Abstract
Traumatic brain injury (TBI) is a major cause of death and disability worldwide. It causes progressive tissue atrophy and consequent neurological dysfunctions. TBI is accompanied by neuroinflammation, a process mediated largely by microglia. CD38 is an ectoenzyme that promotes transmembrane signaling via the synthesis of potent calcium mobilizing agents or via its receptor activity. CD38 is expressed in the brain in various cell types including microglia. In previous studies, we showed that CD38 regulates microglial activation and response to chemokines. In view of the important role of neuroinflammation in TBI and the effects of CD38 on microglial responses, the present study examines the role of CD38 in the recovery of mice from closed head injury (CHI), a model of focal TBI. For this purpose, CD38-deficient and wild-type (WT) mice were subjected to a similar severity of CHI and the effect of the injury on neurobehavioral and cognitive functions was assessed by the Neurological Severity Score (NSS) and the Object Recognition Test, at various time points post-injury. The results show that recovery after CHI (as indicated by the NSS) was significantly lower in CD38-deficient mice than in WT mice and that the object recognition performance after injury was significantly impaired in injured CD38-deficient mice than in WT mice. In addition, we also observed that the amount of activated microglia/macrophages at the injury site was significantly lower in CD38-deficient mice compared with WT mice. Taken together, our findings indicate that CD38 plays a beneficial role in the recovery of mice from CHI and that this effect is mediated, at least in part, via the effect of CD38 on microglia responses.
Key words: CD38, head trauma, microglia
Introduction
Inflammation is the body's defense mechanism against injury and other threats. In the central nervous system, neuroinflammation is generally beneficial and allows the brain to respond to changes in its environment and to dispose of damaged tissue or undesirable substances. However, this beneficial process sometimes gets out of balance. Uncontrolled chronic neuroinflammation is considered to play a key role in the progression of damage in a number of neurodegenerative diseases such as multiple sclerosis and Alzheimer's disease (AD) (Wyss-Coray, 2006).
CD38 is a NAD glycohydrolase and ADP-ribosyl cyclase (Howard et al., 1993). The extracellular enzymatic domain of CD38 uses nicotinamide adenine dinucleotide (NAD+) and NADP+ to catalyze the formation of cyclic adenosine diphosphoribose (cADPR), adenosine diphosphoribose (ADPR), and nicotinic acid adenine dinucleotide (NAADP+) (Schuber and Lund, 2004). These three metabolites function as signaling molecules either by mobilizing calcium from intracellular stores or by activating calcium entry from the extracellular space (Lee, 2004). Accordingly, cADPR and NAADP induce Ca2+ release from intracellular stores by activation of ryanodine receptors (Dammermann and Guse, 2005; Guse, 2004), notably, a novel NAADP receptor has also been suggested. ADPR binds to the TRPM2 cation channel and facilitates Ca2+ and Na+ influx through this channel (Perraud et al., 2001). Notably, CD38 can also act as a cell-surface receptor capable of initiating a signal transduction cascade affecting cell activation, proliferation, and viability in immune cells. (Deaglio et al., 2001; Frasca et al., 2006; Gregorini et al., 2006; Kumagai et al., 1995; Lee, 2000; Lund et al., 1998, 1999, 2006; Silvennoinen et al., 1996). The CD38 ligand in humans is CD31 (Deaglio et al., 2001), whereas in mice it has not yet been identified. CD38 is believed to be physically and functionally linked to supramolecular signaling complexes in the T, B, NK, and myeloid lineages, where according to the complex, cell lineage, differentiation steps, and microenvironment promotes diverse signal cascades (Malavasi et al., 2008). CD38 is expressed in various tissues and cell types (Ceni et al., 2003; Malavasi et al., 2008). Among these cells are hematopoietic-derived cells such as monocytes, dendritic cells (DCs), lymphocytes, and microglia (Franco et al., 2006; Mayo and Stein, 2007) as well as neural-derived cells such as astrocytes and subsets of neurons (Mizuguchi et al., 1995; Yamada et al., 1997). CD38 plays an important role in inflammatory responses, as indicated by attenuated innate and adaptive immune responses of CD38-deficient mice to inflammatory agents and immunogens (Partida-Sanchez et al., 2003, 2004) and their enhanced susceptibly to bacterial infections (Partida-Sanchez et al., 2001). In addition, CD38 regulates microglial responses. Accordingly, it promotes microglial activation and activation-induced cell death (Mayo et al., 2008; Mayo and Stein, 2007) and regulates microglial chemotactic responses (Partida-Sanchez et al., 2004), effects that were mediated via its cADPR metabolite. Since microglia are essential components of neuroinflammation, these results suggest that CD38 may regulate the brain's susceptibility to injuries that are associated with neuroinflammation, such as traumatic brain injury (TBI). Microglia activation and macrophage accumulation are known to follow TBI and to contribute to the post-injury inflammation (Aihara et al., 1995; Morganti-Kossmann et al., 2002; Shein et al., 2008). These microglia/macrophages are derived from resident microglia or bone marrow derived cells (Soulet and Rivest, 2008). Activated microglia transform their morphology, up-regulate certain membrane proteins and express different cytokines and growth factors, which play important roles in the inflammatory processes of TBI (Garden and Moller, 2006; Hanisch and Kettenmann, 2007; Kreutzberg, 1996; Shein et al., 2008). TBI is a major cause of mortality and morbidity in the 15–24-year age group in the Western world (Waxweiler et al., 1995) and is thought to be an environmental risk factor for AD (Guo et al., 2000; Lye and Shores, 2000; Plassman et al., 2000).
The present study examines whether CD38 regulates the brain's susceptibility to TBI using the closed head injury (CHI) model in mice (Chen et al., 1996). CHI is a well-established model of focal TBI, which was shown to trigger a cascade of events that lead to delayed tissue edema, neuroinflammation, impaired neurological function, and cell death (Grosjean et al., 2007; Nadler et al., 2008; Otto et al., 2001; Schmidt et al., 2005; Shapira et al., 1988; Shohami et al., 1997; Stahel et al., 2000).
The effect of CD38 on the brain's susceptibility to CHI was studied in CD38-deficient mice. Our results show that the neurological recovery of the CD38-deficient mice from the injury was impaired and that this impairment was accompanied by a reduced amount of activated microglia in the vicinity of the injured area.
Methods
Animals and reagents
BALB/c CD38-deficient mice (Cd38−/−) (Cockayne et al., 1998; Krebs et al., 2005) were obtained from the Trudeau Institute Breeding Facility (Saranac Lake, NY). Wild-type BALB/c mice were purchased from Harlan (Jerusalem, Israel). Mice were maintained in accordance with all applicable rules and guidelines of the Animal Care and Use Committee of Tel Aviv University.
Antibodies
The Abs used for detecting activated microglia/macrophages were Mac-2, produced from hybridoma (ATCC TIB 166, M3/38.1.2.8.HL.2), kindly provided by Professor I. Witz, Tel-Aviv University, and the rat anti-mouse F4/80 monoclonal antibody (mAb; MCA497; Serotec, Raleigh, NC). Rabbit anti-glial fibrillary acidic protein (GFAP) polyclonal Abs (Z 0334; Dako Cytomation; Carpinteria, CA) was used for detecting astrocytes, and a biotin-conjugated mouse mAb raised against neuronal nuclei (NeuN) (MAB 377B, Chemicon) was used to detect neurons. The secondary Abs used for 3,3′-diaminobenzidine (DAB) chromogenic staining were as follows: biotin-conjugated rabbit anti-rat diluted 1:500 (for Mac-2 staining) or 1:250 (for F4/80 staining; BA-4001; Vector Laboratories, Burlingame, CA) and biotin-conjugated goat anti-rabbit antibody diluted 1:200 (BA-1000; Vector Laboratories).
Closed head injury
The CHI study was conducted according to the National Institutes of Health (NIH) Guidelines for Use and Care of Laboratory Animals, and was approved by the Animal Care Committee of the Hebrew University of Jerusalem. Four-month-old male Cd38−/− mice and BALB/c (wild-type [WT]) mice were used in this study. Experimental CHI was induced under isoflurane anesthesia using a modified weight-drop device (Chen et al., 1996; Yatsiv et al., 2002). Briefly, following anesthesia, a midline longitudinal incision was performed and the skull was exposed. A Teflon-tipped cone (2 mm diameter) was placed 1 mm lateral to the midline in the mid-coronal plane. The head was manually held in place, and a 95-g weight was dropped on the cone from a height that was adjusted to yield a moderate trauma (Neurological Severity Score [NSS] of 6—7) to the left hemisphere. The severity of the trauma was confirmed 1 h later by assessing the NSS values. After recovery from anesthesia, the mice were returned to their home cages with postoperative care and ad libitum access to food and water. WT and Cd38−/− mice (81 and 68, respectively) were subjected to CHI; afterwards, the NSS values of each mouse were determined at different time points. Fractions of the injured mice were scarified at day 1, 3, and 7 post-injury for immunohistochemical analysis. Six different experiments were performed. In three experiments, the NSS was determined at 1 h and 1, 3, 7, 14, 21, and 28 days post- injury. In two experiments, the NSS was determined at 1 h and 1, 2, 3, 7, 14, 21, and 28 days post-injury. In one experiment, NSS was analyzed at 1 h and 1, 2, and 28 days post-injury. Accordingly, the total number of mice assessed for NSS at each of the indicated time points was as follows: WT, 81 at 1 h, 80 at day 1, 42 at 2 days, 59 at 3 days, 29 at 7 days, 23 at 14 days, 23 at 21 days, and 35 at 28 days; Cd38−/−, 68 at 1 h, 66 at day 1, 35 at day 2, 48 at day 3, 22 at day 7, 17 at day 14, 17 at day 21, and 25 at day 28. The number of WT and Cd38−/− mice scarified for the immunohistochemical analysis was 9 and 8 at day 1, 19 and 17 at day 3, and 6 and 5 at day 7 post-injury, respectively. These mice were anesthetized with a mixture of ketamine (250 mg/kg; Ketaset, Fort Dodge, IA) and xylazine (2%; in a ratio of 0.85:0.15, respectively), and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Next, the brains were removed and placed for further fixation in 4% paraformaldehyde for 18 h at 4°C and placed in 30% sucrose for 48 h. Frozen coronal sections (30 μm) were then cut on a sliding microtome and collected serially.
Immunohistochemistry
The sections were immunostained free-floating as described (Matsumori et al., 2006), utilizing the following primary Abs: anti-NeuN mAb (1:500), anti-F4/80 mAb (1:100), anti-Mac-2 mAb (1:300), and anti-GFAP Ab (1:1000). Following a 1-h blocking step in primary Abs dilution buffer (Biomeda Corp., Foster City, CA), primary Abs were applied overnight at 4°C. After having been rinsed in phosphate-buffered saline (PBS) with 0.1% Triton X-100, the bound primary Abs were visualized with the aid of the appropriate biotinylated secondary Abs and horseradish peroxidase (HRP)-conjugated streptavidin (Vectastain Elite ABC Kit (Standard); Vector Laboratories).
Image analysis
The peroxidase-immunostained sections were viewed and photographed with either a Nikon plan ×4/0.10 NA or a Nikon plan ×10/0.25 AN objective and a Nikon DS-5M camera (Nikon Instech, Tokyo, Japan). The density of immunohistochemical staining was determined with the aid of the Image-Pro Plus system (version 5.1; Media Cybernetics, Silver Spring, MD) by expressing the amount of staining as a percentage area stained. Moderate adjustments for contrast and brightness were done after quantification on the images shown in Figures 3–5 below. Notably, equal adjustments were applied to the corresponding WT and Cd38−/− images. For quantifying the F4/80 and Mac-2 staining, for each mouse, images were taken with Nikon plan ×10/0.25 AN objective from four brain sections (spacing approximately 200 micron) containing the area of maximal cavitation (approximately bregma 1.1 to −0.1). The images were taken frame-by-frame in such a way that they covered without overlapping all the surrounding of the cavity. The mean cavity area in the sections of WT or Cd38−/− mice was similar. Three to five images (dependent on the size of the cavity) from the areas surrounding to the cavity were captured in each section. The density of the stained cells was calculated in each of these regions as the percentage area that was stained. For quantitating the GFAP staining, seven brain sections (spacing approximately 200 micron) containing the area of maximal cavitation (approximately bregma 1.1 to −0.1) of each mouse were analyzed. Three to five (depending on the size of the hemisphere) non-overlapping images were taken at a × 4 magnification (Nikon plan × 4/0.10 NA objective), covering the entire injured hemisphere. For each slice, the amount of staining in the injured hemisphere (the sum of staining measured in all the corresponding images) was expressed relative to the area of the injured hemisphere (the sum of the areas covered by all the corresponding images). To prevent overlapping fields, in each image we defined a specific region at the edge of the image as a point of reference. The next image was taken immediately after this marked area. When images were taken at the edge of the brain (and thus contained also white space), the analysis was performed only on the part which contained brain tissue. For quantitating the NeuN staining, three to five brain sections, taken from bregma −1.46 to −2.06 of each mouse, were analyzed. Images were captured with Nikon plan ×10/0.25 AN objective from the hippocampus of the damaged and contralateral hemispheres. The amount of staining in the area of the hippocampus, confined by a line connecting both ends of the dentate gyrus and extending towards the pyramidal cell layer of the hippocampus (CA2/3), was determined. The staining density was determined by expressing the amount of staining as a percentage area stained. The sections of all mice were coded, and all of the sections of each marker, for each time point post-injury, were stained simultaneously.
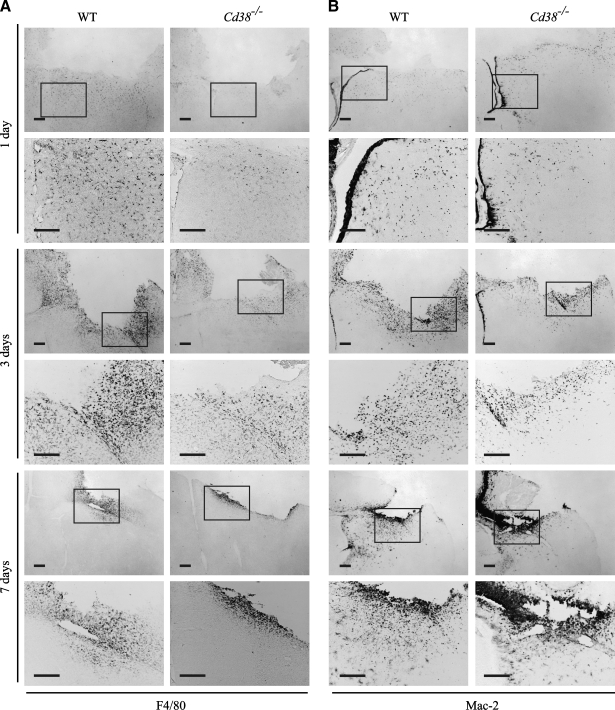
FIG. 3.
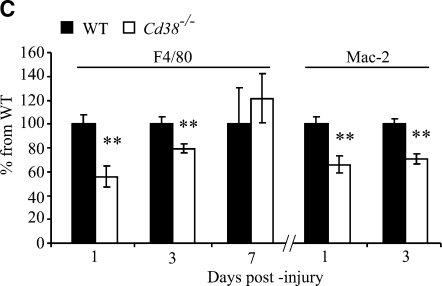
The amount of activated microglia at the injury site is lower in Cd38−/− mice than in wild-type (WT) mice at 1 day and 3 days post-injury. Frozen brain sections, prepared from WT and Cd38−/− mice subjected to closed head injury (CHI) and killed 1, 3, and 7 days post-injury, were immunohistochemically stained with the anti-F4/80 or anti-Mac-2 monoclonal antibody (mAb), as described in the text. Representative images are shown for anti-F4/80 (A) and Mac-2 (B) staining, respectively. The images shown are low-magnification (upper) and high magnification (lower) of the same brain area. The region that was magnified is marked in the upper panel by a rectangle. Scale bar = 250 μm. (C) Quantitation of the density of the F4/80 and the Mac-2-positive cells in the injury boundary. This was performed by computerized densitometry as described in the text. At each time point, the density value obtained for each section (WT and Cd38−/−) was normalized to the average density value of the corresponding WT sections. Images used for the analysis were obtained from coronal sections at bregma 1.1 and −0.1. The data presented are expressed as the means ± standard error of the mean (SEM; bars) of the normalized values from two different experiments (n = 8, 11, and 6; WT) or 7, 10, and 5 (Cd38−/−), at 1, 3, and 7 days postinjury, respectively) **p < 0.01 compared with the corresponding WT (Student's t-test).
FIG. 5.
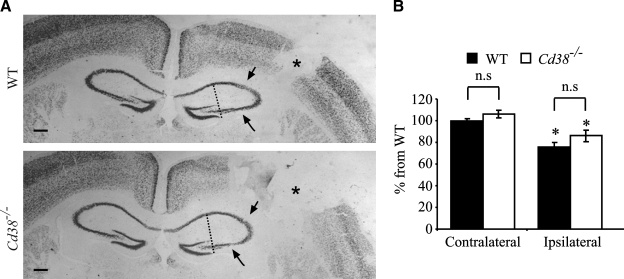
Analysis of neuronal loss in the hippocampal CA2 and CA3 regions. Frozen brain sections, prepared from wild-type (WT) and Cd38−/− mice subjected to closed head injury (CHI) and killed 7 days post-injury, were immunohistochemically stained with the anti–neuronal nuclei (NeuN) monoclonal antibody (mAb), as described in the text. (A) Representative images of NeuN staining of WT and Cd38−/− brain sections are shown. Scale bar = 250 μm. The CA2/CA3 region was defined as the region included between an imaginary line drawn from one tip of the dentate gyrus to the other toward the pyramidal neurons. Arrows indicate areas of neuronal loss, and asterisks indicate the cavity. (B) Computerized densitometric analysis of the density of NeuN staining of the hippocampal CA2/CA3 neurons. This was performed by computerized densitometry, as described in the text. Density values were obtained for the ipsilateral (injured) and contralateral hemispheres. The density value obtained for each section (WT and Cd38−/−) was normalized to the average density value of the contralateral WT sections. Images used for the analysis were obtained from coronal sections at bregma 1.46 and −2.06. The data presented are expressed as means ± standard error of the mean (SEM; bars) of the normalized values from two different experiments (12 WT and 13 Cd38−/− mice. *p < 0.05 compared with the corresponding contralateral value (Student's t-test). No significant difference (n.s.) was observed between the WT and Cd38−/− in the contralateral and ipsilateral.
Neurobehavioral evaluation
The neurological status of the mice was evaluated according to the NSS, by an observer who was unaware of the differences between the mice. This score is a 10-point scale that assesses the functional neurological status based on the presence of some reflexes and the ability to perform motor and behavioral tasks such as beam walking, beam balance, and spontaneous locomotion (Beni-Adani et al., 2001; Tsenter et al., 2008). Animals are awarded one point for failure to perform a task, such that scores ranged from 0 to 10, increasing with the severity of dysfunction. The NSS obtained 1 h following CHI reflects the initial severity of injury. Therefore, the extent of recovery can be calculated as the difference between the NSS at 1 h and at any subsequent time point (ΔNSS).
Object Recognition Test
We used the procedure described by Ennaceur and Delacour (1988) and adapted to mice as described (Biegon et al., 2004; Tsenter et al., 2008). At 24 h before testing, WT and Cd38−/− mice were allowed to explore the testing box for 1 h to reduce neophobic responses and habituate to the stimuli present in the empty arena. Then, in the first trial, two identical objects were placed in the right and left corners of the box. Mice were placed into the box for 5 min, where they explored the two objects, and the exploratory activity (i.e., time spent in object-directed exploration) was recorded manually. Four hours later, mice were re-introduced for 5 min to the same cage, in which one of the objects was replaced by a new one. The cumulative time spent by the mouse exploring each of the objects was recorded. Exploration of an object was defined as follows: directing the nose to the object at a distance of ≤2 cm and/or touching it with the nose; turning around or sitting on the object was not considered as exploratory behavior. The test was performed on days 3, 6, 14, 23, and 29, following the CHI. Mice were assessed by a single observer. Memory was operationally defined by the discrimination ratio for the novel object (DIR), as the proportion of time the animals spent investigating the novel object minus the proportion spent investigating the familiar one during the testing period [Discrimination Ratio, DIR = (Novel Object Exploration Time – Familiar Object Exploration Time) / Total Exploration Time) × 100]. Two experiments were conducted. In the first (n = 9), the test was done before the trauma, and then at days 3 and 6 after trauma. In the second (n = 7–10), the test was done before the trauma, and then at days 3, 14, 23, and 29 after trauma. Accordingly, the total number of mice assessed for the Object Recognition Test (ORT) at each of the indicated time points was as follows: WT, 19 naive mice and 19, 9, 10, 8, and 8 injured mice at 3, 6, 14, 23, and 29 days post-injury, respectively; Cd38−/−, 19 naive mice and 17, 9, 9, 7, and 7 injured mice at 3, 6, 14, 23, and 29 days post-injury, respectively.
Statistical analysis
Statistical evaluation was performed by Mixed Models analysis (for the NSS, ΔNSS, and ORT), using the SPSS software (SPSS Inc., Chicago, IL) followed by Bonferroni corrected Student's t-test, or by the two-tailed Student's t-test (for the analysis of F4/80, Mac-2, GFAP or NeuN staining) using the Statistica software (StatSoft Pacific, Australia). Data was expressed as mean value ± standard error of the mean (SEM). A value of p < 0.05 was considered to be statistically significant.
Results
Effect of closed head injury on neurological functions
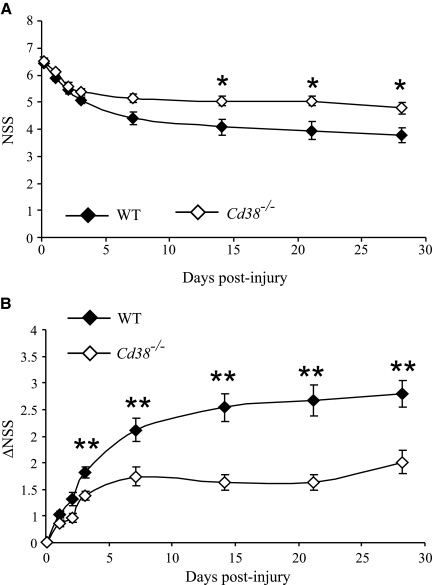
WT and Cd38−/− mice were subjected to CHI. The neurological status of the mice was evaluated using the NSS scoring system (which tests motor and behavioral functions) at 1 h, and 1, 2, 3, 7, 14, 21, and 28 days following trauma. All the WT and Cd38−/− mice exhibited moderate neurological deficits 1 h after the trauma, with a group mean NSS of 6.76 ± 0.08 and 6.82 ± 0.09 for WT and Cd38−/− mice, respectively, indicating a similar initial severity of injury (Tsenter et al., 2008). Evaluation of the NSS values at later time points revealed that the neurological status of both WT and Cd38−/− mice improved over time (a decrease in the NSS values). The improvement effect reached a plateau after 7–14 days post-trauma in both genotypes; however, the improvement was lower in the Cd38−/− mice compared to WT (38% in WT versus 20% in Cd38−/− at plateau; Fig. 1A). Mixed Models analysis of the NSS values revealed a significant effect of treatment (time) and group × treatment (genotype × time; p < 0.001). Further analysis revealed a significant difference between the WT and Cd38−/− mice at 14, 21, and 28 days post-injury (Bonferroni corrected Student's t-test; p < 0.017). Assessment of the extent of recovery, as reflected by ΔNSS, revealed an increase in ΔNSS values over time, which reached a plateau after 7–14 days in both genotypes; however, the ΔNSS values of the Cd38−/− mice at plateau were lower than those of WT mice (Fig. 1B). Mixed Models analysis of the ΔNSS values revealed a significant effect of time and genotype × time (p < 0.001). Further analysis revealed significant differences between the WT and Cd38−/− mice at 3, 7, 14, 21, and 28 days post-injury (Bonferroni corrected Student's t-test; p < 0.004). These results thus suggest that CD38 deficiency leads to impairment in the neurological recovery following CHI.
FIG. 1.
CD38 deficiency worsens post-traumatic neurological recovery. Wild-type (WT) and Cd38−/− mice were subjected to closed head injury (CHI) as described in the text, and their neurological performance was evaluated at the indicated time points by the Neurological Severity Score (NSS). The NSS (A) and the ΔNSS (B) data are expressed as mean score ± standard error of the mean (SEM). Mixed Models analysis of the NSS and ΔNSS results reveled a significant effect of time (p < 0.001) and genotype × time (p < 0.001). *p < 0.017, **p < 0.004 a significant difference between WT and Cd38−/− mice (Bonferroni corrected Student's t-test).
Post-traumatic effect on recognition memory
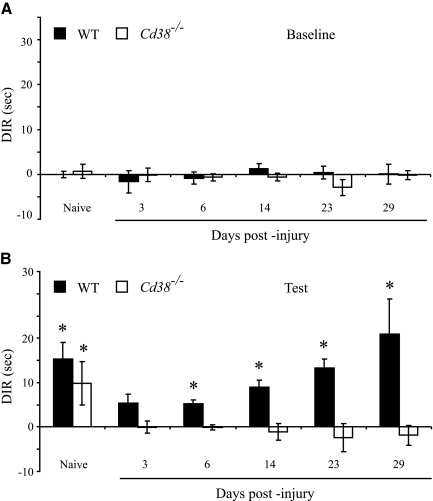
The effect of CD38 deficiency on post-traumatic recognition memory of WT or Cd38−/− mice was tested using the ORT paradigm. Recognition memory was measured in the naive and the injured mice, the latter from day 3 post-injury until day 29. The time spent by the mice exploring two identical reference objects on the testing day (baseline recognition) was first assessed, and then, 4 h later, one of the objects was replaced and the time that the mice spent exploring the familiar or the new objects was assessed (test). Figure 2 depicts the DIR between the two objects in the field (Arque et al., 2008), namely, the difference in exploration time between two identical objects (baseline; A) or between a new and the familiar objects (test; B), divided by the total time that the mouse spent exploring the two objects in the test. Analysis of the baseline DIR values (Fig. 2A) revealed that all animals tested, i.e., naive WT and Cd38−/− or injured WT and Cd38−/−, did not discriminate between the identical objects and spent about the same time at each of the objects (DIR ∼0). Statistical analysis utilizing the Mixed Models analysis of the DIR values during the test period (Fig. 2B) revealed a significant difference of group × treatment (genotype × time) between the DIR values of the two genotypes (p < 0.001). Further analysis revealed that the naive WT and Cd38−/− mice significantly discriminated (DIR > 0, t-test single sample, Bonferroni corrected; p < 0.003) between the familiar and the new objects and spent more time exploring the new object (Fig. 2B). No significant difference in the DIR values was observed between the naive WT and Cd38−/− mice (Bonferroni corrected Student's t-test). These results suggest that both WT and Cd38−/− mice exhibited a similar recognition memory before injury. At 3 days post-injury, the ability of both WT and Cd38−/− mice to discriminate between the familiar and the new objects was significantly impaired (their DIR values were not significantly different from 0; t-test single sample, Bonferroni corrected). At later time points following injury, the WT mice significantly improved their ability to discriminate between the objects (DIR > 0, t-test single sample, Bonferroni corrected; p < 0.003), whereas the Cd38−/− mice did not (the DIR values were not significantly different from 0; t-test single sample, Bonferroni corrected). Taken together, these results suggest that CD38 deficiency impaired recognition memory following a moderate CHI.
FIG. 2.
CD38 deficiency abrogates recognition memory after closed head injury (CHI). Recognition memory was evaluated using the Object Recognition Test (ORT) in naive and injured (3, 6, 14, 23, and 29 days after injury) wild-type (WT) and Cd38−/− mice as described in the text. For each mouse in the different groups, the absolute time spent exploring each of the two objects was recorded, and the differences between the exploration time of the two objects were calculated, first, with identical objects (baseline; A) and then, 4 h later (test; B), with one of the objects replaced by a new one. The results are expressed as discrimination ratio (DIR) values as described in the text. Values shown are means ± standard error of the mean (SEM; bars). Statistical analysis (Mixed Models analysis) of the DIR values of the “Test” results revealed a significant difference of genotype × time (p < 0.001) between the WT and Cd38−/− groups. *p < 0.003, DIR > 0, t-test single sample, Bonferroni corrected.
Effect of CD38 on the appearance of activated microglia/macrophages in the vicinity of the cavity
One of the hallmarks of CHI is the generation of a cavitation lesion. This lesion is surrounded by dense gliosis comprising mainly activated microglia/macrophages in the immediate vicinity of the impact (Erlich et al., 2007). These activated microglia were thought to participate in the post-injury recovery process (Akiyama et al., 1994; Kreutzberg, 1996; Stahel et al., 2000). Since our previous in vitro studies showed that microglial activation is impaired in CD38-deficient microglia (Mayo et al., 2008), we were interested in examining the effect of CD38 deficiency on the appearance of activated microglia at the cavity boundary zone. To this end, WT and Cd38−/− mice were subjected to CHI, and frozen coronal brain sections were prepared at 1, 3, and 7 days post-injury. Activated microglia/macrophages were detected by immunohistochemical staining for the activated microglia markers F4/80 and Mac-2. Figure 3A,B shows that activated microglia/macrophages accumulate around the cavity both in the WT- and Cd38−/−-injured brains, and this effect is evident already at 1 day post-injury. However, close inspection of the density of activated microglia/macrophages (F4/80 or Mac-2-positive cells) around the cavity reveals that it was lower in Cd38−/− mice, compared with WT mice, at 1 and 3 days post-injury (Fig. 3A,B). Quantitation of the F4/80 or Mac-2 staining density around the cavity reveals reduction in the density of F4/80 or Mac-2-positive cells (activated microglia), in Cd38−/− brains compared with WT brains, at 1 and 3 days post-injury (44 ± 8% and 21 ± 6%, or 34 ± 7% and 29 ± 4% staining in WT and Cd38−/− mice at 1 and 3 days post-injury, respectively). At 7 days post-injury, however, no significant difference in the density of F4/80-positive cells was observed between WT and Cd38−/− mice (Fig. 3A,C). Similar results were obtained for Mac-2-positive cells (Fig. 3B); however, the intensity and the high-density staining did not allow quantitation of the Mac-2 staining at day 7. These results thus suggest that CD38 deficiency attenuates the appearance of activated microglia/macrophages at the injury site at early time points following injury.
Effect of CD38 deficiency on the appearance of activated astrocytes
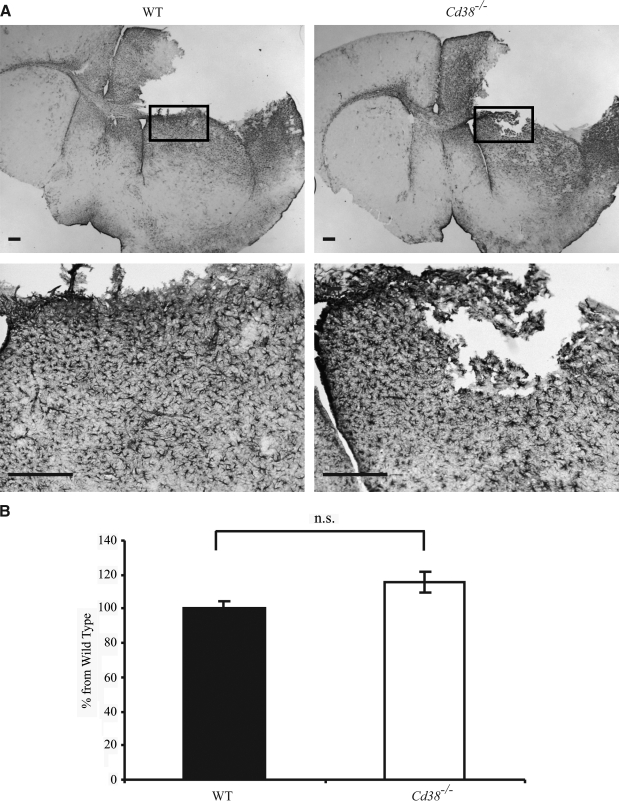
Astrocytes respond to TBI by undergoing activation, a process that is accompanied by enhanced GFAP expression, hypertrophy, and proliferation, and occurs in a gradated fashion in relation to the severity of the injury. Because activated astrocytes also affect recovery from TBI (Floyd and Lyeth, 2007; Laird et al., 2008; Myer et al., 2006), we examined the effect of CD38 expression on the appearance of activated astrocytes following CHI, by monitoring GFAP staining. As shown in Figure. 4A, massive GFAP staining was observed in both injured WT and Cd38−/− mice, at 3 days post-injury. The GFAP staining was detected in both hemispheres; however, it was mainly confined to the injured hemisphere where it was localized in various regions, predominately around the cavity. Since the activated astrocytes were not confined to distinct brain regions but were spread in various regions, the amount of GFAP staining was quantified in the entire injured hemisphere, rather than in a particular region. As shown in Figure 4B, no significant difference was observed in the amount of GFAP staining between WT and Cd38−/− mice. Similar results were obtained when GFAP expression was determined in the injured cortex by quantitative real-time reverse transcription—polymerase chain reaction (RT-PCR) at 4 and 12 h post-injury; namely, GFAP mRNA expression was similar in WT and Cd38−/− mice (data not shown).
FIG. 4.
The appearance of activated astrocytes in wild-type (WT-) and Cd38−/− -injured brains. Frozen brain sections, prepared from WT and Cd38−/− mice subjected to closed head injury (CHI) and killed 3 days post-injury, were immunohistochemically stained with the anti–glial fibrillary acidic protein (GFAP) antibodies (Abs), as described in the text. (A) Representative images of GFAP staining of WT and Cd38−/− brain sections are shown. The images shown are low (upper) and high (lower) magnifications of the same brain area. The region that was magnified is marked in the upper panel by a rectangle. Scale bar = 250 μm. (B) Quantitation of the density of the GFAP staining. This was performed by computerized densitometry, as described in the text. The density value obtained for each section (WT and Cd38−/−) was normalized to the average density value of the corresponding WT sections. Images used for the analysis were obtained from coronal sections at bregma 1.1 and −0.1. The data presented are expressed as means ± standard error of the mean (SEM; bars) of the normalized values from two different experiments (10 WT and nine Cd38−/− mice). No significant difference (Student's t-test) was observed between the WT and Cd38−/− (no significant difference [n.s.]).
Effect of CD38 deficiency on hippocampal neuronal loss
Hippocampal neurons, especially in the CA3 domain, are particularly susceptible to cell death following CHI (Chen et al., 1996). We therefore examined whether CD38 expression affects the susceptibility of the CA2 and CA3 hippocampal neurons to cell death induced by CHI. Neurons were detected by staining for the neuronal marker NeuN. As shown in Figure 5A, CHI caused a neuronal loss (a reduction in NeuN immunoreactivity) in the CA2 and CA3 hippocampal regions of the injured hemispheres in WT and Cd38−/− mice, 7 days post-injury (compared to the CA2 and CA3 regions in the non-injured contralateral hemispheres). However, quantitation of the density of the NeuN staining of these regions, in the non-injured as well as the injured hemispheres, did not reveal a significant difference between WT and Cd38−/− mice.
Discussion
We show here that CD38 deficiency impairs the ability to recovery from head trauma in mice subjected to CHI. The NSS and ORT behavioral tests were used to assess the neurological functions of the mice. The NSS measures motor ability, balancing, and alertness, and is a reliable indicator of the severity of the neurological damage (Reshef et al., 2008; Tsenter et al., 2008). Animals subjected to CHI undergo spontaneous recovery, which is indicated, inter alia, by a progressive decline in their NSS over time and is expressed by the ΔNSS values (Chen et al., 1996). Our results clearly show that the CD38-deficient mice exhibited a significantly lower ability, compared with WT mice, to improve the neurological functions, which are assessed by the NSS test.
ORT is a well-established procedure to measure a specific form of episodic memory in rodents. It is based on the spontaneous behavior of rodents to distinguish between novel and familiar objects and can be considered as a “pure” working-memory non-spatial test, completely free of reference memory components. Failure to show a preference for the novel object after a delay is interpreted as impairment in the neural storage or retrieval of the memory of the previously presented object (Ennaceur and Delacour, 1988; Ennaceur et al., 2005). Our results show that CD38 deficiency significantly impaired the cognitive performance (measured by the ORT) following TBI, suggesting that CD38 has a protective role against the effect of injury on recognition memory.
Taken together the NSS and ORT results suggest that CD38 function plays an important role in injury and repair/recovery processes following TBI.
Numerous mechanisms may account for the effect of CD38 on recovery processes. In the present study, we focused on two potential processes, i.e., neuronal loss and neuroinflammation. Neuronal loss following CHI may result from immediate cell death that occurs at the site of the lesion as well as from secondary neuronal death in parts of the brain that are not directly affected by the impact (Grossman et al., 2003; Reshef et al., 2008). Since the CA2 and CA3 regions of the hippocampus were previously shown to undergo secondary neuronal loss in CHI (Chen et al., 1996; Panikashvili et al., 2001) and ORT is partially controlled by the hippocampus (Akirav and Maroun, 2006; Broadbent et al., 2004), we examined the NeuN staining in these areas. As expected, neuronal loss was detected in these brain regions; however, no difference was observed in the density of the NeuN staining between the WT and Cd38−/− mice, suggesting that the CD38 deficiency did not enhance neuronal death in these hippocampal regions. We cannot exclude, however, the possibility that in other brain regions subtle differences do exist. Alternatively, it is possible that the effect of CD38 is not mediated by affecting the extent of neuronal death but rather, it depends on other features such as synaptic transmission or neurite sprouting in the hippocampus or other brain regions.
It is well established that neuroinflammation, via activated microglia and astrocytes, plays an important role in TBI (Allan and Rothwell, 2001; Ladeby et al., 2005; Laird et al., 2008; Morganti-Kossmann et al., 2007; Streit, 2000). Therefore, the potential role of activated microglia and astrocytes in promoting the CD38 effect was examined by assessing their appearance in the CHI-subjected WT and Cd38−/− mice. In the CHI model, activated microglia are mainly confined to the boundary of the injured cavity, whereas the distribution of reactive astrocytes is much broader (Figs. 3 and 4). Thus, activated microglia were monitored in brain areas adjacent to the injury, whereas reactive astrocytes were monitored in the entire injured hemisphere. Analysis of the GFAP staining did not reveal a significant difference in the density of GFAP staining in the injured hemisphere between WT and Cd38−/− mice. Inspection of GFAP staining in the injured hippocampus also did not reveal a difference between WT and Cd38−/− (data not shown). These results thus suggest that CD38 deficiency does not affect CHI-induced astrocyte activation, at least as indicated by GFAP expression. However, a significant and substantial reduction (∼40%) in the amount of activated microglia (as indicated by two different activated microglial/macrophages markers, F4/80 and Mac-2) was observed in Cd38−/− mice, compared with WT mice, as early as 1 day post-injury. Notably, at later time points, the extent of this difference declined until no difference was observed 7 days post-injury. These results thus suggest that CD38 expression regulates, at least in part, the amount of activated microglia/macrophages in the injured area at early time points following injury. We have previously shown that CD38 and/or its metabolite cADPR is needed for proper microglial activation (Mayo et al., 2008) and response to FPRL1-dependent chemotaxis (Partida-Sanchez et al., 2004). Therefore, the reduced amount of activated microglia/macrophages detected at the site of injury may reflect an impairment in either activation or migration (or both). The latter notion might be supported by the findings that, at 12 h post-injury, Cd38−/− mice exhibited ∼90% increase (compared with injured WT mice) in the expression (measured by qRT PCR) of the chemokine CCL2 in the injured cortex, whereas no difference was observed in the expression of the cytokines IL-1β and TNFα (data not shown). Thus, despite the enhancement of CCL2 expression in the injured area, the amount of activated microglia in this region was lower in the Cd38−/− mice, which may suggest that the microglia were impaired in their ability to be recruited to the injured site. The reason why, at 7 days post-injury, the amount of activated microglia at the site of injury was similar in WT and Cd38−/− mice is not known. A possible explanation might be that at that time point the microglial activation process (including migration) reaches its plateau in WT mice but not in Cd38−/− mice, and thus, in the latter, microglia continue to migrate into the injured site or are activated until they close the gap.
Our findings, indicating that in Cd38−/− mice accumulation of activated microglia is impaired at the site of injury, may suggest that CD38 regulates recovery from TBI, at least in part, by its effect on microglial activation. Indeed, several reports indicated that proliferating microglia have a protective effect against various types of acute brain injury such as excitotoxicity (Simard and Rivest, 2007) and ischemic injury (Lalancette-Hebert et al., 2007). In addition, it was shown that reactive microglia/macrophages can remove inhibitory tissue debris and secrete growth-promoting factors, resulting in regeneration (Streit, 2002). Note, however, that in addition to its effect on microglia, CD38 may affect other yet unknown brain functions that may also play a role in its effect on recovery from trauma. With this regard, it should be noted that it was reported that CD38 ligation can cause death of thymocytes and pro-B cells (Malavasi et al., 2008). The role of infiltrating thymocytes and B cells following TBI has not been fully established, thus the effect of such death process in the recovery from TBI is not known. However, if these infiltrating cells play a harmful effect, then an alternative interpretation of our results would be that the impaired recovery of CD38 deficient mice is also due to a reduction in such CD38-dependent cells death.
The CD38 substrate, NAD, was shown to exert toxic effects in some systems (Adriouch et al., 2007; Ying, 2008) and NAD levels are elevated in some tissues from Cd38−/− mice. Thus, it is possible that this elevated level of NAD may lead to the impaired recovery of Cd38−/− mice from CHI. Although we cannot exclude this possibility, we do not favor it, since mice deficient in either CD38 alone (Cd38−/−) or CD38 and Poly(ADP-ribose) polymerase-1 (PARP-1) (Cd38−/−/PARP-1−/−) (PARP-1 is an enzyme that uses NAD as a substrate to Poly-ADP-ribosylate proteins [Schreiber et al., 2006]) exhibited a similar reduced recovery (as indicated by NSS) from CHI, compared with WT mice (data not shown). Moreover, it was shown that NAD may promote a protective effect in acute brain injuries since intranasal administration of NAD+ decreased ischemic brain injury (Ying et al., 2007).
CD38 may exert its effects on recovery by either its enzymatic activity (via its metabolites, cADPR, ADPR, and NAADP+) or by its receptor activity. Which of these activities or metabolites actually mediates the effect of CD38 on recovery from CHI is presently not known. However, we showed previously that cADPR plays an important role in microglial activation and response to chemotaxis in vitro, whereas agonist Abs did not have any effect (Mayo et al., 2008; Partida-Sanchez et al., 2004). Thus, at least with respect to the CD38 effects that are mediated via microglia, it is reasonable to assume that these effects are mediated via the CD38 ADP-ribosyl cyclase activity.
The results presented here indicate that CD38 plays a beneficial role in recovery/repair processes following CHI. Further studies, however, are needed to determine the mechanism whereby CD38 promotes these effects, although a mechanism that acts via microglia seems to be a promising candidate. TBI is the leading cause of death and disability in young people (Waxweiler et al., 1995), yet no specific pharmacological therapy is currently available to prevent the development of secondary brain injuries. Our findings thus suggest that CD38 and its downstream targets might be useful targets and therefore need to be further investigated in order to develop new efficacious therapeutic approaches to facilitate recovery from head trauma.
Acknowledgments
This work was supported by the Israel Science Foundation (643/02 to R.S.), Israel Ministry of Science, Culture & Sport (Eshkol Foundation to L.M.), Adams Super-Center for Brain Research (to R.S.), U.S.-Israel Binational Science Foundation (2007061 to R.S.), and National Institutes of Health (R01 AI063399 to F.E.L.).
Author Disclosure Statement
The authors have no financial conflict of interest.
References
- Adriouch S. Hubert S. Pechberty S. Koch-Nolte F. Haag F. Seman M. NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J. Immunol. 2007;179:186–194. doi: 10.4049/jimmunol.179.1.186. [DOI] [PubMed] [Google Scholar]
- Aihara N. Hall J.J. Pitts L.H. Fukuda K. Noble L.J. Altered immunoexpression of microglia and macrophages after mild head injury. J. Neurotrauma. 1995;12:53–63. doi: 10.1089/neu.1995.12.53. [DOI] [PubMed] [Google Scholar]
- Akirav I. Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb. Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Akiyama H. Tooyama I. Kondo H. Ikeda K. Kimura H. McGeer E.G. McGeer P.L. Early response of brain resident microglia to kainic acid-induced hippocampal lesions. Brain Res. 1994;635:257–268. doi: 10.1016/0006-8993(94)91447-8. [DOI] [PubMed] [Google Scholar]
- Allan S.M. Rothwell N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Arque G. Fotaki V. Fernandez D. Martinez de Lagran M. Arbones M.L. Dierssen M. Impaired spatial learning strategies and novel object recognition in mice haploinsufficient for the dual specificity tyrosine-regulated kinase-1A (Dyrk1A) PLoS ONE. 2008;3:e2575. doi: 10.1371/journal.pone.0002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beni-Adani L. Gozes I. Cohen Y. Assaf Y. Steingart R.A. Brenneman D.E. Eizenberg O. Trembolver V. Shohami E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 2001;296:57–63. [PubMed] [Google Scholar]
- Biegon A. Fry P.A. Paden C.M. Alexandrovich A. Tsenter J. Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: implications for treatment of neurological and cognitive deficits. Proc. Natl. Acad. Sci. USA. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent N.J. Squire L.R. Clark R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceni C. Pochon N. Brun V. Muller-Steffner H. Andrieux A. Grunwald D. Schuber F. De Waard M. Lund F. Villaz M. Moutin M.J. CD38-dependent ADP-ribosyl cyclase activity in developing and adult mouse brain. Biochem. J. 2003;370:175–183. doi: 10.1042/BJ20020604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Constantini S. Trembovler V. Weinstock M. Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma. 1996;13:557–568. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- Cockayne D.A. Muchamuel T. Grimaldi J.C. Muller-Steffner H. Randall T.D. Lund F.E. Murray R. Schuber F. Howard M.C. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood. 1998;92:1324–1333. [PubMed] [Google Scholar]
- Dammermann W. Guse A.H. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J. Biol. Chem. 2005;280:21394–21399. doi: 10.1074/jbc.M413085200. [DOI] [PubMed] [Google Scholar]
- Deaglio S. Mehta K. Malavasi F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leuk. Res. 2001;25:1–12. doi: 10.1016/s0145-2126(00)00093-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. Delacour J. A new one-trial test for neurobiological studies of memory in rats1: Behavioral data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. Michalikova S. Bradford A. Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav. Brain Res. 2005;159:247–266. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Erlich S. Alexandrovich A. Shohami E. Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol. Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Floyd C.L. Lyeth B.G. Astroglia: important mediators of traumatic brain injury. Prog. Brain Res. 2007;161:61–79. doi: 10.1016/S0079-6123(06)61005-4. [DOI] [PubMed] [Google Scholar]
- Franco L. Bodrato N. Moreschi I. Usai C. Bruzzone S. Scarf i S. Zocchi E. De Flora A. Cyclic ADP-ribose is a second messenger in the lipopolysaccharide-stimulated activation of murine N9 microglial cell line. J. Neurochem. 2006;99:165–176. doi: 10.1111/j.1471-4159.2006.04031.x. [DOI] [PubMed] [Google Scholar]
- Frasca L. Fedele G. Deaglio S. Capuano C. Palazzo R. Vaisitti T. Malavasi F. Ausiello C.M. CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood. 2006;107:2392–2399. doi: 10.1182/blood-2005-07-2913. [DOI] [PubMed] [Google Scholar]
- Garden G.A. Moller T. Microglia biology in health and disease. J. Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gregorini A. Tomasetti M. Cinti C. Colomba D. Colomba S. CD38 expression enhances sensitivity of lymphoma T and B cell lines to biochemical and receptor-mediated apoptosis. Cell Biol. Int. 2006;30:727–732. doi: 10.1016/j.cellbi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Grosjean M.B. Lenzlinger P.M. Stahel P.F. Yatsiv I. Shohami E. Trentz O. Kossmann T. Morganti-Kossmann M.C. Immunohistochemical characterization of Fas (CD95) and Fas ligand (FasL/CD95L) expression in the injured brain: relationship with neuronal cell death and inflammatory mediators. Histol. Histopathol. 2007;22:235–250. doi: 10.14670/HH-22.235. [DOI] [PubMed] [Google Scholar]
- Grossman R. Shohami E. Alexandrovich A. Yatsiv I. Kloog Y. Biegon A. Increase in peripheral benzodiazepine receptors and loss of glutamate NMDA receptors in a mouse model of closed head injury: a quantitative autoradiographic study. Neuroimage. 2003;20:1971–1981. doi: 10.1016/j.neuroimage.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Guo Z. Cupples L.A. Kurz A. Auerbach S.H. Volicer L. Chui H. Green R.C. Sadovnick A.D. Duara R. DeCarli C. Johnson K. Go R.C. Growdon J.H. Haines J.L. Kukull W.A. Farrer L.A. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- Guse A.H. Biochemistry, biology, and pharmacology of cyclic adenosine diphosphoribose (cADPR) Curr. Med. Chem. 2004;11:847–855. doi: 10.2174/0929867043455602. [DOI] [PubMed] [Google Scholar]
- Hanisch U.K. Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Howard M. Grimaldi J.C. Bazan J.F. Lund F.E. Santos-Argumedo L. Parkhouse R.M. Walseth T.F. Lee H.C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- Krebs C. Adriouch S. Braasch F. Koestner W. Leiter E.H. Seman M. Lund F.E. Oppenheimer N. Haag F. Koch-Nolte F. CD38 controls ADP-ribosyltransferase-2-catalyzed ADP-ribosylation of T cell surface proteins. J. Immunol. 2005;174:3298–3305. doi: 10.4049/jimmunol.174.6.3298. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G.W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kumagai M. Coustan-Smith E. Murray D.J. Silvennoinen O. Murti K.G. Evans W.E. Malavasi F. Campana D. Ligation of CD38 suppresses human B lymphopoiesis. J. Exp. Med. 1995;181:1101–1110. doi: 10.1084/jem.181.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladeby R. Wirenfeldt M. Garcia-Ovejero D. Fenger C. Dissing-Olesen L. Dalmau I. Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res. Brain Res. Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Laird M.D. Vender J.R. Dhandapani K.M. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M. Gowing G. Simard A. Weng Y.C. Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.C. Enzymatic functions and structures of CD38 and homologs. Chem. Immunol. 2000;75:39–59. doi: 10.1159/000058774. [DOI] [PubMed] [Google Scholar]
- Lee H.C. Multiplicity of Ca2+ messengers and Ca2+ stores: a perspective from cyclic ADP-ribose and NAADP. Curr. Mol. Med. 2004;4:227–237. doi: 10.2174/1566524043360753. [DOI] [PubMed] [Google Scholar]
- Lund F.E. Cockayne D.A. Randall T.D. Solvason N. Schuber F. Howard M.C. CD38: a new paradigm in lymphocyte activation and signal transduction. Immunol. Rev. 1998;161:79–93. doi: 10.1111/j.1600-065x.1998.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Lund F.E. Muller-Steffner H. Romero-Ramirez H. Moreno-Garcia M.E. Partida-Sanchez S. Makris M. Oppenheimer N.J. Santos-Argumedo L. Schuber F. CD38 induces apoptosis of a murine pro-B leukemic cell line by a tyrosine kinase-dependent but ADP-ribosyl cyclase- and NAD glycohydrolase-independent mechanism. Int. Immunol. 2006;18:1029–1042. doi: 10.1093/intimm/dxl037. [DOI] [PubMed] [Google Scholar]
- Lund F.E. Muller-Steffner H.M. Yu N. Stout C.D. Schuber F. Howard M.C. CD38 signaling in B lymphocytes is controlled by its ectodomain but occurs independently of enzymatically generated ADP-ribose or cyclic ADP-ribose. J. Immunol. 1999;162:2693–2702. [PubMed] [Google Scholar]
- Lye T.C. Shores E.A. Traumatic brain injury as a risk factor for Alzheimer's disease: a review. Neuropsychol. Rev. 2000;10:115–129. doi: 10.1023/a:1009068804787. [DOI] [PubMed] [Google Scholar]
- Malavasi F. Deaglio S. Funaro A. Ferrero E. Horenstein A.L. Ortolan E. Vaisitti T. Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- Matsumori Y. Hong S.M. Fan Y. Kayama T. Hsu C.Y. Weinstein P.R. Liu J. Enriched environment and spatial learning enhance hippocampal neurogenesis and salvages ischemic penumbra after focal cerebral ischemia. Neurobiol. Dis. 2006;22:187–198. doi: 10.1016/j.nbd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Mayo L. Jacob-Hirsch J. Amariglio N. Rechavi G. Moutin M.J. Lund F.E. Stein R. Dual role of CD38 in microglial activation and activation-induced cell death. J. Immunol. 2008;181:92–103. doi: 10.4049/jimmunol.181.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L. Stein R. Characterization of LPS and interferon-gamma triggered activation-induced cell death in N9 and primary microglial cells: induction of the mitochondrial gateway by nitric oxide. Cell Death Differ. 2007;14:183–186. doi: 10.1038/sj.cdd.4401989. [DOI] [PubMed] [Google Scholar]
- Mizuguchi M. Otsuka N. Sato M. Ishii Y. Kon S. Yamada M. Nishina H. Katada T. Ikeda K. Neuronal localization of CD38 antigen in the human brain. Brain Res. 1995;697:235–240. doi: 10.1016/0006-8993(95)00885-t. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann M.C. Rancan M. Stahel P.F. Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann M.C. Satgunaseelan L. Bye N. Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Myer D.J. Gurkoff G.G. Lee S.M. Hovda D.A. Sofroniew M.V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nadler Y. Alexandrovich A. Grigoriadis N. Hartmann T. Rao K.S. Shohami E. Stein R. Increased expression of the gamma-secretase components presenilin-1 and nicastrin in activated astrocytes and microglia following traumatic brain injury. Glia. 2008;56:552–567. doi: 10.1002/glia.20638. [DOI] [PubMed] [Google Scholar]
- Otto V.I. Stahel P.F. Rancan M. Kariya K. Shohami E. Yatsiv I. Eugster H.P. Kossmann T. Trentz O. Morganti-Kossmann M.C. Regulation of chemokines and chemokine receptors after experimental closed head injury. Neuroreport. 2001;12:2059–2064. doi: 10.1097/00001756-200107030-00053. [DOI] [PubMed] [Google Scholar]
- Panikashvili D. Simeonidou C. Ben-Shabat S. Hanus L. Breuer A. Mechoulam R. Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S. Cockayne D.A. Monard S. Jacobson E.L. Oppenheimer N. Garvy B. Kusser K. Goodrich S. Howard M. Harmsen A. Randall T.D. Lund F.E. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S. Iribarren P. Moreno-Garcia M.E. Gao J.L. Murphy P.M. Oppenheimer N. Wang J.M. Lund F.E. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J. Immunol. 2004;172:1896–1906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S. Randall T.D. Lund F.E. Innate immunity is regulated by CD38, an ecto-enzyme with ADP-ribosyl cyclase activity. Microbes Infect. 2003;5:49–58. doi: 10.1016/s1286-4579(02)00055-2. [DOI] [PubMed] [Google Scholar]
- Perraud A.L. Fleig A. Dunn C.A. Bagley L.A. Launay P. Schmitz C. Stokes A.J. Zhu Q. Bessman M.J. Penner R. Kinet J.P. Scharenberg A.M. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- Plassman B.L. Havlik R.J. Steffens D.C. Helms M.J. Newman T.N. Drosdick D. Phillips C. Gau B.A. Welsh-Bohmer K.A. Burke J.R. Guralnik J.M. Breitner J.C. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Reshef A. Shirvan A. Shohami E. Grimberg H. Levin G. Cohen A. Trembovler V. Ziv I. Targeting cell death in vivo in experimental traumatic brain injury by a novel molecular probe. J. Neurotrauma. 2008;25:569–580. doi: 10.1089/neu.2007.0341. [DOI] [PubMed] [Google Scholar]
- Schmidt O.I. Heyde C.E. Ertel W. Stahel P.F. Closed head injury—an inflammatory disease? Brain Res. Brain Res. Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Schreiber V. Dantzer F. Ame J.C. de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- Schuber F. Lund F.E. Structure and enzymology of ADP-ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites. Curr. Mol. Med. 2004;4:249–261. doi: 10.2174/1566524043360708. [DOI] [PubMed] [Google Scholar]
- Shapira Y. Shohami E. Sidi A. Soffer D. Freeman S. Cotev S. Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit. Care Med. 1988;16:258–265. doi: 10.1097/00003246-198803000-00010. [DOI] [PubMed] [Google Scholar]
- Shein N.A. Grigoriadis N. Horowitz M. Umschwief G. Alexandrovich A.G. Simeonidou C. Grigoriadis S. Touloumi O. Shohami E. Microglial involvement in neuroprotection following experimental traumatic brain injury in heat-acclimated mice. Brain Res. 2008;1244:132–141. doi: 10.1016/j.brainres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Shohami E. Gallily R. Mechoulam R. Bass R. Ben-Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J. Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O. Nishigaki H. Kitanaka A. Kumagai M. Ito C. Malavasi F. Lin Q. Conley M.E. Campana D. CD38 signal transduction in human B cell precursors. Rapid induction of tyrosine phosphorylation, activation of syk tyrosine kinase, and phosphorylation of phospholipase C-gamma and phosphatidylinositol 3-kinase. J. Immunol. 1996;156:100–107. [PubMed] [Google Scholar]
- Simard A.R. Rivest S. Neuroprotective effects of resident microglia following acute brain injury. J. Comp. Neurol. 2007;504:716–729. doi: 10.1002/cne.21469. [DOI] [PubMed] [Google Scholar]
- Soulet D. Rivest S. Bone-marrow-derived microglia: myth or reality? Curr. Opin. Pharmacol. 2008;8:508–518. doi: 10.1016/j.coph.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Stahel P.F. Shohami E. Younis F.M. Kariya K. Otto V.I. Lenzlinger P.M. Grosjean M.B. Eugster H.P. Trentz O. Kossmann T. Morganti-Kossmann M.C. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Streit W.J. Microglial response to brain injury: a brief synopsis. Toxicol. Pathol. 2000;28:28–30. doi: 10.1177/019262330002800104. [DOI] [PubMed] [Google Scholar]
- Streit W.J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Tsenter J. Beni-Adani L. Assaf Y. Alexandrovich A.G. Trembovler V. Shohami E. Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J. Neurotrauma. 2008;25:324–333. doi: 10.1089/neu.2007.0452. [DOI] [PubMed] [Google Scholar]
- Waxweiler R.J. Thurman D. Sniezek J. Sosin D. O'Neil J. Monitoring the impact of traumatic brain injury: a review and update. J. Neurotrauma. 1995;12:509–516. doi: 10.1089/neu.1995.12.509. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Yamada M. Mizuguchi M. Otsuka N. Ikeda K. Takahashi H. Ultrastructural localization of CD38 immunoreactivity in rat brain. Brain Res. 1997;756:52–60. doi: 10.1016/s0006-8993(97)00117-0. [DOI] [PubMed] [Google Scholar]
- Yatsiv I. Morganti-Kossmann M.C. Perez D. Dinarello C.A. Novick D. Rubinstein M. Otto V.I. Rancan M. Kossmann T. Redaelli C.A. Trentz O. Shohami E. Stahel P.F. Elevated intracranial IL-18 in humans and mice after traumatic brain injury and evidence of neuroprotective effects of IL-18-binding protein after experimental closed head injury. J. Cereb. Blood Flow Metab. 2002;22:971–978. doi: 10.1097/00004647-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- Ying W. Wei G. Wang D. Wang Q. Tang X. Shi J. Zhang P. Lu H. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front. Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]