Abstract
The objective of this study was to examine the mortality rates in individuals with traumatic brain injury (TBI) who were classified as having experienced late post-traumatic seizures (LPTS) in the first 2 years post-TBI compared to those who were seizure-free (non-LPTS). Participants were a pooled sample (n = 508) from two studies which enrolled individuals with TBI who were injured between March 31, 1992 and December 20, 1999. The first sample was made up of individuals enrolled in a study of risk factors for LPTS development; the second sample was composed of individuals enrolled in the TBI National Database from a single rehabilitation center. Seventy-one (14%) participants had LPTS, of which 27% had died at 8–15 years post-injury, as compared to 10% of non-LPTS participants. Individuals with LPTS died at a younger age (54.1 versus 67.7 years; p = 0.01), but there were no statistically significant differences in either time from date of injury to death or highest GCS score in the first 24 h. Causes of death were variable and not specifically related to epilepsy. Of those with LPTS, risk factors for death include advanced age at time of injury and presence of subdural hematoma. The higher mortality rate and death at younger age with variable causes in TBI individuals with LPTS warrant close medical evaluation and monitoring of these individuals, particularly accessibility and compliance with ongoing general medical care, and education of primary care colleagues of the unique needs of this at-risk population.
Key Words: epilepsy, rehabilitation, traumatic brain injury
Introduction
Traumatic brain injuries (TBI) account for 20% of symptomatic epilepsy in the general population and 5% of all epilepsy (Hauser et al., 1991). Post-traumatic seizures (PTS) are classified thusly: immediate, within the first 24 hours post-injury; early, during the first week post-injury; and late, more than 7 days post-injury (Brain Injury Special Interest Group of the American Academy of Physical Medicine and Rehabilitation, 1998). The incidence of late PTS (LPTS) ranges from 5–19% in civilian populations, and is much higher, 32–50%, in the military (Aiskainen et al., 1999; Englander et al., 2003; Jennett, 1975; Salazar et al., 1985). Approximately one-half to two-thirds of individuals with LPTS experience the initial seizure within the first 12 months post-injury, and more than 75% by the end of the second year (Caveness et al., 1979; DaSilva et al., 1990; Englander et al., 2003; Salazar et al., 1985). The risk factors associated with the development of LPTS are age, penetrating injuries, multiple neurosurgical procedures, injury severity, lesion location, intracranial hemorrhage, and occurrence of early PTS (Annegers et al., 1980; Annegers et al., 1998; Brandvold et al., 1990; Caveness et al., 1979; Desantis et al., 1979; Englander et al., 2003; Glotzner et al., 1983; Jennett, 1975; Kollevold, 1979; Salazar et al., 1987).
Once the first LPTS has occurred, the risk of experiencing others increases dramatically (Annegers et al., 1998; Courjon, 1970; Haltiner et al., 1997; Jennett, 1973). Morbidity for those who experience seizures includes loss of driving privileges, decreased employability, and increased emergency department visits, physician visits, and pharmacy and laboratory costs (Annegers et al., 1998; Courjon, 1970; Haltiner et al., 1997; Jennett, 1973). There is also the added burden to the person with TBI and caregivers in living with epilepsy (Kobau et al., 2007; Wiebe et al., 1999). Mortality rates in individuals with epilepsy of any cause are estimated to be two to five times higher than in the normal population (Lhatoo and Sander, 2005; Nilsson et al., 1997), and it is believed that one of the major causes for the increased mortality rates is the concomitant TBI that caused the post-traumatic epilepsy (Annegers and Coan, 1999). Standardized mortality ratios for symptomatic epilepsy, regardless of cause of death, range from 2.2–4.9 (Cockerell et al., 1994; Hauser et al., 1980; Nilsson et al., 2003; Shackleton et al., 1999). The risk of death following the diagnosis of epilepsy is greater in the first 2 years, a 16-fold higher risk, decreasing to a twofold increased risk 15 years after diagnosis (Shackleton et al., 1999).
The impact of having a TBI and LPTS has not been examined in depth with respect to mortality. In general, life expectancies of individuals with TBI are reduced (Shavelle and Strauss, 2000; Strauss et al., 1999), but the specific causes for the increased mortality rate are largely unclear (Harrison-Felix et al., 2004; Shavelle et al., 2001). A large-scale study in the United Kingdom of individuals with TBI injured between 1948 and 1961 found that there was an increased risk of dying from epilepsy, as well as pneumococcal meningitis, suicide, accidents, and respiratory disease in this population (Roberts, 1979). In a more recent examination of 2320 Californians with long-term TBI of up to 28 years, 12 deaths were reported from seizures, whereas the expected number for this age group without TBI was 0.5, resulting in a standardized mortality ratio of 24.3 [observed/expected deaths (Shavelle and Strauss, 2000)]. Two studies from the TBI Model Systems (TBIMS) national database have examined mortality in individuals with TBI who survive at least 1 year post-injury. The first study determined that the overall mortality rate for individuals in the TBIMS database who had survived the first year post-injury was 7.4% (Harrison-Felix et al., 2004). The occurrence of a seizure during inpatient rehabilitation was associated with a 55% increased risk of dying after 1 year post-TBI; however, when a multivariate regression analysis was performed, the occurrence of a seizure no longer contributed to mortality prediction. Only older age, being unemployed at the time of injury, and greater disability at rehabilitation discharge remained significantly associated with mortality (Harrison-Felix et al., 2004). The study did not examine mortality specifically in relation to when seizures first occurred after rehabilitation discharge; thus the seizure events were a mixture of early and late PTS. The second study examined causes of death after 1 year post-TBI, and it was found that the overall rate of death due to seizure was 4% in the sample; this corresponded to a 37 times higher likelihood of an individual with TBI dying from a seizure than the general population (Harrison-Felix et al., 2006). However, this study did not specifically examine individuals with TBI and known LPTS to determine mortality rate and causes of death compared to individuals with TBI alone.
Recognizing the increased mortality rates in persons with epilepsy, the significant percentage of individuals with TBI who experience LPTS, and the reports that seizures are associated with increased mortality in individuals with TBI, this study was completed to examine the mortality rates in individuals with TBI who were classified as having experienced LPTS in the first 2 years post-TBI compared to those without LPTS. In addition, demographics, injury severity, CT findings, and causes of death were compared to better characterize the risk factors associated with mortality in individuals with TBI and LPTS.
Methods
Participants
Individuals with TBI were eligible for this study via two pathways. In the first pathway, the individual had enrolled, during the years from 1992–1997, in a prospective study of risk factors for the development of LPTS in the first 2 years post-TBI at either the Santa Clara Valley Medical Center (SCVMC; San Jose, CA) or the Denver Health Medical Center (DHMC; Denver, CO). In the second pathway, the individual had enrolled, from 1992–1999, in the TBIMS National Database at SCVMC. The inclusion criteria for the first pathway have been described in detail elsewhere (Englander et al., 2003), and are summarized here briefly: at least 16 years of age at time of injury; inpatient admission to a trauma center with a TBI due to external causes; observed positive CT findings or a best Glasgow Coma Scale score (GCS) in the first 24 h of 10 or less; and provision of informed consent. The inclusion criteria for the second pathway were consistent with the eligibility criteria for the TBIMS National Database, and only differed from the first group in that they all experienced inpatient rehabilitation. Of this pool of potential participants, individuals were classified as LPTS or non-LPTS. LPTS was defined as the occurrence of at least one seizure between 1 week and 2 years post-injury.
Measures
Mortality, as determined through the Social Security Death Index, was the primary outcome measure of interest. Additional variables were gender, age at injury, highest GCS score within the first 24 h of injury, time since injury to first seizure, time since injury to death, time since first seizure to death, and cause of death as listed on the death certificate. CT findings were also examined; these included extent of intracranial compression, presence and location of intracranial hemorrhage, presence of extra-axial collection, single versus multiple contusions, and presence of intraparenchymal bone or bullet fragments.
Analytic plan
The LPTS and non-LPTS groups were compared using chi-square (χ2) and Z-ratio tests for categorical data, and t-tests and analysis of variance (ANOVA) for continuous data. Non-parametric survival analyses (Kaplan-Meier analysis) were used to compare cumulative probabilities of mortality with respect to seizure status. Causes of death were examined descriptively.
Results
Participant characteristics (Table 1)
Table 1.
Characteristics of Individuals with Respect to Late Post-Traumatic Seizure Status
| LPTS (n = 71) | non-LPTS (n = 437) | |
|---|---|---|
| Male | 58 (82%) | 323 (74%) |
| Age at time of injury (y) | 39.6 (SD = 14.4) | 36.7 (SD = 16.9) |
| % Caucasian | 29 (41%) | 183 (42%) |
| % Violent etiology of injury | 15 (21%) | 92 (21%) |
| Mid-line shift >5 mm | 14 (20%) | 70 (16%) |
| Subdural hematoma | 35 (49%) | 163 (37%) |
| Multiple cortical contusionsa | 44 (62%) | 141 (32%) |
| Penetrating fragments | 7 (10%) | 29 (7%) |
p < 0.0001.
The participant pool consisted of 508 individuals with TBI who were injured between March 31, 1992 and December 20, 1999, of which 381 (75%) were male. The largest ethnic groups were Caucasian (n = 214 or 42%) and Hispanic (n = 148 or 29%). The average age at injury was 37.1 years (SD = 16.5), and the mean highest GCS in the first 24 h after injury was 10.9 (SD = 4.0). The most frequent mechanisms of injury were motor vehicle accidents (n = 156 or 31%), followed by falls (n = 101 or 20%) and assaults (n = 90 or 18%). CT test results were reviewed for the first week post-injury. Of the entire sample 84 (17%) had a mid-line shift of >5 mm, 185 (36%) had multiple cortical contusions, 198 (39%) had subdural hematomas, and 36 (7%) had penetrating fragments. Seventy-one (14%) of the participants had LPTS with an average time since injury to first LPTS of 160.8 days (175.0) or nearly 6 months. The mortality rate for the entire sample as of May 2007 was 12.4% (63), with an average age at time of death of 63.9 years (19.4).
Potential demographic, injury severity differences, and CT findings between the LPTS and non-LPTS groups were also analyzed (Table 1). The two groups were not significantly different for age at time of injury, gender, ethnicity, and etiology of injury. There was no significant difference in the proportion of LPTS individuals who had a mid-line shift of >5 mm or the presence of penetrating fragments. There was a significant difference in the presence of multiple cortical contusions, 44 (62%) for the LPTS group versus 141 (32%) in the non-LPTS group (χ2 = 23.3; df (1); n = 508; p < .0001; phi = 0.21). There was a trend towards a higher proportion of LPTS individuals with subdural hematomas, 35 (49%), compared to non-LPTS individuals, 163 (37%) (χ2 = 3.7; df (1); n = 508; p = 0.055; phi = 0.09).
Seizure status and mortality (Table 2)
Table 2.
Characteristics of Expired Individuals with Respect to Late Post-Traumatic Seizure Status
| Expired LPTS (n = 19/71) | Expired non-LPTS (n = 44/437) | |
|---|---|---|
| Malea | 18 (95%) | 31 (70%) |
| Age at time of injury (y)a | 48.4 (SD = 15.9) | 63.6 (SD = 19.7) |
| Duration from injury to death (d) | 1538 (SD = 1317) | 1652 (SD = 1301) |
| Age at death (y)a | 54.1 (SD = 16.0) | 67.9 (SD = 19.6) |
| Highest GCS in first 24 h | 10.9 (SD = 4.2) | 12.1 (SD = 3.8) |
| Mid-line shift >5 mm | 3 (16%) | 8 (19%) |
| Subdural hematoma | 14 (74%) | 24 (56%) |
| Multiple cortical contusions | 11 (58%) | 16 (37%) |
| Penetrating fragments | 1 (5%) | 1 (2%) |
p < 0.01.
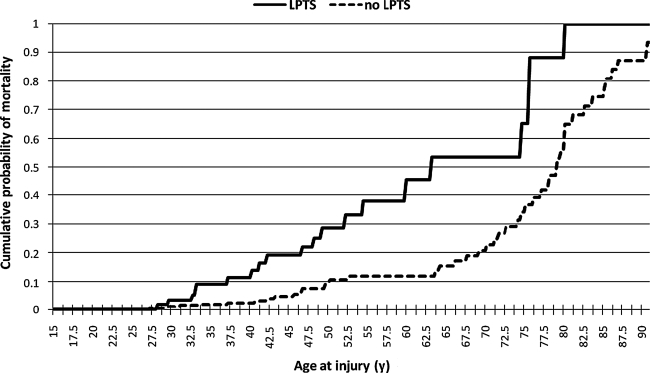
The mortality rates as of May 2007 differed significantly depending upon whether an individual had experienced LPTS; 19 of the 71 (27%) individuals in the LPTS group had died, compared to 44 of 437 (10%) of the non-LPTS group [χ2 = 15.7; df (1); n = 508; p < 0.0003; phi = 0.18]. Proportionately more males in the LPTS group had expired, 18 of 19 (95%), than in the non-LPTS group, 31 of 44 (70%) [χ2 = 4.5; df (1); n = 63; p = 0.03; phi = 0.27]. LPTS individuals who had died were significantly younger at the time of injury, 48.4 years (15.9), than the non-LPTS persons who had died (63.6 years [19.7]) [t = 3.0; df (61); p = 0.0043]. As shown in Figure 1, the cumulative mortality curve for the LPTS group was significantly shifted to the left, toward younger age at injury (Breslow rank test p = 0.0002).
FIG. 1.
Mortality as a function of age at injury and seizure status. The chart indicates the cumulative probability of mortality for the LPTS and no LPTS groups with respect to age at time of injury.
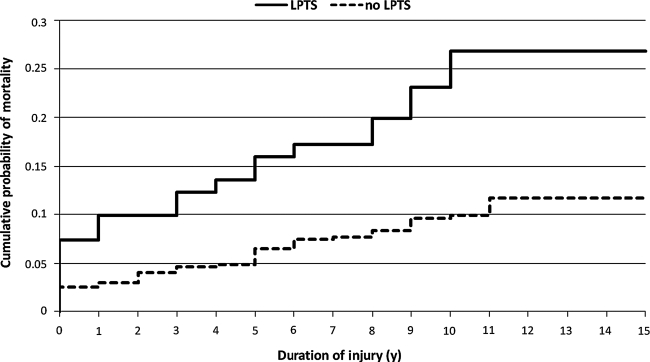
The average duration from date of injury to death was not significantly different between the two groups. Figure 2 shows the cumulative mortality curves with respect to duration since injury and seizure status; while the LPTS curve is shifted significantly upward, reflecting the increased mortality rates in this group, the two curves are essentially parallel, indicating comparable patterns of mortality as a function of duration since injury (Fig. 2). There was also no significant difference in highest GCS score in the first 24 h after injury.
FIG. 2.
Mortality as a function of duration of injury and seizure status. The chart indicates the cumulative probability of mortality for the LPTS and no LPTS groups with respect to duration of injury at either the time of death or of lost to follow-up or end of study.
Data from CT tests performed within the first 7 days after injury revealed interesting differences between the LPTS and non-LPTS groups. There was no significant difference in the proportion of individuals in the expired LPTS group with a mid-line shift of >5 mm and those without. While not reaching statistical significance, there was a larger proportion of individuals with subdural hematomas in the expired LPTS group, 14 (74%), than in the expired non-LPTS group, 24 (56%). There was also a larger proportion of individuals with multiple cortical contusions in the expired LPTS group, 11 (58%), than in the expired non-LPTS group, 16 (37%). There was no statistically significant difference in the proportion of individuals with penetrating fragments between the two groups.
Etiology of death
Table 3 gives detailed information about the causes of death for the LPTS and non-LPTS groups. Death certificates could not be obtained for 2 LPTS subjects and 5 non-LPTS subjects. A higher percentage of those with LPTS died from the original trauma, a new trauma, or acquired immune deficiency syndrome (AIDS), compared to those in the non-LPTS group. The rates reached statistical significance for new trauma (Z-ratio = 1.68; p = 0.049) and AIDS (Z-ratio = 2.20; p = 0.014). Conversely, a higher percentage in the non-LPTS group died from cardiovascular accident (CVA), cardiovascular causes, and chronic obstructive pulmonary disease (COPD) than in the LPTS group; however, these differences were not statistically significant.
Table 3.
Causes of Death for the LPTS and non-LPTS groups
| Cause of death | LPTS (n = 19) | Non-LPTS (n = 47) | p Value |
|---|---|---|---|
| Original trauma | 3 (16%) | 4 (9%) | NS |
| New trauma | 3 (16%) | 2 (4%) | 0.049 |
| Substance abuse (alcohol/methamphetamine) | 2 (10%) | 3 (6%) | NS |
| Acquired immune deficiency syndrome | 2 (10%) | 0 (0%) | 0.014 |
| CVA | 1 (5%) | 5 (11%) | NS |
| Cardiovascular | 1 (5%) | 8 (17%) | NS |
| Cancer | 1 (5%) | 3 (6%) | NS |
| Renal disease | 1 (5%) | 1 (2%) | NS |
| Seizure | 0 (0%) | 1 (2%) | NS |
| Pneumonia | 1 (5%) | 4 (9%) | NS |
| Neurodegenerative | 0 (0%) | 1 (2%) | NS |
| COPD | 0 (0%) | 3 (6%) | NS |
| Other | 2 (10%) | 7 (15%) | NS |
| Unknown | 2 (10%) | 5 (11%) | NS |
CVA, cardiovascular accident; COPD, chronic obstructive pulmonary disease; NS, not significant.
Mortality within the LPTS group (Table 4)
Table 4.
Characteristics of the Late Post-Traumatic Seizure Group with Respect to Mortality
| Expired (n = 19/71) | Alive (n = 52/71) | |
|---|---|---|
| Male | 18 (95%) | 40 (77%) |
| Age at time of injury (y)a | 48.4 (SD = 15.9) | 36.3 (SD = 12.4) |
| Duration from injury to first LPTS (d) | 158.4 (SD = 170.6) | 187.5 (SD = 180.3) |
| Highest GCS in first 24 h | 10.9 (SD = 4.2) | 9.5 (SD = 3.9) |
| Mid-line shift >5 mm | 3 (16%) | 11 (22%) |
| Subdural hematomaa | 14 (74%) | 21 (43%) |
| Multiple cortical contusions | 11 (58%) | 33 (67%) |
| Penetrating fragments | 1 (5%) | 6 (12%) |
p < 0.05.
Demographic and injury characteristics were compared in the LPTS group for those who had died (n = 19) and those who were alive as of May 2007 (n = 52). There was a trend towards a higher proportion of men in the expired LPTS group, 18 of 19 (95%), than in the alive LPTS group, 40 of 52 (77%) [χ2 = 2.9; df (1); n = .71; p = 0.086; phi = 0.20]. LPTS individuals who had died were significantly older at the time of injury, 48.4 years (15.9), than those still alive, 36.3 years (12.4) [t = 3.4; df (69); p = 0.0013]. There was no difference between the expired and non-expired LPTS groups in the duration between the date of injury, onset of LPTS, and the highest GCS score in the first 24 h after injury.
CT findings from the first 7 days after injury revealed differences between the expired and alive LPTS groups. There was a significantly higher proportion of expired individuals with subdural hematomas, 14 (74%), compared to individuals still alive in May 2007, 21 (42%) [χ2 = 5.5; df (1); n = 69; p = 0.019; phi = 0.28]. There was no significant difference in the proportion of LPTS individuals who had expired or were still alive with respect to a mid-line shift of >5 mm, the presence of multiple cortical contusions, or the presence of penetrating fragments.
Mortality within the non-LPTS group (Table 5)
Table 5.
Characteristics of the Non-Late Post-Traumatic Seizure Group with Respect to Mortality
| Expired (n = 44/437) | Alive (n = 393/437) | |
|---|---|---|
| Male | 31 (70%) | 292 (74%) |
| Age at Time of Injury (years)b | 63.6 (SD = 19.7) | 33.7 (SD = 13.5) |
| Highest GCS in first 24 h | 12.1 (SD = 3.8) | 10.9 (SD = 4.0) |
| Mid-line shift >5 mm | 8 (18%) | 62 (16%) |
| Subdural hematomaa | 25 (57%) | 138 (35%) |
| Multiple cortical contusions | 17 (39%) | 124 (31%) |
| Penetrating fragments | 1 (2%) | 28 (7%) |
p < 0.005.
p < 0.0001.
Demographic and injury characteristics were compared in the non-LPTS group for those who had died (n = 44) and those who were alive as of May 2007 (n = 393). There was no significant difference in the proportion of men in the expired non-LPTS group compared to the alive non-LPTS group. Non-LPTS individuals who had died were significantly older at the time of injury, 63.6 years (19.7), than those still alive, 33.7 (13.5) [t = 13.2; df (434); p < 0.0001]. There was also no statistically significant difference between the expired and alive non-LPTS groups in the highest GCS score in the first 24 h after injury.
CT findings from the first 7 days after injury revealed differences between the expired and alive non-LPTS groups. There was a significantly higher proportion of expired individuals with subdural hematomas, 25 (57%), compared to individuals still alive in May 2007, 138 (35%) [χ2 = 8.0; df (1); n = 437; p = 0.0048; phi = 0.14}. There were no significant differences between the expired and alive non-LPTS groups with respect to midline shift of >5 mm, the presence of multiple cortical contusions, or the presence of penetrating fragments.
Discussion
The results of this study indicate that individuals with LPTS have a very high mortality rate at a younger age compared to individuals with TBI and no LPTS and those with epilepsy in general. Even without adjusting for age, those with TBI and LPTS have nearly three times the mortality rate of those with TBI alone, 27% versus 10% in this study. They die 15 years younger than those who have TBI alone, but do not differ significantly in duration post-TBI or in initial severity of injury as measured by GCS or the severity of mid-line shift. In both the LPTS and non-LPTS groups, individuals who had expired were older at the time of injury and were more likely to have experienced a subdural hematoma during the original injury.
While it is known that individuals with TBI experience increased mortality compared to those without TBI, on the order of 2–5 times the rate, and that those with undifferentiated epilepsy have higher mortality rates, the mortality risk of the combined conditions has not been studied recently in a civilian population (Baguley et al., 2000; Harrison-Felix et al., 2004; Shackleton et al., 2002). Walker and colleagues (1971) found that World War I and World War II veterans with TBI had a higher mortality rate than the general population, and that veterans with TBI and epilepsy had a mortality rate that was higher than that of those with TBI alone; this increased rate of mortality became apparent at the age of 55 and older. The authors did not make any assertions about the role of epilepsy in further increasing mortality rates in these veterans with TBI, although they did note that those with TBI and epilepsy had more severe injuries than those without epilepsy (Walker et al., 1971). A similar pattern occurred in our study population; while individuals with LPTS had similar etiology of injuries, GCS scores, and mid-line shift compared to the non-LPTS group, the LPTS group had a higher incidence of subdural hematomas and multiple contusions.
In this study, the causes of mortality were variable; however, more individuals in the LPTS group died from new trauma and complications from HIV-AIDS compared to those in the non-LPTS group, who were more likely to die from COPD, CVA, and cardiovascular complications. The most obvious conclusion is that the LPTS group was more likely to engage in risk-taking behaviors that eventually result in death than those in the older non-LPTS group, who died more typically from age-related problems. Interestingly, both the expired LPTS and non-LPTS groups were significantly older at the time of injury and had a higher percentage of subdural hematomas compared to those still alive. The striking difference between the expired LPTS and non-LPTS group was gender; the expired LPTS group was 95% male. One can postulate that these younger males are more likely to engage in the kinds of activities that can lead to HIV-AIDS or subsequent trauma, with resultant illness and/or death. Substance abuse also makes these events more likely to occur.
Only one death was attributed to seizures; that was in a person who died 10 years post-injury and was not previously classified as having LPTS, that is, with the first seizure occurring within 2 years post-TBI. Only one individual with LPTS had a death due to “cardiovascular disease,” which occurred at the age of 44, 3 years post-TBI. At first glance it does not appear that this population is at undue risk of sudden unexplained death due to epilepsy (SUDEP). However, Langan and associates (2002) outlined the challenges of detecting SUDEP with post-mortem investigations, and our study did not involve extensive investigation of post-mortem findings.
Some risk stratification is possible for those with LPTS. Older individuals and those who have experienced subdural hematoma have a higher risk of mortality. However, those individuals with LPTS that died were typically less than 55 years old. One could surmise that those deaths due to AIDS may be less likely in this decade; similarly, there are more treatment options for hepatitis C. However, in order for treatment to be successful for LPTS and other diseases such as AIDS or hepatitis C, diligent adherence to treatment protocols and regular follow-up are crucial.
One can conclude that the individual with TBI and LPTS is at high risk for early death, and requires not only diligent follow-up for control of seizures, but also close medical follow-up for the occurrence of other medical conditions that can lead to early demise. It is generally recognized by patients, families, insurance carriers, and the general public that those with chronic conditions such as hypertension, diabetes, cancer, AIDS, renal disease, spinal cord injury, and connective tissue diseases are at high risk of morbidity and mortality related to their underlying disease state. In TBI, the common co-morbidities of depression, decreased self-awareness, late PTS, and fatigue are recognized and typically treated by mental health specialists, neurologists, and physiatrists. However, in the first several years after injury, most people with TBI experience improvement in their general sense of well-being as well as in their capabilities for mobility, self-care, and even cognition, which may lull them, as well as medical practitioners, into a false sense of relatively good general health.
Given the findings here that individuals with TBI and LPTS are dying of a variety of causes not specifically related to epilepsy, it seems prudent to make sure that these individuals have access to and follow-through with ongoing general medical care and prescribed medications. We also need to educate our primary care colleagues regarding the unique needs of this at-risk population. The younger individuals in this study were at risk of dying with subsequent trauma, AIDS, and substance abuse, whereas the older individuals were not surprisingly at risk for circulatory disorders.
Especially for those with LPTS, basic immunizations for pneumococcus, influenza, and hepatitis should be administered, and safe sexual practices reinforced. The facts that substance abuse increases the likelihood of subsequent seizures and new trauma and may have lethal consequences must be strongly reinforced by anyone caring for this population.
Limitations
Death certificates were not obtainable for seven individuals, which limits some of our generalizations regarding cause of death. Verification of primary cause of death through post-mortem examination would have been the best possible source for determining primary cause of death; however, due to differences in state laws concerning autopsy, this was not possible for the majority of cases. It was decided that the next best source, with acknowledgment of its inherent limitations, would be the death certificate, and that future studies should be designed to utilize more accurate sources for determining primary cause of death. Our data were incomplete with regard to the role of substance abuse in the initial trauma, and whether substance abuse persisted post-TBI. For those that expired, we have no information regarding the management of their seizure disorder; subsequent analyses regarding seizure control and other medical and social morbidities for those who are still living with LPTS are underway.
Acknowledgment
This work was supported by the U.S. Department of Education, Office of Special Education and Rehabilitative Services, National Institute on Disability and Rehabilitation Research (grant no. H133A020524).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Aiskainen I. Kaste M. Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcomes. Epilepsia. 1999;40:584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Annegers J.F. Coan S.P. SUDEP: Overview of definitions and review of incidence data. Seizure. 1999;8:347–352. doi: 10.1053/seiz.1999.0306. [DOI] [PubMed] [Google Scholar]
- Annegers J.F. Grabow J.D. Broover R.V. Laws E.R. Elveback L.R. Kurland L.T. Seizures after head trauma: a population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- Annegers J.F. Hauser A. Coan S.P. Rocca W.A. A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Baguley I. Sleva-Younan S. Lazarus R. Green A. Long-term mortality trends in patients in patients with traumatic brain injury. Brain Injury. 2000;14:505–512. doi: 10.1080/026990500120420. [DOI] [PubMed] [Google Scholar]
- Brain Injury Special Interest Group of the American Academy of Physical Medicine and Rehabilitation. Practice parameter: antiepileptic drug treatment of posttraumatic seizures. Arch. Phys. Med. Rehabil. 1998;79:594–597. [PubMed] [Google Scholar]
- Brandvold B. Levi L. Feinsod M. George E.D. Penetrating craniocerebral injuries in the Israeli involvement in the Lebanese conflict 1982–1985. Analysis of a less aggressive surgical approach. J. Neurosurg. 1990;72:15–21. doi: 10.3171/jns.1990.72.1.0015. [DOI] [PubMed] [Google Scholar]
- Caveness W.F. Meirowsky A.M. Rish B.L. Mohr J.P. Kistler J.P. Dillon J.D. Weiss G.H. The nature of post-traumatic epilepsy. J. Neurosurg. 1979;50:545–553. doi: 10.3171/jns.1979.50.5.0545. [DOI] [PubMed] [Google Scholar]
- Cockerell O.C. Johnson A.L. Sander J.W. Hart Y.M. Goodridge D.M. Shorvon S.D. Mortality from epilepsy: results from a prospective population-based study. Lancet. 1994;344:918–921. doi: 10.1016/s0140-6736(94)92270-5. [DOI] [PubMed] [Google Scholar]
- Courjon J. A longitudinal electro-clinical study of 80 cases of post-traumatic epilepsy observed from the time of the original trauma. Epilepsia. 1970;11:29–36. doi: 10.1111/j.1528-1157.1970.tb03863.x. [DOI] [PubMed] [Google Scholar]
- DaSilva A.M. Vaz A.R. Ribeiro I. Melo A.R. Nune B. Correia M. Controversies in post-traumatic epilepsy. Acta Neurochir. Suppl. 1990;50:48–51. doi: 10.1007/978-3-7091-9104-0_9. [DOI] [PubMed] [Google Scholar]
- Desantis A. Cappricci E. Granata G. Early post-traumatic seizures in adults. J. Neurosurg. Sci. 1979;23:207–210. [PubMed] [Google Scholar]
- Englander J.E, Bushnik T.Duong T.T.Cifu D.X.Zafonte R.Wright J.Hughes R.Bergman W.2003Analyzing risk factors for late posttraumatic seizures: A prospective, multi-center investigation Arch Phys. Med. Rehabil. 84365–373. [DOI] [PubMed] [Google Scholar]
- Glotzner F.L. Haubitz I. Miltner F. Kapp G. Pflughaupt K.W. Epilepsy prophylaxis with carbamazepine in severe brain injuries. Neurochirurgia. 1983;26:66–79. doi: 10.1055/s-2008-1053615. [DOI] [PubMed] [Google Scholar]
- Haltiner A.M. Temkin N.R. Dikmen S.S. Risk of seizure recurrence after the first late posttraumatic seizure. Arch Phys. Med. Rehabil. 1997;78:835–840. doi: 10.1016/s0003-9993(97)90196-9. [DOI] [PubMed] [Google Scholar]
- Harrison-Felix C. Whiteneck G. DeVivo M. Hammond F.M. Jha A. Mortality following rehabilitation in the Traumatic Brain Injury Model Systems of Care. NeuroRehabilitation. 2004;19:45–54. [PubMed] [Google Scholar]
- Harrison-Felix C. Whiteneck G. DeVivo M.J. Hammond F.M. Jha A. Causes of death following 1 year post-injury among individuals with traumatic brain injury. J. Head Trauma Rehabil. 2006;21:22–33. doi: 10.1097/00001199-200601000-00003. [DOI] [PubMed] [Google Scholar]
- Hauser W.A. Annegers J.F. Elveback L.R. Mortality in patients with epilepsy. Epilepsia. 1980;21:399–412. doi: 10.1111/j.1528-1157.1980.tb04088.x. [DOI] [PubMed] [Google Scholar]
- Hauser W.A. Annegers J.F. Kurland L.T. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32:429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- Jennett B. Epilepsy after non-missile head injuries. Scot. Med. J. 1973;18:8–13. doi: 10.1177/003693307301800103. [DOI] [PubMed] [Google Scholar]
- Jennett W.B. Epilepsy After Non-Missile Head Injuries. William Heinemann Medical Books: London; 1975. [Google Scholar]
- Kobau R. Zahran H. Grant D. Thurman D.J. Price P.H. Zack M.M. Prevalence of active epilepsy and health-related quality of life among adults with self-reported epilepsy in California: California Health Interview Survey, 2003. Epilepsia. 2007;48:1904–1913. doi: 10.1111/j.1528-1167.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- Kollevold T. Immediate and early cerebral seizures after head injuries: Part IV. J. Oslo City Hosp. 1979;29:35–47. [PubMed] [Google Scholar]
- Langan Y. Nashef L. Sander A.S. Certification of deaths due to epilepsy. J. Neurol. Neurosurg. Psychiatry. 2002;73:751–752. doi: 10.1136/jnnp.73.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhatoo S.D. Sander J.W. Cause-specific mortality in epilepsy. Epilepsia. 2005;46:36–39. doi: 10.1111/j.1528-1167.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- Nilsson L. Ahlbom A. Farahmand B.Y. Tomson T. Mortality in a population-based cohort of epilepsy surgery patients. Epilepsia. 2003;44:575–581. doi: 10.1046/j.1528-1157.2003.03302.x. [DOI] [PubMed] [Google Scholar]
- Nilsson L. Tomson T. Farahmand B.Y. Diwan V. Persson P.G. Cause-specific mortality in epilepsy. Epilepsia. 1997;38:1062–1068. doi: 10.1111/j.1528-1157.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- Roberts A. Severe Accidental Head Injury: An Assessment of Long-Term Prognostics. Macmillan: London; 1979. pp. 140–179. [Google Scholar]
- Salazar A.M. Grafman J. Jabbari G. Vance S.C. Amin D. Epilepsy and cognitive loss after penetrating head injury. Adv. Epilep. 1987;16:627–631. [Google Scholar]
- Salazar A.M. Jabbari B. Vance S.C. Grafman J. Amin D. Dillon J.D. Epilepsy after penetrating head injury: I. Clinical correlates. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Shackleton D.P. Westendorp R.G.J. Kasteleijn-Nolst Trenite D.G.A. de Craen A.J.M. Vandernbroucke J.P. Survival of patients with epilepsy: An estimate of the mortality risk. Epilepsia. 2002;43:445–450. doi: 10.1046/j.1528-1157.2002.10301.x. [DOI] [PubMed] [Google Scholar]
- Shackleton D.P. Westendorp R.G. Kasteleijn-Nolst Trenite D.G.A. Vandenbroucke J.P. Mortality in patients with epilepsy: 40 years of follow up in a Dutch cohort study. J. Neurol. Neurosurg. Psychiatry. 1999;66:636–640. doi: 10.1136/jnnp.66.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavelle R.M. Strauss D.J. Comparative mortality of adults with traumatic brain injury in California, 1988–1997. J. Insurance Med. 2000;32:163–166. [PubMed] [Google Scholar]
- Shavelle R.M. Strauss D. Whyte J. Day S.M. Yu Y.L. Long-term causes of death after traumatic brain injury. Am. J. Phys. Med. Rehabil. 2001;80:510–516. doi: 10.1097/00002060-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Strauss D.J. Shavelle R.M. DeVivo M.J. Life tables for persons with traumatic brain injury. J. Insurance Med. 1999;31:104–105. [Google Scholar]
- Walker A.E. Leuchs H.K. Lechtape-Gruter H. Caveness W.F. Kretschman C. Life expectancy of head injured men with and without epilepsy. Arch. Neurol. 1971;24:95–100. doi: 10.1001/archneur.1971.00480320023001. [DOI] [PubMed] [Google Scholar]
- Wiebe S. Bellhouse D.R. Fallahay C. Eliasziw M. Burden of epilepsy: the Ontario Health Survey. Can. J. Neurol. Sci. 1999;26:263–270. doi: 10.1017/s0317167100000354. [DOI] [PubMed] [Google Scholar]