Abstract
Objective
Bacterial vaginosis (BV) is associated with preterm delivery, but there is little evidence that treatment improves pregnancy outcomes. We examined whether oral or vaginal metronidazole treatment for BV in early pregnancy was more effective in restoring the normal vaginal environment.
Methods
This was a randomized controlled trial comparing oral and intravaginal metronidazole for treatment of BV in early pregnancy (<20 weeks). Vaginal samples collected at baseline and 4 weeks after treatment were evaluated using gram stain, culture, colorimetric detection of sialidase, and immunoassay for measurement of proinflammatory cytokines interleukins-1β, -6, -8 (IL-1β, IL-6, IL-8) and secretory leukocyte protease inhibitor (SLPI). We compared the effect of treatment between groups (using chi-square and t test) and within individuals (McNemar's test).
Results
Of 126 subjects, 108 (86%) completed follow-up (55 oral, 53 intravaginal). Of the study population, 34% achieved therapeutic cure, and this was not different between treatment groups. BV-associated bacteria were significantly reduced in both groups, but few subjects regained colonization with protective lactobacilli. Among women who achieved therapeutic cure, the level of IL-1β dropped significantly (p < 0.001) and SLPI increased (p = 0.003). More women in the vaginal treatment group had undetectable sialidase after treatment (p = 0.013).
Conclusions
Treatment with oral or intravaginal metronidazole in early pregnancy reduced colonization with BV-associated bacteria but was not effective in achieving therapeutic cure or in restoring healthy vaginal lactobacilli.
Introduction
Bacterial vaginosis (BV) is a common cause of vaginal discharge characterized by a shift from dominant normal vaginal lactobacilli to a vaginal microbiota dominated by anaerobes and gram-negative bacteria.1 BV has been associated with preterm delivery2 even after correction for demographic and obstetrical risk factors, although treatment for BV in pregnancy does not consistently reduce the risk of preterm delivery.3–5 Some hypothesize that preterm delivery may not be related to BV per se but rather to an inflammatory immune response, which may not diminish with treatment for infection.6
Studies among women in preterm labor have consistently demonstrated associations between BV and positive amniotic fluid cultures, increased levels of proinflammatory amniotic fluid cytokines, and histological chorioamnionitis.7,8 BV is associated with higher concentrations of vaginal proinflammatory cytokines, specifically interleukin-1β (IL-1β) and IL-8, among both nonpregnant and pregnant women9–12; these vaginal proinflammatory cytokines have also been linked with preterm delivery.13,14 A separate virulence factor is sialidase, a microbial hydrolytic enzyme that may facilitate binding of bacteria to the vaginal epithelium and stimulation of cytokine response. Pregnant women with BV and detectable levels of sialidase have been shown to be at increased risk of preterm birth.15
BV may also decrease the presence of vaginal protective factors. Secretory leukocyte protease inhibitor (SLPI) is a host defense molecule produced by epithelial cells that is thought to control damage resulting from the inflammatory process.16,17 SLPI has significantly decreased concentrations among women with BV and other vaginal infections.18
Oral and vaginal metronidazole treatments have been shown to be equally effective in treatment of BV in nonpregnant women.19,20 However, oral metronidazole has not been effective at decreasing rates of preterm delivery in low-risk women with BV.3 At the time this trial was designed, no studies had been published that evaluated the use of vaginal metronidazole in pregnancy.21 Therapeutic cure of BV, as defined by the U.S. Food and Drug Administration (FDA), includes elimination of anaerobic bacteria as well as resolution of clinical signs and symptoms of BV.22 For antibiotic treatment to maximally decrease the risk of preterm delivery in BV, it should not only decrease vaginal colonization with pathogenic bacteria but also decrease inflammation and the presence of bacterial virulence factors. The lack of efficacy of oral metronidazole in decreasing preterm delivery rates in low-risk women raised the question of whether vaginal metronidazole might have a differential effect on genital tract inflammation.
In this analysis, we compared the therapeutic cure rate and the presence of pathogenic bacteria, inflammatory markers, and bacterial virulence factors among pregnant women with BV who received either oral or vaginal metronidazole therapy. We hypothesized that systemic, oral metronidazole would be more likely to increase therapeutic cure and reduce pathogenic bacteria, inflammation, and bacterial virulence factors than local application of vaginal metronidazole.
Materials and Methods
Study design
This was a randomized, double-blind, placebo-controlled trial comparing oral (250 mg three times daily for 7 days) and intravaginal (5 g of 0.75% gel twice daily for 5 days) metronidazole treatment of BV in early pregnancy. From May 2000 to September 2004, pregnant women were recruited from public health clinics in Seattle, Washington, where they were screened for BV by gram stain.23 Women were excluded from study participation if they were >20 weeks' gestation, were <16 years of age, used antibiotics within 7 days prior to their screening visit, had a history of preterm birth (<37 weeks), had a multiple gestation pregnancy, or had major medical complications, such as chronic hypertension or preexisting diabetes, recent alcohol dependency, or allergy to metronidazole. Women who tested positive for BV and met the eligibility criteria were invited to enroll in the treatment trial.
After providing written informed consent, study participants were randomized to treatment with oral metronidazole (Centers for Disease Control and Prevention [CDC]-recommended regimen: 250 mg three times daily for 7 days) plus intravaginal placebo or intravaginal metronidazole (5 g of 0.75% gel twice daily for 5 days) plus oral placebo. The oral and vaginal placebos were indistinguishable from active therapy. Randomization to study group used random number tables, with a 1:1 ratio between study groups. The Investigational Drug Service at the University of Washington performed the randomization and provided the treatment assignments in opaque, sealed envelopes. Neither the subjects nor the study personnel assessing treatment effect were aware of which active agent had been assigned. This study was approved by the University of Washington and CDC Institutional Review Boards and registered with ClinicalTrials.gov, number NCT00153517.
At baseline, demographic, obstetrical, medical, and lifestyle data were collected using a 30-minute standardized personal interview. A physical examination was done, data were collected on Amsel's clinical criteria,24 and five Dacron swabs were collected from the posterior vaginal fornix for gram stain, bacterial culture, and analysis of cytokines, sialidase, and SLPI. Study participants returned for follow-up 4 and 8 weeks after completion of treatment and again at delivery. At the follow-up visits, a structured interview was conducted, and information was gathered about treatment adherence (by patient report and medication diary), adverse effects, tolerability of the medication (including such side effects as metallic taste, which might have compromised blinding to treatment assignment), and the presence of any symptoms of infection. The physical examination and sample collection were repeated, and BV was assessed by gram stain. Information about gestational age at delivery, mode of delivery, and postpartum complications was assessed by chart abstraction using a standardized form. All outcomes reported in this analysis are from the 4-week follow-up visit unless otherwise noted.
Five Dacron swabs were collected from the vagina at each examination. One swab was used to create the gram stain slide for diagnosis of BV. Two swabs were placed in a Port-a-Cul anaerobic transport device (Becton Dickinson, Cockeysville, MD) and delivered to the laboratory for quantitative culture setup within 24 hours of collection.25 Anaerobic microorganisms were identified after 48–72 hours of incubation at 37°C in 5%–10% CO2. Plates for recovery of anaerobes were incubated within the anaerobic chamber (Bactron IV, Sheldon Manufacturing, Cornelius, OR) at 37°C for 5–7 uninterrupted days. All isolates were identified by standard biochemical methods.26 The two remaining swabs were eluted in 900 μL of phosphate-buffered saline (PBS) (yielding a 1:10 dilution) and frozen at −80°C until further analysis.
Successful microbiological treatment was defined as a normal vaginal gram stain (score of 0–3) 4 weeks after completion of antibiotics. Successful clinical treatment was defined as the absence of all four clinical signs at follow-up (no homogeneous discharge, no amine odor after the addition of potassium hydroxide, no clue cells on saline microscopy, and pH <4.5). Therapeutic cure was defined as achievement of both clinical and microbiological cure, consistent with published guidelines.22 Treatment failure was defined as persistent BV by gram stain (score of 7–10) at 4-week follow-up or persistence of any clinical symptoms. Patients with persistent BV after treatment were notified, as were their obstetrical care providers. Decisions about repeat treatment were made by the obstetrical care provider.
BV was diagnosed by gram stain using Nugent's criteria: a score of 0–3 indicated normal flora, 4–6 intermediate flora, and 7–10 BV.23 All slides were scored by the same reader (K.A.). Trichomonas vaginalis was diagnosed by culture (In-Pouch™ TV, Biomed Diagnostics, White City, OR). The proinflammatory cytokines IL-6, IL-8, and IL-1β and the mucosal defense molecule SLPI were measured using standard enzyme-linked immunosorbent assay (ELISA). Samples were first tested for the presence of semen using azo dye for acid phosphatase.27 Any specimens positive for acid phosphatase were excluded from SLPI analysis, as SLPI can be found at high concentrations in seminal plasma, although semen does not impact concentrations of cytokines.27,28 SLPI was assayed using a commercial ELISA kit (catalog DP100) with a reported sensitivity of 0.25 pg/mL from R&D Systems (Minneapolis, MN). All other cytokines were assayed an independently validated ELISA29 with a sensitivity of 0.5 pg/mL using the following commercially available matched antibody pairs: IL-6 MAB206 and BAF206 from R&D Systems, IL-1β MAB601 and BAF201 from R&D Systems, and IL-8 M331 and M330B from Fisher Scientific (Pittsburgh, PA). Sialidase was detected using a 4-methylumbelliferyl spot test, modified for use with vaginal fluid; 10 μL of vaginal fluid was spotted to Whatman no. 2 filter paper, saturated with sialidase reagent (1 mg 2′-(4-methylumbelliferyl) a-D-N-acetylneuraminic acid sodium salt hydrate) (Sigma, St Louis, MO) dissolved in 6.6 mL dH20), and incubated for 30 minutes. Development of a blue fluorescence when exposed to ultraviolet light was indicative of a positive test and the presence of sialidase.
The original planned sample size was 250 subjects (125 per treatment arm), which would have provided sufficient power to detect a 20% difference in the rate of therapeutic cure of BV between the two arms, with a planned interim analysis at 50% accrual. All analyses comparing oral and intravaginal treatment groups were intent-to-treat. As the planned interim analysis showed no difference in either the primary outcome (BV therapeutic cure) or in preterm birth between treatment groups, the decision was made to stop the trial early for futility. The rest of the analyses presented in this report compared the effect of metronidazole on vaginal flora and host defenses using a within-subject approach, pooling data from the oral and intravaginal treatment arms. Secondary analyses also compared the effects of oral vs. intravaginal metronidazole on vaginal colonization with BV-associated and other bacteria, change in proinflammatory cytokines and sialidase, and effect on SLPI concentrations. A post hoc power calculation showed that we had 80% power to detect a 0.2 log difference in pretreatment and posttreatment cytokine concentrations within subjects (i.e., paired t test) and 80% power to detect a 0.5 log difference in cytokine concentrations between the vaginal and oral treatment groups.
Chi-square and Fisher's exact tests (two-tailed) were used to compare categorical variables and treatment outcomes between the two treatment groups. All analysis was performed in an intention-to-treat manner. The presence or absence of each bacterial species and sialidase before and after treatment were compared using McNemar's test on pooled data from the entire study population. Categorical variables were created to describe the response of each bacterium to treatment—never present, gain of bacterial species, loss of bacterial species, or persistence of bacterial species—and these were compared between the oral and vaginal treatment groups using the chi-square test. Cytokine values were not normally distributed and so were log10 transformed. Student's t test was used to compare changes in log-transformed cytokine concentrations. A categorical variable was created for change in sialidase—present at baseline only, present at follow-up only, present at both visits or neither visit (i.e., no change in sialidase)—and this was compared between groups using the chi-square test. Statistical analyses were performed using SPSS statistical software, version 11.0 (SPSS Inc., Chicago, IL).
Results
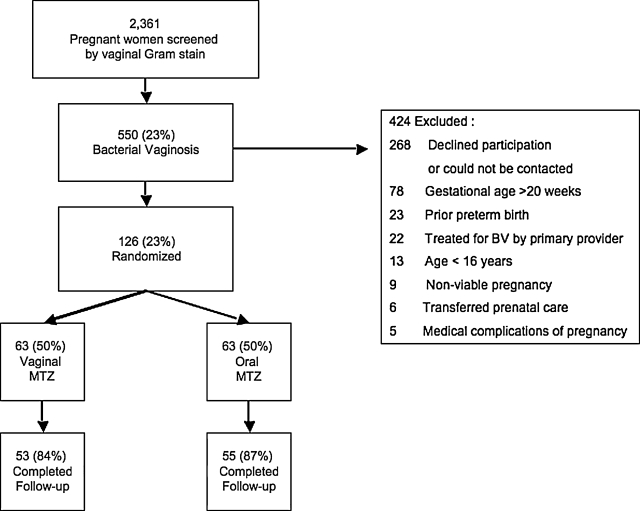
Over 52 months of recruitment, 2361 pregnant women were screened for BV, of whom 550 (23%) tested positive. One hundred twenty-six (23%) of these women enrolled in the study, and 108 women completed follow-up. Of the women who completed follow-up, 55 were randomized to the oral metronidazole treatment group and 53 were randomized to the vaginal metronidazole treatment group (Fig. 1). The rest of the women either declined participation or could not be contacted (n = 268), had been treated for BV by their provider (n = 22), transferred prenatal care outside of our system (n = 6), or were ineligible for the study for the following reasons: maternal age <16 years (n = 13), gestational age >20 weeks (n = 78), prior preterm birth (n = 23), medical complication of pregnancy (n = 5), or nonviable pregnancy (n = 9). The median age for the cohort was 23 years (range 16–41), and the median gestational age at enrollment was 16 weeks (9–20).
FIG. 1.
Study flow diagram. Of 2361 women screened, 550 were diagnosed with bacterial vaginosis by gram stain. Of these, 268 declined participation, 156 were excluded for a variety of reasons, and 126 agreed to participate in the trial and were randomized to either oral metronidazole and vaginal placebo or vaginal metronidazole and oral placebo.
Demographic characteristics at enrollment for the oral and vaginal treatment groups are shown in Table 1. The groups were similar in all demographic characteristics except for parity at enrollment. There were significantly more primiparous women in the vaginal treatment arm than in the oral treatment arm (p = 0.03). There was no difference between women randomized to oral or vaginal treatment in the rates of preterm delivery (16% vs. 13%, p = 0.45), low birth weight infants (11% vs. 6%, p = 0.32), or cesarean delivery (18% vs. 23%, p = 0.74). Adherence to medication was high in this study population, with mean number of missed doses equal to 0, ranging from 0 to 12 for the oral formulation and from 0 to 6 for the vaginal formulation. Of those taking oral metronidazole, 24% reported problems with the medication, as did 21% of patients using vaginal metronidazole (p = 0.72). Twelve percent of those taking oral placebo reported problems related to the oral medication, and 13% of those with vaginal placebo reported problems related to this medication (p = 0.94).
Table 1.
Baseline Demographic Characteristics and Pregnancy Outcomes by Oral or Vaginal Metronidazole Treatment Arm
| |
Treatment arm |
|
|
|---|---|---|---|
| Characteristic | Vaginal n = 53 | Oral n = 55 | p valuea |
| Maternal age, median (range) | 22 (16–39) | 23 (16–41) | 0.67 |
| Weeks gestation at screening, median (range) | 16 (9–20) | 15.5 (10–20) | 0.98 |
| Race/ethnicity, n (%) | |||
| African American | 13 (24.5) | 17 (30.9) | 0.45 |
| Hispanic | 13 (24.5) | 9 (16.4) | |
| Asian/Pacific Islander | 11 (20.8) | 13 (23.6) | |
| Native American | 1 (1.9) | – | |
| White | 14 (26.4) | 14 (25.5) | |
| Other/unknown | 1 (1.9) | 2 (3.6) | |
| Education, n (%) | |||
| <12th grade | 20 (37.7) | 21 (38.2) | 0.99 |
| 12th grade or GED | 13 (24.5) | 14 (25.5) | |
| >12th grade | 20 (37.7) | 20 (36.4) | |
| Smoker, n (%) | 21 (39.6) | 25 (45.5) | 0.60 |
| Nulliparous, n (%) | 24 (45.3) | 14 (25.5) | 0.03 |
Chi-square test for categorical variables; Mann-Whitney test for continuous variables.
All women had a Nugent score >7 when invited to enroll in the trial. At the 4-week follow-up visit after treatment, 49% of women randomized to oral metronidazole and 47% of those randomized to vaginal treatment had normal flora on vaginal gram stain (p = 0.98) (Table 2). At 8 weeks, 51% of those in the oral group and 53% in the vaginal metronidazole group had a Nugent score <3 (p = 0.95). Sixty percent of women in the oral treatment arm and 55% of those in the vaginal treatment arm achieved clinical cure at the 4-week follow-up visit (p = 0.89), although only 33% of the oral treatment group and 34% of the vaginal treatment group met the strict standard for therapeutic cure at 4 weeks (p = 1.0).
Table 2.
Microbiological, Clinical, and Therapeutic Cure Rates at Follow-Up among Women Treated with Oral vs. Intravaginal Metronidazole. Data are presented as n (%)
| Type of cure | Oral n = 55 | Vaginal n = 53 | p valuea |
|---|---|---|---|
| Gram stain score | 0.98 | ||
| 0–3 (microbiological cure) | 27 (49)b | 25 (47) | |
| 4–6 | 11 (20) | 11 (21) | |
| 7–10 | 17 (31) | 17 (32) | |
| Abnormal clinical signs | 0.89 | ||
| 0 (clinical cure) | 30/50 (60) | 27/49 (55) | |
| 1–2 | 11/50 (22) | 12/49 (25) | |
| 3–4 | 9/50 (18) | 10/49 (20) | |
| Therapeutic cure | |||
| Gram stain 0–3 and no symptoms | 17/51 (33) | 17/50 (34) | 1.000 |
Fisher's exact test.
n (%).
The bacteria most commonly detected at study entry were Gardnerella vaginalis (detected in 92 of 99, or 93% of specimens), Ureaplasma urealyticum (detected in 79 of 99, or 90% of specimens), and Prevotella spp. (detected in 67 of 75, or 79% of specimens). When treatment effect was compared for each bacterium in four categories (never present, gain of bacterial colonization, loss of bacterial colonization, persistence of bacterial colonization), no differences were seen between the treatment groups (p values not shown). However, when overall prevalence of bacteria in the whole study population was compared before and 4 weeks after treatment, significantly greater numbers of women lost rather than gained colonization with G. vaginalis (36 vs. 4, p <0.001), Mycoplasma hominis (23 vs. 6, p = 0.002), and U. urealyticum (23 vs. 8, p = 0.01) (Table 3). Conversely, fewer women lost than gained colonization with Enterococcus spp. (11 vs. 26, p = 0.02). Very few women (15 of 73, 21%) recolonized the vagina with protective, hydrogen peroxide-producing lactobacilli after treatment for BV.
Table 3.
Effect of Oral or Intravaginal Metronidazole Treatment on Vaginal Bacterial Colonization between Visit 1 (with BV) and Visit 2 (after Treatment for BV)
| Isolate | n | Never present n (%) | Gain n (%) | Loss n (%) | Persistent n (%) | p valuea |
|---|---|---|---|---|---|---|
| H2O2 positive Lactobacilli | 99 | 73 (74) | 15 (15) | 7 (7) | 4 (4) | 0.13 |
| Escherichia coli | 99 | 63 (64) | 16 (16) | 13 (13) | 7 (7) | 0.71 |
| Black Pigmented GNR | 99 | 70 (71) | 4 (4) | 11 (11) | 14 (14) | 0.12 |
| Gardnerella vaginalis | 99 | 7 (7) | 4 (4) | 36 (36) | 52 (53) | <0.001 |
| Mycoplasma hominis | 99 | 60 (61) | 6 (6) | 23 (23) | 10 (10) | 0.002 |
| Prevotella bivia | 85 | 18 (18) | 12 (12) | 24 (24) | 31 (31) | 0.07 |
| Enterococcus | 99 | 46 (46) | 26 (26) | 11 (11) | 16 (16) | 0.02 |
| Ureaplasma | 99 | 20 (20) | 8 (8) | 23 (23) | 48 (48) | 0.01 |
| Candida albicans | 99 | 67 (68) | 10 (10) | 10 (10) | 12 (12) | 1.0 |
McNemar's test for comparison of pretreatment and posttreatment prevalence.
There was no difference in the change in log10 transformed vaginal cytokine concentrations between the oral and vaginal treatment groups 4 weeks after treatment, nor was there a difference in pretreatment or posttreatment concentrations between the two treatment groups (data not shown). However, significantly more women in the vaginal treatment group had a loss of vaginal sialidase compared with the oral group (19 of 51 vs. 7 of 52, p = 0.013). There were no significant differences in baseline cytokine concentrations between women who did and did not achieve therapeutic cure of BV. Among the group of women with therapeutic cure, there was a significant decrease in the mean concentrations of IL-1β (−252 ±1927 ng/mL, p<0.001) and increase in mean SLPI (177,688 ±498,788 ng/mL, p = 0.003) after treatment (Table 4). In the group that did not achieve therapeutic cure, there were no significant changes in cytokine concentrations after treatment. Both cured and noncured groups experienced a significant decrease in the presence of sialidase: 12 of 13 women with sialidase present at baseline in the therapeutic cure group and 14 of 25 in the uncured group (p = 0.013 and p = 0.004, respectively) were negative for sialidase at follow-up. When this analysis was repeated for women who had normal flora, intermediate flora, and persistent BV after treatment, women with normal flora had a similar significant drop in IL-1β (−205 ± 1701 ng/mL, p = 0.001) and an increase in SLPI (105,195 ± 469,827 ng/mL, p = 0.03), women with intermediate flora had a significant drop only in IL-1β (−521.8 ±1141 ng/mL, p = 0.003), and women with persistent BV had no change in cytokine concentrations.
Table 4.
Comparison of Change in Vaginal Cytokine Concentrations before and after Treatment for Bacterial Vaginosis, Stratified by Treatment Successa
| Therapeutic cure (n = 32) | No cure (n = 63) | |
|---|---|---|
| IL-6, ng/mL | ||
| Mean change | −1 ± 85 (p = 0.57b) | −4.5 ± 78 (p = 0.17) |
| Median change | 0 | −1.9 |
| IL-8, ng/mL | ||
| Mean change | −7743 ± 30641 (p = 0.52) | 1604 ± 9892 (p = 0.51) |
| Median change | −107 | 54 |
| IL-1β, ng/mL | ||
| Mean change | −252 ± 1927 (p < 0.001) | −68 ± 1110 (p = 0.32) |
| Median change | −64 | −17.5 |
| SLPI, ng/mL | ||
| Mean change | 177688 ± 498788 (p = 0.003) | 935 ± 486062 (p = 0.12) |
| Median change | 91,264 | 46,614 |
| Sialidase Loss | 12/13 (p = 0.01c) | 14/25 (p = 0.004) |
Therapeutic cure is defined as gram stain score <3 and absence of any clinical signs or symptoms.
Paired t test for log-transformed values.
McNemar's test.
Discussion
Although treatment for BV during early pregnancy with vaginal or oral metronidazole is associated with a significant decrease in vaginal colonization with G. M. hominis, and U. urealyticum, cure was achieved infrequently. Thirty-four percent of women achieved the strict definition of cure for BV (normal gram stain and no clinical symptoms) when evaluated 4 weeks after treatment; when the definition is relaxed to include women with one or two clinical symptoms, however, 44% of women would be considered cured. This is lower than reported in many other trials.21,30 However, most earlier trials defined BV cure as less than three clinical symptoms. Additionally, in this study, we treated women with the CDC-recommended regimen for pregnant women at the time of the study of 250 mg oral metronidazole three times daily, which may not provide adequate antibiotic concentrations in pregnancy to eradicate BV.30 An oral metronidazole dose of 500 mg twice daily might have increased our cure rate. No difference between the oral and vaginal metronidazole treatment groups was seen in terms of cure rates or pregnancy outcomes, and both groups had preterm delivery rates of approximately 12%, in keeping with past studies.3,30,31
Microbiological and therapeutic cures were associated with a significant decrease in the vaginal concentrations of the proinflammatory cytokine IL-1β but not IL-6 or IL-8. Women who did not meet criteria for cure did not show any decrease in vaginal cytokine concentrations. IL-1β is part of the initial trigger for inflammation in the normal inflammatory response and has been correlated with increased concentrations of vaginal IL-8 and increased numbers of neutrophils.32 The decrease in IL-1β with treatment suggests that the overall inflammatory stimulus may be diminished, but the persistence of IL-8 may demonstrate ongoing inflammation that could be associated with subsequent preterm delivery and is not affected by treatment. Data about the relationship between IL-6 and BV are mixed,11,33 but elevated intra-amniotic concentrations of IL-6 are clearly associated with increased risk of preterm birth.34 In this study, however, no relationship was seen between treatment arm or response and IL-6 concentrations.
Sialidase detection decreased in women both with and without therapeutic cure and in both oral and vaginal treatment groups. This is somewhat surprising, as sialidase is thought to be the product of the bacteria associated with BV (specifically Prevotella spp. and G. vaginalis35,36), and, thus, we would expect it to decrease with cure but remain detectable if bacteria are still present. The harmful effects of sialidase are presumed to result from its ability to hydrolyze cervical mucus and remove a barrier to bacterial colonization of the upper genital tract.37,38 Thus, whether or not it decreases after treatment, the damage may already be done.
Concentrations of the protective enzyme SLPI increased by 0.2 logs in women who achieved therapeutic cure. SLPI has been associated with a decreased risk of HIV acquisition39 and is thought to act as an anti-inflammatory molecule. In previous studies, SLPI has been associated with the presence of a normal vaginal gram stain,40,41 but it is unclear whether the presence of lactobacilli promotes SLPI production or the presence of BV-associated pathogens inhibits SLPI. Results from this study do not offer a definitive answer but suggest the latter, as SLPI concentrations rose even in the absence of Lactobacillus recolonization. Treatment did not promote colonization with hydrogen peroxide-producing lactobacilli: only 17% of women without lactobacilli present at baseline were colonized at follow-up. This suggests that additional measures may be necessary to completely restore a healthy vaginal environment.
Women in our study used a vaginal placebo gel, which was designed to be inert. However, given the results of studies in the vaginal microbicide field showing anti-inflammatory properties of simple gel preparations,42 there may actually have been an effect of the vaginal placebo that diminished any differences between the two groups. Additional limitations of this study include its small size, as the trial was halted early for futility reasons.
Conclusions
This study offers additional insight into the mechanisms of how BV may increase the risk of preterm delivery, combining measurements of bacterial colonization, proinflammatory cytokines, sialidase, and the protective compound SLPI. The results suggest, as many have hypothesized, that despite the clinical presentation, BV is associated with inflammation that does not necessarily resolve after treatment of the bacterial infection.
Acknowledgments
We thank Jan Aura, ARNP, for her work in managing the study and collecting samples from patients.
This study was supported by ASPH/CDC/ASTDR S1179 and S2239. 3M Pharmaceuticals donated vaginal Metrogel and the vaginal placebo compound. C.M. is supported by NICHD HD-01-264, Women's Reproductive Health Research Career Development Award. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NICHD.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Allsworth JE. Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–120. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 2.Hillier SL. Nugent RP. Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 28. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 3.Carey JC. Klebanoff MA. Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 4.Kekki M. Kurki T. Pelkonen J. Kurkinen-Raty M. Cacciatore B. Paavonen J. Vaginal clindamycin in preventing preterm birth and peripartal infections in asymptomatic women with bacterial vaginosis: A randomized, controlled trial. Obstet Gynecol. 2001;97:643–648. doi: 10.1016/s0029-7844(01)01321-7. [DOI] [PubMed] [Google Scholar]
- 5.Leitich H. Brunbauer M. Bodner-Adler B. Kaider A. Egarter C. Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: A meta-analysis. Am J Obstet Gynecol. 2003;188:752–758. doi: 10.1067/mob.2003.167. [DOI] [PubMed] [Google Scholar]
- 6.Romero R. Espinoza J. Goncalves LF. Kusanovic JP. Friel L. Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravett MG. Hummel D. Eschenbach DA. Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67:229–237. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL. Krohn MA. Cassen E. Easterling TR. Rabe LK. Eschenbach DA. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clin Infect Dis. 1995;20(Suppl 2):S276–278. doi: 10.1093/clinids/20.supplement_2.s276. [DOI] [PubMed] [Google Scholar]
- 9.Basso B. Gimenez F. Lopez C. IL-1beta, IL-6 and IL-8 levels in gyneco-obstetric infections. Infect Dis Obstet Gynecol. 2005;13:207–211. doi: 10.1080/10647440500240664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedges SR. Barrientes F. Desmond RA. Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193:556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 11.Losikoff P. Fichorova R. Snyder B, et al. Genital tract interleukin-8 but not interleukin-1beta or interleukin-6 concentration is associated with bacterial vaginosis and its clearance in HIV-infected and HIV-uninfected women. Infect Dis Obstet Gynecol. 2007 doi: 10.1155/2007/92307. ;2007:Article ID 92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigi RH. Yudin MH. Cosentino L. Meyn LA. Hillier SL. Cytokines, pregnancy, and bacterial vaginosis: Comparison of levels of cervical cytokines in pregnant and nonpregnant women with bacterial vaginosis. J Infect Dis. 2007;196:1355–1360. doi: 10.1086/521628. [DOI] [PubMed] [Google Scholar]
- 13.Genc MR. Witkin SS. Delaney ML, et al. A disproportionate increase in IL-1beta over IL-1ra in the cervicovaginal secretions of pregnant women with altered vaginal microflora correlates with preterm birth. Am J Obstet Gynecol. 2004;190:1191–1197. doi: 10.1016/j.ajog.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez Bosquet E. Ferrer I. Valls C. Borras M. Lailla JM. The value of interleukin-8, interleukin-6 and interleukin-1beta in vaginal wash as predictors of preterm delivery. Gynecol Obstet Invest. 2005;59:175–178. doi: 10.1159/000084279. [DOI] [PubMed] [Google Scholar]
- 15.Cauci S. Hitti J. Noonan C, et al. Vaginal hydrolytic enzymes, immunoglobulin A against Gardnerella vaginalis toxin, and risk of early preterm birth among women in preterm labor with bacterial vaginosis or intermediate flora. Am J Obstet Gynecol. 2002;187:877–881. doi: 10.1067/mob.2002.127454. [DOI] [PubMed] [Google Scholar]
- 16.Abe T. Kobayashi N. Yoshimura K, et al. Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J Clin Invest. 1991;87:2207–2215. doi: 10.1172/JCI115255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogelmeier C. Hubbard RC. Fells GA, et al. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest. 1991;87:482–488. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draper DL. Landers DV. Krohn MA. Hillier SL. Wiesenfeld HC. Heine RP. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am J Obstet Gynecol. 2000;183:1243–1248. doi: 10.1067/mob.2000.107383. [DOI] [PubMed] [Google Scholar]
- 19.Bistoletti P. Fredricsson B. Hagstrom B. Nord CE. Comparison of oral and vaginal metronidazole therapy for nonspecific bacterial vaginosis. Gynecol Obstet Invest. 1986;21:144–149. doi: 10.1159/000298944. [DOI] [PubMed] [Google Scholar]
- 20.Ferris DG. Litaker MS. Woodward L. Mathis D. Hendrich J. Treatment of bacterial vaginosis: A comparison of oral metronidazole, metronidazole vaginal gel, and clindamycin vaginal cream. J Fam Pract. 1995;41:443–449. [PubMed] [Google Scholar]
- 21.Koumans EH. Markowitz LE. Hogan V. Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: A synthesis of data. Clin Infect Dis. 2002;35(Suppl 2):S152–172. doi: 10.1086/342103. [DOI] [PubMed] [Google Scholar]
- 22.Administration USFad. Guidance for industry. www.fda.gov/cder/guidance/2572dft.pdf. [May 26;2008 ];Bacterial vaginosis—Developing antimicrobial drugs for treatement.
- 23.Nugent RP. Krohn MA. Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amsel R. Totten PA. Spiegel CA. Chen KC. Eschenbach D. Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 25.Eschenbach DA. Thwin SS. Patton DL, et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30:901–907. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 26.Hillier SL. Martius J. Krohn M. Kiviat N. Holmes KK. Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 27.Agnew KJ. Aura J. Nunez N, et al. Effect of semen on vaginal fluid cytokines and secretory leukocyte protease inhibitor. Infect Dis Obstet Gynecol. 2008;2008:820–845. doi: 10.1155/2008/820845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlsson K. Bjartell A. Lilja H. Secretory leucocyte protease inhibitor in the male genital tract: PSA-induced proteolytic processing in human semen and tissue localization. J Androl. 1995;16:64–74. [PubMed] [Google Scholar]
- 29.Williams MA. Mahomed K. Farrand A, et al. Plasma tumor necrosis factor-alpha soluble receptor p55 (sTNFp55) concentrations in eclamptic, preeclamptic and normotensive pregnant Zimbabwean women. J Reprod Immunol. 1998;40:159–173. doi: 10.1016/s0165-0378(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 30.Yudin MH. Landers DV. Meyn L. Hillier SL. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: A randomized trial. Obstet Gynecol. 2003;102:527–534. doi: 10.1016/s0029-7844(03)00566-0. [DOI] [PubMed] [Google Scholar]
- 31.Hanson JM. McGregor JA. Hillier SL, et al. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. J Reprod Med. 2000;45:889–896. [PubMed] [Google Scholar]
- 32.Cauci S. Guaschino S. De Aloysio D, et al. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003;9:53–58. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 33.Sawada M. Otsuki K. Mitsukawa K. Yakuwa K. Nagatsuka M. Okai T. Cervical inflammatory cytokines and other markers in the cervical mucus of pregnant women with lower genital tract infection. Int J Gynaecol Obstet. 2006;92:117–121. doi: 10.1016/j.ijgo.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Menon R. Camargo MC. Thorsen P. Lombardi SJ. Fortunato SJ. Amniotic fluid interleukin-6 increase is an indicator of spontaneous preterm birth in white but not black Americans. Am J Obstet Gynecol. 2008;198(1):71.e1–7. doi: 10.1016/j.ajog.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 35.Briselden AM. Moncla BJ. Stevens CE. Hillier SL. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J Clin Microbiol. 1992;30:663–666. doi: 10.1128/jcm.30.3.663-666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGregor JA. French JI. Jones W, et al. Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: Results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol. 1994;170:1048–1059. doi: 10.1016/s0002-9378(94)70098-2. discussion 1059–1060. [DOI] [PubMed] [Google Scholar]
- 37.Wiggins R. Hicks SJ. Soothill PW. Millar MR. Corfield AP. Mucinases and sialidases: Their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex Transm Infect. 2001;77:402–408. doi: 10.1136/sti.77.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olmsted SS. Meyn LA. Rohan LC. Hillier SL. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex Transm Dis. 2003;30:257–261. doi: 10.1097/00007435-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Wahl SM. McNeely TB. Janoff EN, et al. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 1997;3(Suppl 1):S64–69. doi: 10.1111/j.1601-0825.1997.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 40.Taggart CC. Greene CM. McElvaney NG. O'Neill S. Secretory leucoprotease inhibitor prevents lipopolysaccharide-induced IkappaBalpha degradation without affecting phosphorylation or ubiquitination. J Biol Chem. 2002;277:33648–33653. doi: 10.1074/jbc.M203710200. [DOI] [PubMed] [Google Scholar]
- 41.Weldon S. McGarry N. Taggart CC. McElvaney NG. The role of secretory leucoprotease inhibitor in the resolution of inflammatory responses. Biochem Soc Trans. 2007;35:273–276. doi: 10.1042/BST0350273. [DOI] [PubMed] [Google Scholar]
- 42.Bollen LJ. Blanchard K. Kilmarx PH, et al. No increase in cervicovaginal proinflammatory cytokines after Carraguard use in a placebo-controlled randomized clinical trial. J Acquir Immune Defic Syndr. 2008;47:253–257. doi: 10.1097/QAI.0b013e31815d2f12. [DOI] [PubMed] [Google Scholar]