L. Hsia C.J. Tisherman S.A. Early antioxidant therapy with Tempol during hemorrhagic shock increases survival in rats. J. Trauma. 2002;53:968–977. doi: 10.1097/00005373-200211000-00025. [DOI] [PubMed] [Google Scholar]
- King D.R. Cohn S.M. Proctor K.G. Changes in intracranial pressure, coagulation, and neurologic outcome after resuscitation from experimental traumatic brain injury with hetastarch. Surgery. 2004;136:355–363. doi: 10.1016/j.surg.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Leker R.R. Teichner A. Lavie G. Shohami E. Lamensdorf I. Ovadia H. The nitroxide antioxidant tempol is cerebroprotective against focal cerebral ischemia in spontaneously hypertensive rats. Exp. Neurol. 2002;176:355–363. doi: 10.1006/exnr.2002.7910. [DOI] [PubMed] [Google Scholar]
- Li H. Ma L. Hsia C.J.C. Zweier J.L. Kuppusamy P. Polynitroxyl-albumin (PNA) enhances myocardial infarction therapeutic effect of tempol in rat hearts subjected to regional ischemia-reperfusion. Free Rad. Biol. Med. 2002;32:712–719. doi: 10.1016/s0891-5849(02)00762-1. [DOI] [PubMed] [Google Scholar]
- Matsushita Y. Bramlett H.M. Kuluz J.W. Alonso O. Dietrich W.D. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathologic consequences of traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2001;21:847–856. doi: 10.1097/00004647-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Miller J.D. Becker D.P. Secondary insults to the injured brain. J. R. Coll. Surg. Edinb. 1982;27:292–298. [PubMed] [Google Scholar]
- Myburgh J. Cooper D.J. Finfer S. Bellomo R. Norton R. Bishop N. Lo S.K. Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N. Engl. J. Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- Ohtaki M. Tranmer B.I. Role of hypervolemic hemodilution in focal cerebral ischemia of rats. Surg. Neurol. 1993;40:196–206. doi: 10.1016/0090-3019(93)90068-c. [DOI] [PubMed] [Google Scholar]
- Prough D.S. Whitley J.M. Taylor C.E. Deal D.D. DeWitt D.S. Small-volume resuscitation from hemorrhagic shock in dogs: Effects on systemic hemodynamics and systemic blood flow. Crit. Care Med. 1991;19:364–372. doi: 10.1097/00003246-199103000-00015. [DOI] [PubMed] [Google Scholar]
- Russell J. Okayama N. Alexander J.S. Granger D.N. Hsia C.J. Pretreatment with polynitroxyl albumin (PNA) inhibits ischemia-reperfusion induced leucocyte-endothelial cell adhesion. Free Radic. Biol. Med. 1998;25:153–159. doi: 10.1016/s0891-5849(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Schirmer-Mikalsen K. Vik A. Gisvold S.E. Skandsen T. Hynne H. Klepstad P. Severe head injury: control of physiologic variables, organ failure, and complications in the intensive care unit. Acta Anaesthesiol. Scand. 2007;51:1194–1201. doi: 10.1111/j.1399-6576.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- Shackford S.R. Zhuang J. Schmoker J. Intravenous fluid tonicity: effect on intracranial pressure, cerebral blood flow, and cerebral oxygen delivery in focal brain injury. J. Neurosurg. 1992;76:91–98. doi: 10.3171/jns.1992.76.1.0091. [DOI] [PubMed] [Google Scholar]
- Sugawara T. Yu F. Ma L. Hsia C.J. Chan P.H. Delayed treatment with polynitroxyl albumin reduces infarct size after stroke in rats. Neuroreport. 2001;12:3609–3612. doi: 10.1097/00001756-200111160-00047. [DOI] [PubMed] [Google Scholar]
- Trembovler V. Gallily R. Horowitz M. Shohami E. Antioxidants attenuate acute toxicity of tumor necrosis factor-alpha induced by brain injury in rat. J. Interferon Cytokine Res. 1999;19:791–795. doi: 10.1089/107999099313640. [DOI] [PubMed] [Google Scholar]
- Tu Y.K. Heros R.C. Candia G. Hyodo A. Lagree K. Callahan R. Zervas N.T. Karacostas D. Isovolemic hemodilution in experimental focal cerebral ischemia. Part 1: Effects on hemodynamics, hemorheology, and intracranial pressure. J. Neurosurg. 1988;69:72–81. doi: 10.3171/jns.1988.69.1.0072. [DOI] [PubMed] [Google Scholar]
- Tu Y.K. Heros R.C. Karacostas D. Liszczak T. Hyodo A. Candia G. Zervas N.T. Lagree K. Isovolemic hemodilution in experimental focal cerebral ischemia. Part 2: Effects on regional cerebral blood flow and size of infarction. J. Neurosurg. 1988;69:82–91. doi: 10.3171/jns.1988.69.1.0082. [DOI] [PubMed] [Google Scholar]
- Walsh J.C. Zhuang J. Shackford S.R. A comparison of hypertonic to isotonic fluid in the resuscitation of brain injury and hemorrhagic shock. 1991;50:284–292. doi: 10.1016/0022-4804(91)90192-o. [DOI] [PubMed] [Google Scholar]