Abstract
Background
Knowledge of factors and mechanisms contributing to the inherent radioresistance of pancreatic cancer may improve cancer treatment. Irradiation inhibits glycogen synthase kinase 3β (GSK3β) by phosphorylation at serine 9. In turn, release of cytosolic membrane β-catenin with subsequent nuclear translocation promotes survival. Both GSK3β and β-catenin have been implicated in cancer cell proliferation and resistance to death.
Methods
We investigated pancreatic cancer cell survival after radiation in vitro and in vivo, with a particular focus on the role of the function of the GSK3β/β-catenin axis.
Results
Lithium chloride, RNAi-medicated silencing of GSK3β, or the expression of a kinase dead mutant GSK3β resulted in radioresistance of Panc1 and BxPC3 pancreatic cancer cells. Conversely, ectopic expression of a constitutively active form of GSK3β resulted in radiosensitization of Panc1 cells. GSK3β silencing increased radiation-induced β-catenin target gene expression asmeasured by studies of AXIN2 and LEF1 transcript levels. Western blot analysis of total and phosphorylated levels of GSK3β and β-catenin showed that GSK3β inhibition resulted in stabilization of β-catenin. Xenografts of both BxPC3 and Panc1 with targeted silencing of GSK3β exhibited radioresistance in vivo. Silencing of β-catenin resulted in radiosensitization, whereas a nondegradable β-catenin construct induced radioresistance.
Conclusions
These data support the hypothesis that GSK3β modulates the cellular response to radiation in a β-catenin-dependent mechanism. Further understanding of this pathway may enhance the development of clinical trials combining drugs inhibiting β-catenin activation with radiation and chemotherapy in locally advanced pancreatic cancer.
Introduction
Roughly 37,000 US patients are diagnosed annually with pancreatic cancer, with the annual death rate from pancreatic cancer approaching its incidence. Aggressive treatment of locally advanced pancreatic cancer patients with highly conformal radiation and chemotherapy produces a moderate beneficial effect on local control of the cancer, albeit with possible increases in toxicity and a minor impact on patient survival. Unfortunately, the addition of agents targeting either Ras [1] (zarnestra, tipifarnib), matrix metalloproteases [2] (marimistat), or EGFR [3] (erlotinib) has not dramatically increased overall survival either. Thus, elucidation of the mechanisms underlying pancreatic cancer radioresistance may lead to improved targeted therapies, which may improve clinical outcomes.
Depending on the context and cell type under study, glycogen synthase kinase 3β (GSK3β) can promote cell survival or apoptosis after cytotoxic insults [4]. Active GSK3β can induce mitochondrial release of cytochrome c, leading to the activation of the intrinsic apoptosis pathway [5]. Active GSK3β phosphorylates β-catenin, a transcription factor involved in survival and proliferation, at serine 33, which primes β-catenin for ubiquitination and subsequent proteosome-mediated degradation [6]. Survival signals such as binding of Wnt ligands or growth factors to their respective receptors leads to inhibition of GSK3β through phosphorylation at serine 9. Inhibition of GSK3β then leads to the stabilization of β-catenin resulting in nuclear translocation and heterodimerization of β-catenin with T-cell factor family members thus promoting transcription of β-catenin target genes and cell survival [7].
We previously conducted a study that showed that the protein kinase Cβ inhibitor enzastaurin prevents radiation-induced phosphorylation of protein kinase Cβ and leads to radiosensitization through persistent activation of GSK3β in pancreatic cancer cells. In addition, we demonstrated that radiation induces phosphorylation of GSK3β at serine 9, a site known to inhibit GSK3β activity [8,9]. Our current study expands on that observation to test the hypothesis that GSK3β mediates the radiation resistance of pancreatic cancer by suppression of β-catenin. Our findings establish that the GSK3β/β-catenin pathway modulates radiation resistance of pancreatic cancer and suggest potential targets to increase efficacy of radiation therapy in pancreatic cancer.
Materials and Methods
Cell Line Generation
Panc-1 and BxPc3 human pancreatic cancer cells were obtained from the American Type Culture Collection (Manassas, VA) and were maintained according to standard tissue culture conditions. We generated lentivirus particles for transduction of shRNA to silence GSK3β or β-catenin. Mission short hairpin RNA (shRNA) lentiviral plasmids (Sigma, St Louis, MO) contain a U6 promoter transcribing nonspecific (NS), GSK3β, or β-catenin shRNA along with a puromyocin resistance gene for selection. We collected supernatants after cotransfection of HEK293T cells with mission shRNA and packaging plasmids [10]. BxPC3 or Panc1 cells were then transduced with NS, GSK3β, or β-catenin shRNA particles and selected under 2 µg/ml puromycin.
We used lentivirus transduction to express kinase inactive GSK3β [11]. We subcloned the GSK3βKK(85,86)MA KKMA insert using KpnI (5′ end) and XbaI (3′ end) from the pCMV4 vector to pLentilox-IRES-GFP. The resulting pLL- GSK3βKK(85,86)MA-IGFP lentiviral plasmid uses a CMV promoter to drive expression of a single messenger RNA with both insert and GFP for the identification of transduced cells. We collected supernatants after cotransfection of HEK293T cells with empty vector control or pLL- GSK3βKK(85,86)MA-IGFP and packaging plasmids. BxPC3 or Panc1 cells were then transduced with empty vector control or pLL GSK3βKK(85,86)MA-IGFP particles and analyzed by flow cytometry. Only cell populations with more than 90% GFP expression were used.
We used stable transfection to generate cells expressing nondegradable β-catenin [12]. We subcloned the β-cateninS33Y-FLAG insert using BamHI (5′ end) and XbaI (3′ end) from the pCMV4 vector to pcDNA3.1 (+). Cells were transfected with 1 µg of empty vector control or pcDNA3.1 (+)β-cateninS33Y-FLAG and then selected with G418. Stable pooled populations of individual clones were verified by Western blot analysis for FLAG.
Colony Formation Assays
After irradiation, cells were trypsinized, counted, and plated at predetermined clonal densities. Two weeks later, cells were fixed with a methanol/acetic acid mixture (7:1) and stained with crystal violet. Colony counting was done using an automated counter. Data were then analyzed by determining the surviving fraction at each dose of radiation. Cell survival curves were fit using the linear-quadratic equation. Radiation sensitivity is expressed in mean inactivation dose (MID), which represents the area under the cell survival curve [13]. MID was calculated for control and each experimental manipulation, and the enhancement ratio was calculated as the MID in the control curve divided by the MID in the experimental curve.
Reverse Transcription-Polymerase Chain Reaction
A Qiagen RN easy RNA extraction kit was used to collect RNA for reverse transcription-polymerase chain reaction (RT-PCR). RT-PCR was performed in duplicate using a Qiagen Quantitect Syber Green RT-PCR kit on GAPDH[14], AXIN2 [15], and Lef1 [16] using previously published primer sequences. CT values for each unknown were compared with a standard curve made of serially diluted RNA from wild-type BxPC3 and Panc1 cells in logarithmic phase growth. AXIN2 and Lef1 values were normalized to the level of GAPDH in each sample.
Antibodies and Immunoblot Analysis
Antibodies to GSK3β (Cell Signaling, Danvers, MA), phospho-Ser9 GSK3β (Cell Signaling), β-catenin (Cell Signaling), phospho-Ser33 β-catenin (Cell Signaling), and β-actin (Sigma) were used at dilutions per the manufacturer. Cell lysate production with RIPA buffer and immunoblot analysis were performed using detailed protocols from Cell Signaling. Xenograph samples were taken after treatment and frozen in a dry ice bath. A mortar and pestle was then used to grind the xenograph samples. β-Actin was used as a control to show that total protein quantities were equal among the groups. Each Western blot was performed three independent times from unique lysates; representative films are shown in Figures 1A, 2A, and 3A.
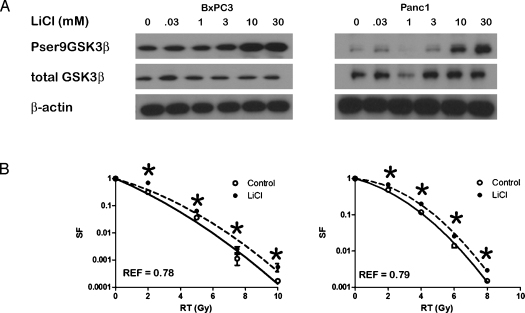
Figure 1.
(A) BxPC3 and Panc1 cells were treated with LiCl for 6 hours, and Western blot analysis for total and phosphorylated GSK3β was performed. (B) Clonogenic survival of control (○) or LiCl-pretreated (●) BxPC3 and Panc1 cells. *P ≤ 0.05. Error bars are SD of three independent experiments performed in triplicate and are smaller than the symbols at some data points.
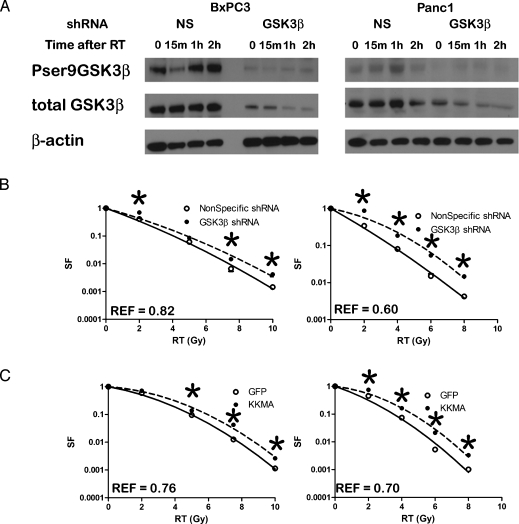
Figure 2.
(A) BxPC3 and Panc1 cells expressing NS or GSK3β shRNA were treated with 2 Gy, and Western blot analysis for total and phosphorylated GSK3β was performed. The blots were confirmed in at least three independent experiments. (B) Clonogenic survival of NS (○) or GSK3β shRNA (●) BxPC3 and Panc1 cells. (C) Clonogenic survival of empty vector control (○) or GSK3βKK(85,86)MA (●) BxPC3 and Panc1 cells. *P ≤ 0.05. Error bars are SD of three independent experiments performed in triplicate and are smaller than the symbols at some data points.
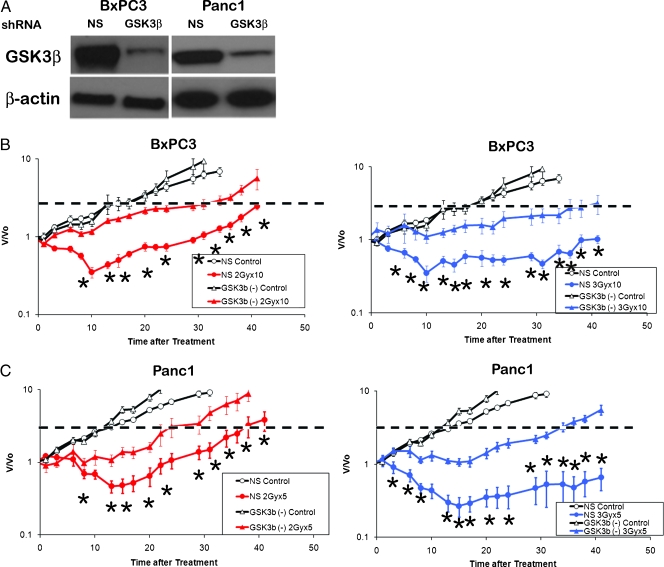
Figure 3.
(A) Xenografts from BxPC3 and Panc1 cells expressing NS or GSK3β shRNA were analyzed for expression of GSK3β. The blots were confirmed in at least three independent experiments. BxPC3 NS shRNA and GSK3β knockdown xenografts were treated with ten 2-Gy fractions (B) or ten 3-Gy fractions (C) and were compared with unirradiated controls. Panc1 NS shRNA and GSK3β knockdown xenografts were treated with five 2-Gy fractions (D) or five 3-Gy fractions (E) and were compared with unirradiated controls. *P ≤ 0.05 between the NS versus GSK3β knockdown. Error bars are SEM of the 10 tumors per treatment arm. The dashed line indicates a four-fold increase in tumor size, used to determine the enhancement ratio.
Xenografts
Animals used in this study were maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care in accordance with current regulations and standards of the United States Department of Agriculture and Department of Health and Human Services. Under an institutionally approved protocol, 4-week-old female athymic nude mice were implanted with 5 x 107 BxPC3 or Panc1 cells subcutaneously. Tumor volume (TV) was calculated according to the following equation: TV = Π/6 x a x b2, where a and b are the longer and shorter dimensions of the tumor, respectively. When the average tumor volume achieved 100 mm3, mice were randomized to treatment groups.
Irradiation
Cells or xenografts were irradiated using a Phillips 250 orthovoltage unit at approximately 2 Gy/min for cells or 1.4 Gy/min for mice in the Irradiation Core of the University of Michigan Cancer Center. Dosimetry is carried out using an ionization chamber connected to an electrometer system, which is directly traceable to a National Institute of Standards and Technology calibration. Mice were anesthetized with a mixture of ketamine 60 mg/kg and xylazine 3 mg/kg and positioned such that the apex of each flank tumor was at the center of a 2.4-cm aperture in the secondary collimator and irradiated with the rest of the mouse being shielded from radiation.
Statistical Analysis
The clonogenic assays were conducted on three independent occasions in triplicate. Mean and SD from the three independent experiments are displayed in Figures 1A, 2, B and C, and 6. A two-tailed t-test was used to analyze differences between mean values of in vitro assays, with α values less than 0.05 considered significant. The radiation enhancement factor (REF) was calculated as previously described [17], with numbers less than 1 indicating radioprotection and numbers greater than 1 indicating radiosensitization.
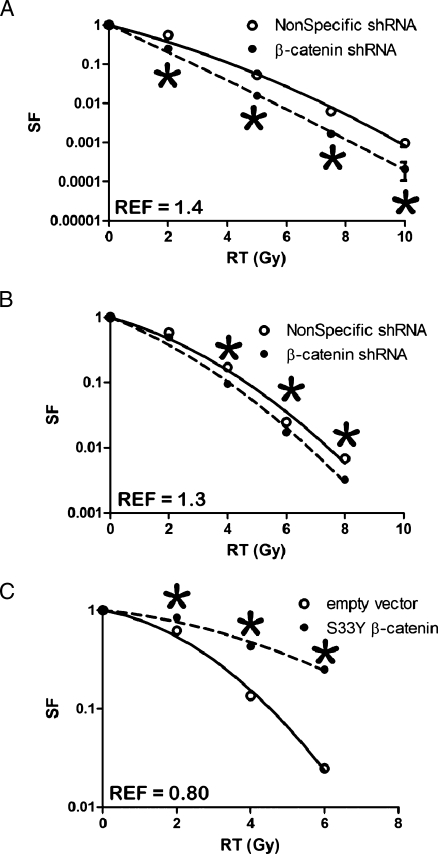
Figure 6.
Clonogenic survival of NS, (○) or β-catenin shRNA, (●) BxPC3, (A) and Panc1, (B) cells. Clonogenic survival of empty vector control, (○) or β-cateninS33YFLAG (●) Panc1 cells, (C). Error bars are SD of three independent experiments performed in triplicate and are smaller than the symbols at some data points.
The RT-PCR data in Figure 5A represent the mean and SD values of three independent experiments performed in triplicate after irradiation. A two-tailed t-test was used to analyze differences between mean values at each time point, with α values less than 0.05 considered significant.
Figure 5.
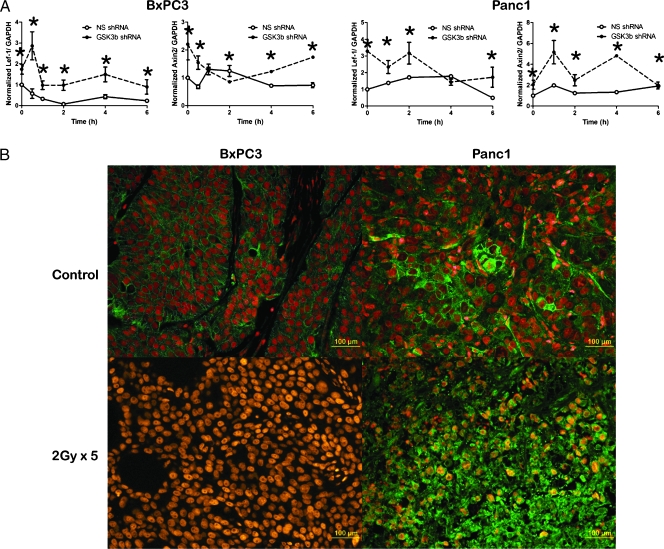
(A) Time course of Lef1 and Axin2 levels in NS (○) or GSK3β shRNA (●) BxPC3 and Panc1 cells subjected to 2-Gy radiation. Mean of three experiments with SDs, *P ≤ 0.05. (B) BxPC3 or Panc1 xenografts were treated with 2-Gy radiation and were stained for β-catenin (green) and propidium idodide (red). Yellow indicates overlap of red and green, consistent with nuclear β-catenin.
The in vivo experiments were designed with a power of 80% to detect a 20% difference in tumor growth delay between the control versus irradiated tumors, resulting in a sample size of 10 tumors per group. Tumor volumes are plotted relative to the pretreatment volume in Figure 3, B and C. A two-tailed t-test was used to analyze differences between mean values at each measurement, with α values less than 0.05 considered significant.
Results
GSK3β Signaling Modulates Radiation Resistance In Vitro
Inhibition of GSK3β by phosphorylation at Ser9 has been previously observed after irradiation of pancreatic cancer cells [17], potentially underscoring their observed radioresistance. We examined the phenotypic effects of GSK3β modulation on radiation response in vitro. Previous studies have shown that lithium chloride (LiCl) is a pharmacological inhibitor of GSK3β, with inhibition correlating with increased phosphorylation of GSK3β at serine 9 [18]. We determined the concentration of LiCl needed to increase GSK3β phosphorylation and found that 30 mM was associated with phosphorylation in BxPC3 and Panc1 cells (Figure 1A). Inhibition of GSK3β by a 6-hour exposure to LiCl before radiation led to an increase in survival in response to radiation in both BxPC3 and Panc1 cells (REFs: 0.78 and 0.79, respectively, P < .05; Figure 1B). Because pharmacologic inhibition such as LiCl treatment may have unintended off-target effects, we also used genetic approaches to test our hypothesis that GSK3β inhibition promotes radioresistance in pancreatic cancer.
To further characterize the role of GSK3β in radiation survival, we transduced BxPC3 and Panc1 cells with a lentivirus construct expressing an shRNA designed to inhibit GSK3β expression. We generated polyclonal populations of BxPC3 and Panc1 cells expressing the shRNA construct and then determined the effect of GSK3β knockdown on survival after radiation. Radiation delivered to pancreatic cancer cells expressing an NS shRNA construct resulted in serine 9 phosphorylation, similar to wild-type cells. Ser9 GSK3β phosphorylation was increased with a peak at 1 hour after a 2-Gy radiation (Figure 2A). Silencing of GSK3β prevented radiation-induced GSK3β serine phosphorylation in response to a 2-Gy radiation and produced radioresistance (REFs of 0.82 in BxPC3 and 0.60 in Panc1, P < .05; Figure 2B) similarly to pharmacological inhibition of GSK3β. These data indicate that inhibition of GSK3β promotes survival in response to irradiation.
As a second genetic approach, we generated polyclonal populations of cells stably expressing GSK3βKK(85,86)MA, which has an inactive substrate phosphorylation domain. Expression of the kinase dead GSK3βKK(85,86)MA inhibited radiation cytotoxicity compared with cells transduced with empty vector (REFs of 0.76 in BxPC3 and 0.70 in Panc1; Figure 2C). These data show that radiation resistance of pancreatic cancer cells in vitro can be modulated through manipulation of GSK3β.
GSK3β Signaling Modulates Radiation Resistance In Vivo
After observing the radioprotective effect of GSK3β inhibition in vitro, we studied the consequences of GSK3β inhibition in vivo. Polyclonal populations of BxPC3 and Panc1 cells expressing GSK3β shRNA maintained knockdown of GSK3 10 weeks after subcutaneous implantation, whereas those with NS shRNA retained the expression of GSK3β (Figure 3A). Control BxPC3 xenografts expressing NS shRNA exhibited a 26-day growth delay with ten 2-Gy fractions (Figure 3B) and a 61-day growth delay with ten 3-Gy fractions (Figure 3C). Silencing of GSK3β leads to shortened growth delay from both the 2-Gy (17 days, REF of 0.65) and the 3-Gy (25 days, REF of 0.40) treatment courses (P < .05 for both). Similarly, control Panc1 xenografts expressing NS shRNA exhibited a 24-day growth delay with five 2-Gy fractions (Figure 3D) and a 43-day growth delay with five 3-Gy fractions (Figure 3E). Silencing of GSK3β leads to shortened growth delay from the 2-Gy (16 days, REF of 0.64) and the 3-Gy (23 days, REF of 0.53) treatment courses (P < .05 for both). Thus, tumors without GSK3β were less sensitive to radiation, similar to the results from the in vitro clonogenic assays.
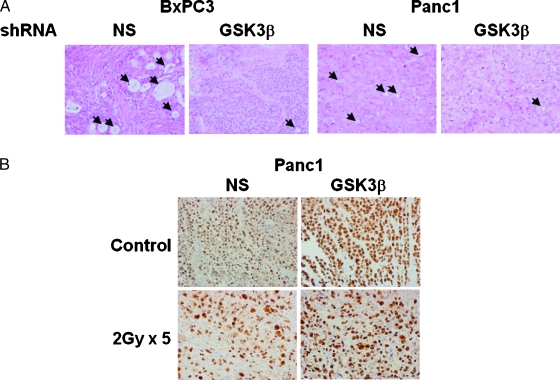
To determine changes in vivo induced by radiation, a separate experiment with identical arms was conducted; tumors were collected immediately after the last fraction of radiation, and staining for hematoxylin and eosin (H&E) and Ki67 was performed (Figure 4). H&E staining revealed that knock down of GSK3β resulted in increased nuclear-to-cytoplasmic ratio and decreased production of mucin, suggesting cellular dedifferentiation, a phenotype consistent with β-catenin activation. Radiation reduced the proliferation index from 95% to 30% in NS shRNA tumors, whereas GSK3β shRNA tumors had a less pronounced reduction from 98% to 65% (Figure 4B).
Figure 4.
Xenografts from BxPC3 and Panc1 cells expressing NS or GSK3β shRNA were analyzed by H&E (A). Black arrows indicate glandular structures present in the NS shRNA xenografts, which are absent in GSK3β shRNA xenografts. Panc1 xenografts with and without radiation were analyzed for proliferation by Ki67 (B). Original magnification, x400.
The decreased tumor growth delay, increased tumor cell density, and increased proliferation in the GSK3β knockdown tumors all correlate with the in vitro observation that inhibition of GSK3β promotes radiation resistance.
Modulation of the Radiation Response through β-Catenin
We hypothesized that GSK3β modulates the radiation response through a β-catenin-dependent gene transcription. We first tested whether radiation induced β-catenin activity. In BxPC3 and Panc1 cells expressing NS shRNA, radiation induced the transcription of Lef1 and Axin2 [19], two well-characterized β-catenin target genes, in a time-dependent manner as measured by quantitative RT-PCR. Targeted silencing of GSK3β resulted in both higher basal and radiation-induced levels of Lef1 or Axin2 gene transcription (Figure 5A). Because we observed that radiation affects β-catenin transcriptional activity in vitro through GSK3β, we hypothesized that radiation would have similar effects in vivo (Figure 5B). Before irradiation, β-catenin localized to the cytosolicmembrane. After radiation of xenografts, β-catenin translocated to the nucleus, suggesting induction of β-catenin signaling. Radiation induction of β-catenin nuclear translocation correlates with the observation in vitro that GSK3β phosphorylation modulates β-catenin-dependent gene transcription.
If GSK3β modulates pancreatic cancer cell response to radiation through β-catenin, then modulation of β-catenin activity may influence cell survival after radiation. Therefore, we transduced BxPC3 and Panc1 cells with lentivirus encoding shRNA targeting β-catenin. Compared with cells transduced with NS shRNA, cells with silenced β-catenin were more sensitive to radiation as shown by reduced clonogenic survival (REFs of 1.4 in BxPC3 and 1.25 in Panc1; Figure 6).
On the basis of these experiments, constitutive activation of β-catenin would be predicted to render pancreatic cancer cells resistant to radiation. Therefore, a β-cateninS33Y-FLAG vector was used to create cells expressing constituently active β-catenin. The S33Y mutation prevents GSK3β-mediated phosphorylation at Ser33, thus preventing ubiquitination and subsequent degradation [20]. Cells expressing constituently active β-cateninS33Y showed increased clonogenic survival (REF for Panc1 cells was 0.8; Figure 6C). The effects of nondegradable β-cateninS33Y were analogous to those resulting from GSK3β inhibition or silencing because both showed an increased resistance to radiation together with an increased level of β-catenin activity. Thus, increased β-catenin activity results in greater radiation resistance of pancreatic cancer cells, whereas loss of β-catenin through RNAi-mediated silencing results in increased radiation sensitivity.
Discussion
In this study, we found that inhibition of GSK3β, by either genetic or pharmacological methods, induces radiation resistance of pancreatic cancer cells in vitro, reduces the duration of radiation-induced tumor growth delay, and leads to increased cell proliferation in vivo. Similarly, the expression of a constituently active β-catenin in pancreatic cancer cells increases the resistance to radiation. Our results reinforce and expand on previous studies of radiation effects on GSK3β.
We have previously demonstrated that radiation induces phosphorylation of GSK3β at Ser9, an event known to inhibit GSK3β kinase activity [17], and that abrogation of this phosphorylation resulted in radiosensitization. Radiation was also shown to inhibit GSK3β activity in SAOS-2 cells [21], although the phenotypic consequences in sensitivity to radiation were not investigated. Irradiation of A549 cells induced phosphorylation of GSK3β at Ser9, and this effect was reduced when cells were plated on fibronectin [22]. The authors suggested that GSK3β is involved in the interaction of cells with the extracellular matrix after radiation to modulate the cytotoxicity of radiation. These studies implicate GSK3β as a mediator of radiation sensitivity.
We hypothesized that GSK3β modulates radiation cytotoxicity, at least in part, through its downstream effector β-catenin. Herein, we show that radiation induces the transcription of Lef1 and Axin2, two well-characterized β-catenin target genes, and targeted silencing of GSK3β results in both higher basal and radiation-induced levels of Lef1 or Axin2 gene transcription. Furthermore, we show that radiation induces translocation of cytosolic β-catenin to the nucleus in Panc1 and BxPC3 xenographs, an observation consistent with the in vitro induction of transcription of Lef1 and Axin2. Finally, we show that cells with silenced β-catenin are more sensitive,whereas cells expressing constituently active β-cateninS33Y are more resistant to radiation. β-Catenin has been shown to prevent epithelial cell death after radiation or anoikis [23]. These findings suggest that β-catenin is involved in determining clonogenic survival of pancreatic cancer cells after irradiation.
Our studies potentially explain the relationship between Wnt signaling and radiation cytotoxicity in other tumor sites. Activation of the Wnt signaling pathway resulted in β-catenin cytoplasmic accumulation with translocation to the nucleus in head and neck cancer cell lines expressing COX-2 [24]. In turn, up-regulation of Ku expression leads to increased radioresistance. Blocking COX-2 signaling led to the suppression of β-catenin-induced Ku expression and consequent radiation sensitivity. Others have suggested that the radioresistance observed clinically in glioblastoma depends in part on the activation of β-catenin in putative cancer stem cells [25]. In a mouse model of breast development, radiation selectively enriched for mammary epithelial progenitors isolated from transgenic mice with activated Wnt/β-catenin signaling but not for background-matched controls [26]. We conversely showed that suppressing β-catenin using shRNA correlated with an increase in radiation sensitivity.
Our data reinforce observations from others that GSK3β inhibition protects normal tissue from radiation toxicity. Radiation-induced GSK3β activation results in mouse hippocampal neuronal apoptosis and subsequent neurocognitive decline. The expression of kinase-inactive GSK3β or pharmacologic inhibition before irradiation significantly attenuated radiation-induced apoptosis in hippocampal neurons, leading to improved cognitive function in irradiated animals [27]. Mice treated with lithium chloride, a known GSK3β inhibitor, had decreased neurocognitive impairment after irradiation as well [28]. Akt serves to inhibit GSK3β after irradiation in normal vascular endothelium [29], and administration of recombinant growth factors known to activate Akt may prevent normal tissue toxicity. However, any pharmacologic strategy to reduce normal tissue damage must be carefully weighed against the risk of tumor protection.
Our results are consistent with radioprotection caused by active β-catenin. A reporter mouse model demonstrated that ionizing radiation activates β-catenin-mediated, T-cell factor-dependent transcription both in vitro and in vivo. Mouse-derived fibroblast cultures expressing stabilized β-catenin formed more colony-forming units than wild-type or null cells after irradiation. β-Catenin levels in irradiated wounds correlated with tensile strength of the wound, and lithium chloride treatment also increased β-catenin levels and increased wound strength [30]. The newly identified R-Spondin1 augments canonical Wnt/β-catenin signaling and causes nuclear translocation of β-catenin. R-Spondin1 reduced mucosal ulceration after whole-body or snoutonly irradiation inmouse models [31]. Therefore, in normal cells,GSK3β inhibition with β-catenin activation may be a radioprotective mechanism. Pancreatic cancer cells potentially invoke a similar mechanism to evade the cytotoxic effects of radiation.
Our results help explain an apparent contradiction present in the literature regarding pancreatic cancer and β-catenin. Mutations in APC leading to β-catenin nuclear accumulation have been well characterized to play a role in colon cancer. However, mutations in APC [32] or β-catenin [33] have not been found in pancreatic cancer. The published literature suggests that constitutive activation of β-catenin does not play a role in pancreatic cancer development. In fact, our results also demonstrate similar findings, as unirradiated tumors lacked nuclear β-catenin, and we did not find evidence of increased β-catenin target gene expression without irradiation. However, we did find that pancreatic cancer cells activate β-catenin in response to radiation to promote survival. Our results may therefore explain in part the clinically observed radioresistance of pancreatic cancer; specifically, it may not be the basal level of β-catenin but rather the induction of β-catenin by radiation that promotes pancreatic cancer cell survival.We plan to test this hypothesis by immunoflorescence of pancreatic cancer specimens treated with neoadjuvant radiation to determine whether activation of β-catenin occurs in patients.
The implications of this work identify a link between radiation and a pathway central to tumor growth, invasion, and metastasis of pancreatic cancer. By further discovering the molecular signaling cascades upstream and downstream of GSK3β, we will also start to gain insight into the potential interactions with other signaling pathways that are known to be involved in radioresistance. Further understanding of this pathway will also help develop clinical trials combining drugs inhibiting β-catenin activation with radiation and cytotoxic agents in locally advanced pancreatic cancer.
Acknowledgement
The authors thank Steven Kronenberg for his graphical expertise.
Footnotes
These studies were supported by an American Society of Therapeutic Radiology and Oncology Resident Seed Grant (A.S.), and National Institutes of Health R03 CA127050-01 (E.B.J.). These studies were conducted in partial fulfillment for the requirements (R.W.) for graduation with Honors, College of Literature, Science, and the Arts, from the University of Michigan. A. Spalding has been designated a B. Leonard Holman Pathway Fellow by the American Board of Radiology, and this work was presented in part at the 2008 Gastrointestinal Malignancies Symposium.
References
- 1.Macdonald JS, McCoy S, Whitehead RP, Iqbal S, Wade JL, III, Giguere JK, Abbruzzese JL. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group, (SWOG 9924) study. Invest New Drugs. 2005;23:485–487. doi: 10.1007/s10637-005-2908-y. [DOI] [PubMed] [Google Scholar]
- 2.Jones L, Ghaneh P, Humphreys M, Neoptolemos JP. The matrix metalloproteinases and their inhibitors in the treatment of pancreatic cancer. Ann N Y Acad Sci. 1999;880:288–307. doi: 10.1111/j.1749-6632.1999.tb09533.x. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 4.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, et al. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 7.Chan TA, Wang Z, Dang LH, Vogelstein B, Kinzler KW. Targeted inactivation of CTNNB1 reveals unexpected effects of β-catenin mutation. Proc Natl Acad Sci USA. 2002;99:8265–8270. doi: 10.1073/pnas.082240999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 β by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296(pt 1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294(pt 3):625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldar-Finkelman H, Argast GM, Foord O, Fischer EH, Krebs EG. Expression and characterization of glycogen synthase kinase-3 mutants and their effect on glycogen synthase activity in intact cells. Proc Natl Acad Sci USA. 1996;93:10228–10233. doi: 10.1073/pnas.93.19.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant β-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 14.Simpson DA, Feeney S, Boyle C, Stitt AW. Retinal VEGF mRNA measured by SYBR green I fluorescence: a versatile approach to quantitative PCR. Mol Vis. 2000;6:178–183. [PubMed] [Google Scholar]
- 15.Koinuma K, Yamashita Y, Liu W, Hatanaka H, Kurashina K, Wada T, Takada S, Kaneda R, Choi YL, Fujiwara SI, et al. Epigenetic silencing of AXIN2 in colorectal carcinoma with microsatellite instability. Oncogene. 2006;25:139–146. doi: 10.1038/sj.onc.1209009. [DOI] [PubMed] [Google Scholar]
- 16.Kahler RA, Galindo M, Lian J, Stein GS, van Wijnen AJ, Westendorf JJ. Lymphocyte enhancer-binding factor 1, (Lef1) inhibits terminal differentiation of osteoblasts. J Cell Biochem. 2006;97:969–983. doi: 10.1002/jcb.20702. [DOI] [PubMed] [Google Scholar]
- 17.Spalding AC, Watson R, Davis ME, Kim AC, Lawrence TS, Ben-Josef E. Inhibition of protein kinase C{beta} by enzastaurin enhances radiation cytotoxicity in pancreatic cancer. Clin Cancer Res. 2007;13:6827–6833. doi: 10.1158/1078-0432.CCR-07-0454. [DOI] [PubMed] [Google Scholar]
- 18.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 β phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 19.Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by β-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Kolligs FT, Hottiger MO, Mosavin R, Fearon ER, Nabel GJ. Regulation of β-catenin transformation by the p300 transcriptional coactivator. Proc Natl Acad Sci USA. 2000;97:12613–12618. doi: 10.1073/pnas.220158597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turenne GA, Price BD. Glycogen synthase kinase3 β phosphorylates serine 33 of p53 and activates p53's transcriptional activity. BMC Cell Biol. 2001;2:12. doi: 10.1186/1471-2121-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordes N, Beinke C, Plasswilm L, van Beuningen D. Irradiation and various cytotoxic drugs enhance tyrosine phosphorylation and β(1)-integrin clustering in human A549 lung cancer cells in a substratum-dependent manner in vitro. Strahlenther Onkol. 2004;180:157–164. doi: 10.1007/s00066-004-1144-2. [DOI] [PubMed] [Google Scholar]
- 23.Orford K, Orford CC, Byers SW. Exogenous expression of β-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol. 1999;146:855–868. doi: 10.1083/jcb.146.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang HW, Roh JL, Jeong EJ, Lee SW, Kim SW, Choi SH, Park SK, Kim SY. Wnt signaling controls radiosensitivity via cyclooxygenase-2-mediated Ku expression in head and neck cancer. Int J Cancer. 2008;122:100–107. doi: 10.1002/ijc.23069. [DOI] [PubMed] [Google Scholar]
- 25.Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–8984. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 26.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thotala DK, Hallahan DE, Yazlovitskaya EM. Inhibition of glycogen synthase kinase 3 β attenuates neurocognitive dysfunction resulting from cranial irradiation. Cancer Res. 2008;68:5859–5868. doi: 10.1158/0008-5472.CAN-07-6327. [DOI] [PubMed] [Google Scholar]
- 28.Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO, Jr, Boone B, Shinohara ET, Hallahan DE. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66:11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 29.Tan J, Geng L, Yazlovitskaya EM, Hallahan DE. Protein kinase B/Akt-dependent phosphorylation of glycogen synthase kinase-3β in irradiated vascular endothelium. Cancer Res. 2006;66:2320–2327. doi: 10.1158/0008-5472.CAN-05-2700. [DOI] [PubMed] [Google Scholar]
- 30.Gurung A, Uddin F, Hill RP, Ferguson PC, Alman BA. β-Catenin is a mediator of the response of fibroblasts to irradiation. Am J Pathol. 2009;174:248–255. doi: 10.2353/ajpath.2009.080576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Kim KA, De Vera J, Palencia S, Wagle M, Abo A. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/β-catenin pathway. Proc Natl Acad Sci USA. 2009;106:2331–2336. doi: 10.1073/pnas.0805159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caca K, Kolligs FT, Ji X, Hayes M, Qian J, Yahanda A, Rimm DL, Costa J, Fearon ER. β- and γ-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ. 1999;10:369–376. [PubMed] [Google Scholar]
- 33.Gerdes B, Ramaswamy A, Simon B, Pietsch T, Bastian D, Kersting M, Moll R, Bartsch D. Analysis of β-catenin gene mutations in pancreatic tumors. Digestion. 1999;60:544–548. doi: 10.1159/000007704. [DOI] [PubMed] [Google Scholar]