Abstract
To survive and metastasize, tumors interact with surrounding tissues by secreting growth factors and cytokines. In return, surrounding host tissues respond by changing their secretome. Numerous factors theoretically function as therapeutic targets or biomarkers of cancer growth and metastatic risk. However, it is unclear if these factors are tumor-derived or actually represent the host defense. To analyze the concentrations of tumor- and microenvironmentderived factors associated with neoplastic growth, we used ELISA-based arrays specific for murine or human proteins to establish a profile of tumor- or host-derived factors circulating in the plasma or within the platelets upon human tumor implantation into mice. Many factors characterized as tumor-derived were actually secreted by host tissues. This study uncovered the origin of various cytokines and revealed their circulation methods. We found that tumor-produced cytokines are predominantly sequestered in platelets. Sequestered proteins are protected from degradation and, thus, may be functional at metastatic sites. These findings identify tumor-specific targets for the detection and prevention of tumor growth and metastasis. As predicted by our model, monocyte chemotactic protein 1 and tumor necrosis factor α may be biomarkers for human cancers. Thus, our study identified several potential biomarkers that might be predictive of prostate cancer.
Introduction
The mechanisms of tumor growth and metastasis have been studied for decades, and yet, in 2008, more people died of cancer than from cardiovascular diseases, thus making cancer the number one cause of death in the United States.Many aspects of tumor development remain enigmatic, precluding development of efficient diagnostic tests and treatments. The intricate interactions of a growing tumor with its microenvironment and macroenvironment make cancerous tissue the most elusive part of an organism. It seems that tumor functions as an ultimate parasite and uses an organism's resources to promote its own growth and to invade into distant locations. The growing tumor secretes a number of growth factors, cytokines, and proteases, which are transported by the host vascular system, reaching multiple organs and tissues.Many factors seem to be secreted by the tumor secretomes of various cancers, such as vascular endothelial growth factors (VEGFs) to promote tumor vascularization [1,2], matrix metalloproteinases (MMPs) to modify the extracellular matrix [1,3], cytokines to attract hematopoietic cells from bone marrow [4,5], and growth factors involved in bone turnover to prepare future metastatic sites. Tumor activity triggers diverse reactions in host tissues, including angiogenic processes, recruitment of inflammatory cells, and changes in hemostasis. As a result, the host organism changes its own secretome, possibly as a defensive measure. Yet, many factors produced by surrounding tissues might promote tumor growth and its invasion rather than inhibit it. Although many factors circulating in the blood of a tumor-bearing organism have been identified and even proposed as diagnostic markers [1–3,6,7], it is unclear whether they are part of the tumor or host secretome.
In many instances, the tumor secretome is aimed at communication with distant organs, and therefore, many components should be “hidden” and protected while being transported to their target. Indeed, it was recently shown that whereas some factors circulate freely within the plasma, others are sequestered within platelets and might be selectively released on platelet activation [8]. Depleting platelets in tumorbearing mice triggers intratumor hemorrhaging and stimulates tumor cell apoptosis within the hemorrhagic area [9]. In addition to the effects on tumor stability, thrombocytopenia diminished tumor cell proliferation. Thus, platelets seem to be required for continued tumor growth. In addition, platelets can directly bind to cells within the tumor, which, in turn, may permit the loading of platelets with tumor-derived factors [10] and promote tumor cell migration and invasiveness. Platelets also bind tumor cells in the circulation, which may assist tumor cells in evading the immune system [11]. Thus, it is not surprising that inhibition of platelet-tumor cell interactions diminishes the formation of metastases [10,11].
In this study, we compared the tumor secretome with the host response to cancer growth by measuring not only freely circulating growth factors but also the ones stored and released by platelets. Further, on the basis of our animal model data, we predicted that monocyte chemotactic protein-1/CCL2 (MCP-1) and tumor necrosis factor α (TNFα) might serve as markers of tumor presence. Indeed, this was confirmed in patients with prostate cancer.
Materials and Methods
Mouse Injection
Eight-week-old male NOD.CB17-Prkdcscid/J (Jackson Laboratory, Bar Harbor,ME)mice were injected subcutaneously with PBS (control; n = 5) or 4 x 105 LNCaP-C4-2 human prostate cancer cells in PBS per side (n = 5). LNCaP-C4-2 cells were provided by Dr Lloyd A. Culp (Case Western Reserve University, Cleveland, OH). Tumors were permitted to grow for 28 days before mice were euthanized.
Platelet Isolation and Activation
While mice were anesthetized, the vena cava of age- and sex-matched mice was exposed, and blood was collected from the vein into acid-citrate-dextrose buffer containing 1 µg/ml prostaglandin E1 (Sigma, St Louis, MO). Platelets and plasma were separated from the plateletrich plasma of blood pooled from four to five mice by gel filtration, as previously described [12]. The activated supernatant was collected by centrifugation after platelets were treated with 100 nM phorbol 12-myristate 13-acetate (PMA; Sigma), 1mMprotease inhibitors (completeMini; Roche, Indianapolis, IN), and 500 µM mouse PAR-4-amide (Bachem, Torrance, CA) at 37°C for 45 minutes to stimulate release of granular contents.
Protein Arrays
The plasma and activated platelet supernatants were assayed with custom-designedQuantibody protein arrays from RayBiotech (Norcross, GA). A human-specific array was used to detect circulating proteins derived from the tumor. A mouse-specific array was used to detect circulating proteins derived from the host. Arrays were assayed according to the manufacturer's protocol. Fluorescently labeled arrays (green fluorescence, Cy3 channel; 555 nm excitation and 565 nm emission) were analyzed using an Axon 4000B (Molecular Devices, Sunnyvale, CA) scanner in the LRI Genomics Core. Values of four replicates for each protein were extracted using the Axon Gene Pix Pro 4.1 software and analyzed using the RayBiotech Custom Raybio Q-Analyzer software, which uses standardized dilutions of each protein to create standard curves used in determining the concentration (pg/ml) of each protein in the samples.
Clinical Samples
An approval from the Cleveland Clinic Institutional Review Board was obtained before the initiation of blood sample collection from patients undergoing radical prostatectomy at the Cleveland Clinic Glickman Urological and Kidney Institute. A patient consent form was specifically created for the study with clearly stated goals and for describing the purpose of our research. Whole blood (3–4 ml) was collected by venipuncture in Na2EDTA tubes (BD Biosciences, San Jose, CA) from patients before surgery and 3 months postoperatively. Plasma and platelets were isolated from whole blood by centrifugation and gel filtration, as previously described [12]. Platelets were activated with 50 µM human TRAP-6-amide (Bachem), 1 mM protease inhibitors, and 100 nM PMA to stimulate granule release.
ELISAs
Isolated plasma and platelet releasates from six patients were assayed using the RayBiotech Human MCP-1 ELISA or Human TNFα ELISA according to the manufacturer's instructions. Two separate samples from each patient at each time point were analyzed and compared with a standard curve to obtain the concentration (pg/ml) of each protein in the samples.
Statistical Analysis
Student's t test analysis or one-way ANOVA with Newman-Keuls posttest was used to determine statistical significance using GraphPad Prism 4.03 software (La Jolla, CA). *P < .05, **P < .01, and ***P < .005.
Results
To compare the tumor secretome to the host tissue defense, we injected human prostate cancer cells into immunocompromised mice (mice without tumors served as controls) and performed a protein array analysis of human or murine proteins in two compartments: plasma and platelet releasates. Candidate proteins were chosen on the basis of their potential roles in cancer progression, invasion, and bone metastasis. Our species-specific system allowed for the detection and quantitative analysis of tumor and host secretomes separately. On the basis of antibody specificity, we measured 15 and 22 proteins of host and tumor origin, respectively. No cross-reaction of antibodies was observed because control samples from mice without tumors were negative for human proteins. A complete list of assayed growth factors and cytokines secreted by host or tumor and their respective values in plasma and platelets is presented in Table 1.
Table 1.
Growth Factor and Cytokine Concentration Values in Plasma and Platelet Releasates.
| Cytokine | Host-Derived | Tumor-Derived | ||||||
| Control Plasma (pg/ml) | Control Releasate (pg/ml) | Tumor Plasma (pg/ml) | Tumor Releasate (pg/ml) | Control Plasma (pg/ml) | Control Releasate (pg/ml) | Tumor Plasma (pg/ml) | Tumor Releasate (pg/ml) | |
| G-CSF | 33,687.5 ± 2321.4* | 9842.4 ± 1452.2 | 57,659.1 ± 9312.4* | 9076.3 ± 2028.7 | 0 ± 0 | 0 ± 0 | 1004.3 ± 72.5† | 59.9 ± 40.6† |
| GM-CSF | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 269.4 ± 8.2‡ | 282.5 ± 55.4‡ |
| HGF | 5965.6 ± 1268.4§ | 12,119.0 ± 594.1 | 27,017.2 ± 4024.8§ | 5519.9 ± 1472.5 | 0 ± 0 | 0 ± 0 | 7292.9 ± 2161.0† | 4235.5 ± 673.9† |
| IL-1β | 5275.7 ± 665.7§ | 3422.1 ± 842.5* | 20,152.1 ± 5530.1§ | 5622.0 ± 1918.3* | 0 ± 0 | 0 ± 0 | 78.5 ± 25.4¶ | 88.1 ± 6.8¶ |
| IL-6 | 0 ± 0# | 19,622.4 ± 2220.8 | 9506.7 ± 3220.1# | 23,512.8 ± 2197.8 | 0 ± 0 | 0 ± 0 | 5739.5 ± 1998.4** | 18,078.0 ± 745.4** |
| MCP-1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0‡,†† | 7100.5 ± 777.0‡,†† |
| M-CSF | 0 ± 0# | 449.8 ± 57.3* | 1337.4 ± 320.8# | 1315.5 ± 171.6* | 0 ± 0 | 0 ± 0 | 81.8 ± 5.5** | 1332.3 ± 74.8** |
| MMP-1 | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 12,763.8 ± 1309.5¶ | 13,742.7 ± 1341.5¶ |
| MMP-2 | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 0 ± 0†† | 2100.4 ± 256.5†† |
| MMP-3 | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 5410.1 ± 1753.6¶ | 4603.3 ± 284.1¶ |
| MMP-9 | 33,502.1 ± 3601.8* | 18,717.9 ± 2046.6 | 48,258.0 ± 9952.3* | 18,911.5 ± 3208.0 | 0 ± 0 | 0 ± 0 | 350.1 ± 165.0¶ | 267.6 ± 135.6¶ |
| MMP-13 | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 1027.6 ± 278.2† | 573.7 ± 81.3† |
| OPG | 40,446.4 ± 4821.8* | 5139.4 ± 360.5 | 50,616.8 ± 2534.0* | 3764.5 ± 263.0 | —‡‡ | —‡‡ | —‡‡ | —‡‡ |
| OPN | 260,695.0 ± 6186.7* | 1182.9 ± 77.1 | 385,459.5 ± 5903.4* | 985.5 ± 92.3 | —‡‡ | —‡‡ | —‡‡ | —‡‡ |
| RANK | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 0 ± 0†† | 5957.0 ± 1714.9†† |
| RANKL | 95,875.4 ± 9753.8 | 37,442.4 ± 6209.7 | 148,956.2 ± 19,582.6 | 43,341.1 ± 4373.9 | 0 ± 0 | 0 ± 0 | 0 ± 0†† | 5890.6 ± 607.3†† |
| SDF-1α | 0 ± 0# | 2568.3 ± 1013.9 | 2209.0 ± 1010.2# | 2621.8 ± 825.0 | 0 ± 0 | 0 ± 0 | 4437.3 ± 1480.6** | 17,563.6 ± 1039.0** |
| TGF-β1 | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 428,248.4 ± 83,344.0** | 839,997.0 ± 54,189.0** |
| TIMP-1 | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 0 ± 0†† | 1540.2 ± 275.9†† |
| TIMP-2 | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 606.9 ± 126.9¶ | 552.1 ± 59.0¶ |
| TNFα | 0 ± 0 | 14,503.4 ± 2195.0§§ | 0 ± 0 | 5773.9 ± 840.0§§ | 0 ± 0 | 0 ± 0 | 3299.1 ± 294.2** | 8489.0 ± 534.6** |
| TPO | 0 ± 0 | 4901.5 ± 808.7 | 0 ± 0 | 3446.3 ± 278.7 | 0 ± 0 | 0 ± 0 | 46,585.5 ± 29,310.7** | 73,686.7 ± 2940.7** |
| uPAR | —‡‡ | —‡‡ | —‡‡ | —‡‡ | 0 ± 0 | 0 ± 0 | 17,876.1 ± 6969.3¶ | 17,665.3 ± 2108.6¶ |
| VEGF | 2697.2 ± 227.2* | 769.3 ± 61.4 | 3802.3 ± 145.7* | 668.5 ± 145.7 | 0 ± 0 | 0 ± 0 | 1821.7 ± 488.0† | 939.3 ± 180.1† |
Plasma and platelet releasates were isolated from mice bearing LNCaP-C4-2 tumors (Tumor) and without tumors (Control) as described in Materials and Methods. Species-specific microarrays for murine (Host-derived) and human (Tumor-derived) proteins were used to determine cytokine and growth factor concentrations, represented as mean ± SEM.
For host derived proteins: 1.1- to 3-fold increase by tumor (VEGF, OPN, OPG, MMP-9, IL-1β, G-CSF, and RANKL).
For tumor-derived proteins: proteins present in plasma at the higher levels than in platelets (G-CSF, HGF, MMP-13, and VEGF).
For tumor-derived proteins: factors produced by tumor but not by the host (GM-CSF and MCP-1).
For host-derived proteins: four- to six-fold increase by tumor (HGF and IL-1β).
For tumor-derived proteins: proteins at equal levels in plasma and platelets.
For host derived proteins: secreted by host only in response to tumor (M-CSF, IL-6, and SDF-1α) with negligible amounts present in control mice.
For tumor-derived proteins: proteins present in platelets at the higher levels compared with plasma (IL-6, M-CSF, SDF-1α, TGF-β1, TNFα, and TPO).
For tumor-derived proteins: tumor-derived factors accumulated in platelets but absent in plasma.
Not determined due to appropriate antibodies being unavailable.
For host-derived proteins: total amount of protein was decreased in the presence of tumor (TNFα).
Tumor-Derived Proteins Enter the Circulation
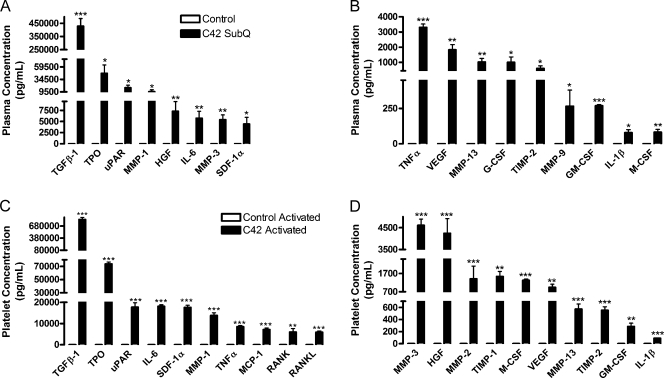
Concentrations of proteins secreted by tumor are presented in Table 1 (right column). Owing to the specificity of the arrays for human proteins, the cytokines studied were not detected in control samples, which were without human tumors (Figure 1). To further determine the impact of each tumor-derived cytokine, we calculated the percent contributions of tumor and host to the total concentrations of circulating cytokines and growth factors (Figure 2). Several important factors, such as thrombopoietin (TPO), TNFα, granulocyte-macrophage colony-stimulating factor (GM-CSF), and stromal-derived factor-1α (SDF-1α), were secreted primarily by the tumor with a negligible amount produced by host. In plasma, TPO, TNFα, and GMCSF were solely of tumor origin (Figure 2, A and B). The tumor-derived proteins present at the highest levels in plasmawere transforming growth factor (TGF)-β1, TPO, soluble urokinase-type plasminogen activator receptor (uPAR), hepatocyte growth factor (HGF), interleukin (IL)-6, and SDF-1α, with concentrations ranging from approximately 9000 to 430,000 pg/ml (Figure 1A; Table 1). The MMPs-1, -3, -9, and -13 were secreted at high concentrations (350-12,750 pg/ml) into the plasma of mice bearing tumors (Figure 1, A and B; Table 1). Further, TNFα, VEGF, granulocyte colony-stimulating factor (G-CSF), tissue inhibitor of metalloproteinase (TIMP)-2, GM-CSF, IL-1β, and macrophage colony-stimulating factor (M-CSF) circulated at lower levels (80-3300 pg/ml) in the plasma (Figure 1B; Table 1). Interestingly, MMP-13 was 1.79-fold higher in the plasma compared with the platelets. Tumor-derived G-CSF, MMP-9, and IL-1β were present at concentrations more than 50-fold lower than their host-derived counterparts in the plasma and the platelets (Table 1). Thus, tumor-derived cytokines can enter the host circulation.
Figure 1.
Concentrations of prostate tumor-derived cytokines circulating in plasma and platelets. Plasma (A, B) and releasates from platelets (C, D) activated with 500 µM PAR-4 and 100 nM PMA were isolated from mice injected subcutaneously with LNCaP-C4-2 (C42, black columns) or PBS (Control, white columns). A species-specific array for human proteins was used to determine the concentrations of tumor-derived cytokines; represented as mean values ± SEM. No tumor-derived proteins were found in the plasma or platelets of control mice, demonstrating the specificity of the array. *P < .05, **P < .01, and ***P < .005 versus control by one-way ANOVA.
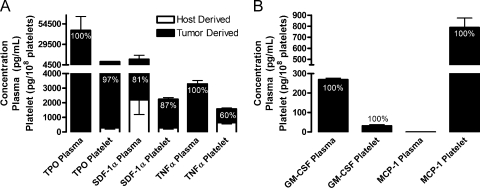
Figure 2.
Predominantly tumor contributions to cytokine concentrations in plasma and platelet releasate. (A and B) Plasma and releasates from platelets activated with 500 µM PAR-4 and 100 nM PMA (Platelets) were isolated from mice injected subcutaneously with human LNCaP-C4-2 cells. Species-specific protein arrays were used to determine the concentrations of murine (Host Derived, white columns) and human (Tumor Derived, black columns) cytokines and growth factors. Concentrations are represented as mean values ± SEM. Percentages represent the tumor contribution to the total concentration of each protein in plasma or platelets.
Tumor-Derived Proteins Are Sequestered within Platelets
In platelet releasates, 100% of the GM-CSF, 97% of the TPO, and 60% of the TNFα were tumor-derived (Figure 2, A and B). GM-CSF was exclusively tumor-produced and was present at equal concentrations in the plasma and platelets (Table 1). Tumor-derived TNFα and TPO were preferentially stored in platelets where their levels were 2.57- and 1.58-fold higher, respectively, than in plasma (Table 2). Interestingly, MCP-1 was completely tumor-derived and localized exclusively to platelets (Figure 2B), with no tumor-derived MCP-1 found in plasma and no host-derived MCP-1 present in platelets or plasma (Table 1). Thus, platelet-stored MCP-1 might serve as a promising marker of tumor presence. Likewise, tumor-derived soluble receptor activator of NF-κB (RANK), RANK ligand (RANKL), MMP-2, and TIMP-1 were present entirely in the platelet compartment and not in the plasma (Table 1). Therefore, they were present at a concentration 1000- to 5800-fold higher in the platelets compared with the plasma (Table 2). In platelets, tumor-derived TPO, uPAR, IL-6, SDF-1α, and MMP-1 concentrations ranged from approximately 13,750 to 73,750 pg/ml (Figure 1C; Table 1), whereas the amount of TNFα, MCP-1, soluble RANK, RANKL, MMP-3, and HGF in platelet releasate from mice bearing tumors ranged from 4000 to 8500 pg/ml (Figure 1, C and D; Table 1). MMP-2, TIMP-1, M-CSF, and VEGF were present at concentrations between approximately 950 and 2100 pg/ml (Figure 1D; Table 1). At the lowest concentration in platelets were tumor-derived MMP-13, TIMP-2, GM-CSF, and IL-1β, with concentrations from approximately 85 to 575 pg/ml (Figure 1D; Table 1). Tumor-derived VEGF is 1.4-fold higher in platelets compared with host, demonstrating that tumor-derived proteins can be preferentially stored in platelets. In addition, tumor-derived M-CSF and IL-6 were 12.21- and 4.35-fold higher, respectively, in platelets compared with plasma (Table 2). These observations indicate that the uptake of certain growth factors by platelets is not driven by their concentration gradient but that it seems to be selective.
Table 2.
Fold Change of Tumor-Derived Proteins in Activated Platelets over Plasma in Tumor Bearing Mice.
| Cytokine | Fold Change Activated Releasate: Plasma |
| IL-6 | 4.35 |
| MCP-1 | 7100.49 |
| M-CSF | 12.21 |
| MMP-2 | 1050.19 |
| RANK | 4467.74 |
| RANKL | 5890.61 |
| SDF-1α | 3.96 |
| TGF-β1 | 1.96 |
| TIMP-1 | 1155.15 |
| TNFα | 2.57 |
| TPO | 1.58 |
Plasma and releasates from platelets activated with 500 µM PAR-4 and 100 nM PMA were isolated from mice bearing subcutaneous LNCaP-C4-2 tumors. A human-specific microarray was used to analyze the protein concentrations that are represented as fold change of mean activated platelet releasate concentrations over mean plasma.
Remarkably, TGF-β1 was present at the highest concentration in the platelet compartment (Figure 1C). TGF-β1 promotes tumor progression and skeletal metastasis of several cancers [6,7]. Most studies describe increased TGF-β1 within the plasma of cancer patients; however, the origin of TGF-β1 in patient plasma has remained an open question. Our analysis showed that TGF-β1 was produced by the tumor reaching concentrations of 428,248 and 839,997 pg/ml in plasma and platelets, respectively, of tumor-bearing mice (Figure 1, A and C; Table 1). Interestingly, tumor-derived TGF-β1 was 1.96-fold higher in the platelets compared with the plasma (Table 2). Thus, TGF-β1 was found at the highest concentration of all the proteins tested in both the plasma and platelets. In agreement with previous studies, it seems that platelets serve as a key reservoir of TGF-β1 in the circulation [4,13].
Previous studies have postulated that SDF-1α is produced by the stromal cells surrounding the tumor [14]. In our study, SDF-1α circulated in the plasma and platelets and was predominantly produced by the tumor (87% and 81%, respectively; Figure 2A). The tumor-derived SDF-1α concentration was 3.96-fold higher in the platelets of mice compared with plasma (Table 2). Platelet-derived SDF-1α has been shown to stimulate the adhesion and recruitment of bone marrow-derived endothelial progenitors to arterial thrombi [15]. This mechanism may also be used by tumors to promote vascularization and support tumor growth, thus accounting for the high levels of SDF-1α produced by the tumor.
Host-Derived Proteins Are Concentrated in Plasma
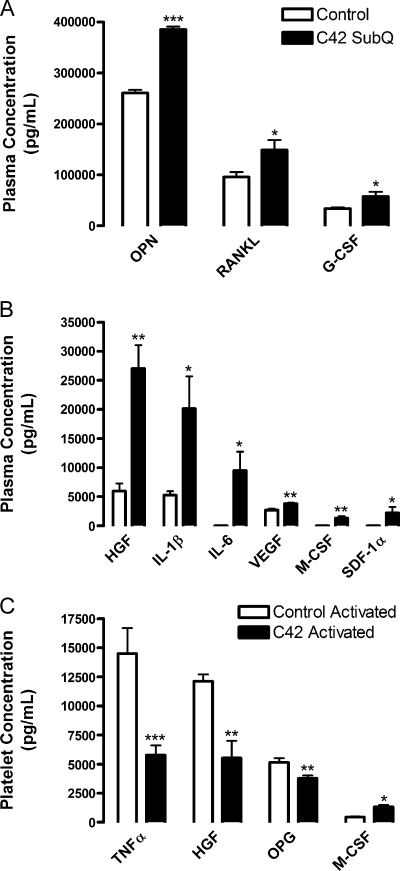
As shown in Table 1, host tissues responded to the presence of tumor and contributed significantly to the pool of factors in plasma as well as in platelets (left column). Many of these factors were increased in response to tumor growth in the plasma of mice (Figure 3, A and B). Previous studies have shown that both the tumor and the microenvironment were able to secrete several cytokines, including HGF, IL-6, SDF-1α, M-CSF, and VEGF [14,16–18]. Remarkably, we found that HGF, M-CSF, IL-6, and even VEGF were largely host-derived in the plasma: 79%, 71%, 62%, and 68%, respectively (Figure 4, A and B). At lower concentrations, ranging from 2000 to 27,000 pg/ml, host-derived HGF, IL-6, VEGF, and M-CSF were increased in the plasma of mice bearing tumors (Figure 3B; Table 1). Host-derived HGF levels were 4.53-fold higher in the plasma of mice with tumors compared with control (Table 3). Host-derived SDF-1α levels in plasma were increased from 0 to 2209 pg/ml in response to tumor presence, whereas the platelet concentrations did not change (Table 1). In addition, host-derived M-CSF and IL-6 were present only in the plasma of animals with tumor (Table 1). Thus, some proteins in the plasma of mice with tumors are produced by both the host and the tumor.
Figure 3.
Concentrations of host tissue-derived cytokines circulating in plasma and platelets in the presence or absence of tumor. Plasma (A, B) and releasates from platelets (C) activated with 500 µM PAR-4 and 100 nM PMA were isolated from mice injected subcutaneously with LNCaP-C4-2 tumor cells (C42, black columns) or PBS (Control, white columns). A species-specific array for murine proteins was used to determine the concentrations of microenvironment-derived cytokines represented as mean values ± SEM. *P < .05, **P < .01, and ***P < .005 versus control by one-way ANOVA.
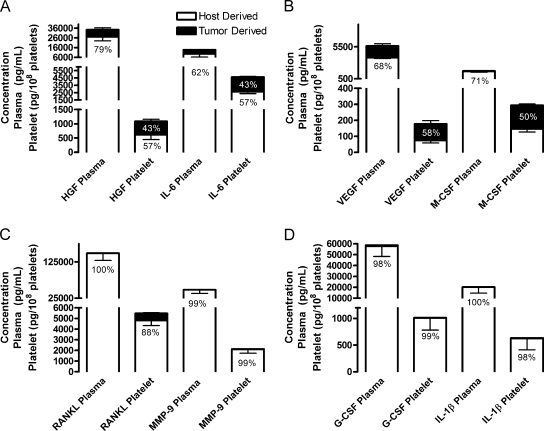
Figure 4.
Predominantly host contributions to cytokine concentrations in plasma and platelet releasate. (A–D) Plasma and releasates from platelets activatedwith 500 µM PAR-4 and 100 nMPMA (Platelets) were isolated frommice injected subcutaneouslywith human LNCaP-C4-2 cells. Species-specific protein arrays were used to determine the concentrations ofmurine (Host Derived, white columns) and human (Tumor Derived, black columns) cytokines and growth factors. Concentrations are represented as mean values ± SEM. Percentages represent the contribution of host (in black) or tumor (in white) to the total protein in plasma or platelets.
Table 3.
Fold Change of Host-Derived Proteins in Mice Bearing Tumors over Control Mice.
| Cytokine | Plasma | Activated Platelets |
| G-CSF | 1.71 | 0.94 |
| HGF | 4.53 | 0.46 |
| IL-1β | 3.82 | 1.67 |
| IL-6 | 9406.73 | 1.21 |
| M-CSF | 1337.45 | 2.97 |
| MMP-9 | 1.44 | 1.02 |
| OPG | 1.25 | 0.74 |
| OPN | 1.47 | 0.84 |
| RANKL | 1.55 | 1.17 |
| SDF-1α | 2208.98 | 1.03 |
| TNFα | —* | 0.40 |
| TPO | —* | 0.70 |
| VEGF | 1.41 | 0.88 |
Plasma and releasates from platelets activated with 500 µM PAR-4 and 100 nM PMA were isolated from mice injected with subcutaneous LNCaP-C4-2 tumors or PBS (control). A murine-specific microarray was used to analyze the protein concentrations that are represented as fold change of mean tumor concentrations over mean control.
None detected.
In response to the presence of a tumor, host tissues upregulated secretion of other factors, including RANKL, G-CSF, MMP-9, and IL-1β. These proteins can interact with the immune system and may control the host tissue's defense against invading cancer cells. Host-derived RANKL, G-CSF, MMP-9, and IL-1β represented 100%, 98%, 99%, and 99.6%, respectively, of the total amount of protein secreted into plasma (Figure 4, C and D). In the plasma, host-derived osteopontin (OPN), RANKL, and G-CSF circulated at high concentrations in response tumor; ranging from approximately 57,500 to 385,500 pg/ml (Figure 3A; Table 1). Osteoprotegrin (OPG) and MMP-9 circulated at concentrations around 50,000 pg/ml in the plasma of mice with tumors (Table 1). Host-derived G-CSFandMMP-9 were 1.71 and 1.44 higher, respectively, in the plasma of mice with tumors compared with controls but were unchanged in the platelets (Table 3). In addition, hostderived IL-1β and RANKL were 3.82- and 1.55-fold higher, respectively, in the plasma of mice with tumors compared with controls (Table 3). Another two factors involved in tumor metastasis were upregulated in the presence of tumor: host-derived OPG and OPN by 1.25- and 1.47-fold, respectively (Table 3). Thus, several factors are upregulated by the host tissue in response to the tumor, although some may exacerbate tumor growth.
VEGF is the most studied tumor-secreted proangiogenic factor, and it was shown to be present at high levels in plasma and in platelets of cancer patients [1,2,8,19]. These studies, however, do not differentiate between tumor- and host-derived VEGF. Surprisingly, more than a half of VEGF in plasma was secreted by the host tissues (Figure 4B). In response to tumor, host tissues upregulated their VEGF secretion into the plasma from 2697 to 3802 pg/ml (Figure 3B), whereas no changes occurred in platelet VEGF concentrations (Table 1). Both host- and tumor-derivedVEGFs were at higher concentrations (5.69- and 1.94-fold, respectively) in the plasma compared with platelets of mice bearing tumors (Table 1). Thus, our data demonstrate that the changes seen in the plasma VEGF levels of patients may represent the combined effect of tumor- and host-produced VEGF.
Host-Derived Proteins Do Not Accumulate in Platelets
Interestingly, only the concentrations of TNFα, HGF, OPG, and M-CSF were altered significantly in the platelet releasate of mice bearing tumors compared with control (Figure 3C; Table 1). Of these factors, only host-derived M-CSF increased 2.97-fold (Table 3) in response to tumor in the platelet compartment from 450 to 1315 pg/ml (Table 1), whereas OPG, HGF, and TNFα were reduced (Figure 3C). Host-derived G-CSF, MMP-9, IL-1β, and RANKL were 99%, 99%, 98%, and 88%, respectively, of the total platelet compartment contents (Figure 4, C and D); however, their concentrations were largely unaffected by the tumor presence. Host-derived IL-1β, IL-6, andRANKL were 1.67-, 1.21-, and 1.17-fold higher, respectively, in the platelets of mice with tumors compared with controls (Table 3), although the change was not significant. The platelet levels of HGF, M-CSF, IL-6, and VEGF were approximately 50% tumor-derived and 50% hostderived (Figure 4, A and B). Overall, the amount of total host-derived HGF was increased by 1.80-fold in the presence of tumor. Hostderived TNFα and TPO were absent in the plasma, and their levels in platelets were reduced in the presence of tumors by 2.5- and 1.40-fold, respectively (Table 3). Thus, our data indicate that host-derived proteins circulate at greater concentrations in the plasma in response to tumor growth and are not preferentially sequestered in platelets. Some cytokines and growth factors were produced by host tissues as a defense mechanism against further tumor growth and invasion.However,many of these factors can also promote tumor growth and invasiveness, rendering the host defense futile.
On the basis of our results, it seems that tumor-derived proteins were more likely to be selectively accumulated in platelets compared with hostderived proteins. In general, 11 (50%) of 22 tested tumor-derived proteins were enriched in the platelet compartment over plasma compared with 3 (20%) of 15 tested host-derived proteins. Several tumor-derived factors, such as MMP-1,MMP-3,MMP-13, uPAR, and TIMP-2, were of approximately equal concentrations in plasma and platelets.
MCP-1 and TNFα as Biomarkers for Tumor Presence in Prostate Cancer Patients
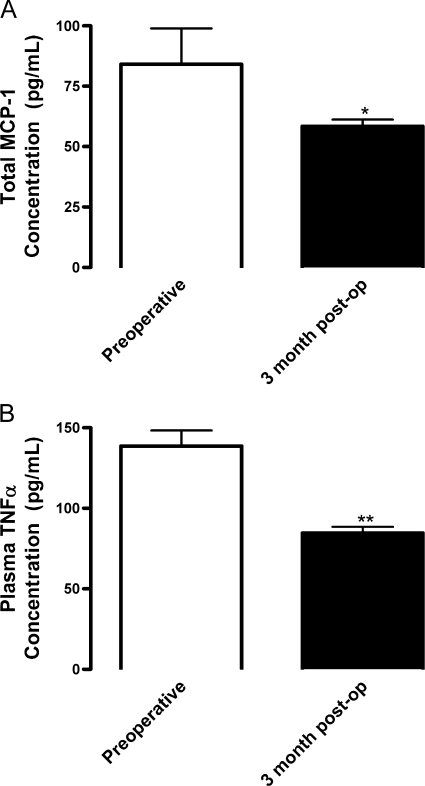
Our studies demonstrated that MCP-1 was secreted solely by the tumor and was present exclusively in the platelets of tumor-bearing mice (Figure 2B). TNFα was also completely tumor-derived within the plasma, and tumor-secreted TNFα comprised 60%of the platelet compartment (Figure 2A). To assess the validity of our findings in cancer patients, we determined the levels of MCP-1 and TNFα in prostate cancer patients before surgery and 3 months after radical prostatectomy. Owing to the timing of blood collection from patients, platelets were partially activated (28%) before processing and, at least in part, had released their α-granular contents into plasma (data not shown). Indeed, we were able to detect MCP-1 not only in the platelet compartment (2.38 ± 0.07 pg/108 platelets) but also in the plasma (26.40 ± 4.83 pg/ml) of patients with prostate cancer before surgery. Nevertheless, as predicted on the basis of our animal model, total MCP-1 was present at a 1.44-fold higher concentration in patients before surgery (84.09 ± 14.86 pg/ml) than 3 months after surgery (58.47 ± 2.77 pg/ml; Figure 5A). Thus, MCP-1 in the blood was largely produced by the tumor, comparable to our mouse model.
Figure 5.
MCP-1 and TNFα concentrations decrease after tumor removal in prostate cancer patients. Plasma and releasate from platelets activated with 50 µM TRAP-6 and 100 nM PMA were isolated from whole blood collected from patients undergoing radical prostatectomy (Preoperative) and 3 months after tumor removal (3 month post-op) and analyzed by ELISA (n = 12). (A) Mean concentrations ± SEM of combined plasma and releasate concentrations are shown. (B) Mean concentrations ± SEM of plasma concentrations are shown. *P < .05 and **P < .01 versus Preoperative by Student's t test.
TNFα was also present in both the plasma and the platelets of patients with prostate cancer before surgery. In our mouse model, tumor implantation resulted in decreased host-derived TNFα in platelets compared with control animals (Figure 3C). Conversely, tumor-derived TNFα compensated for the decrease in host production, comprising 100% of plasma TNFα and 60% of platelet TNFα (Figure 2A). Thus, in ourmousemodel, total platelet TNFα concentrations remained constant (Table 1). Conversely, TNFα in the plasma of tumor-bearing animals was solely tumor-derived (Figure 1C), and TNFα was absent in the plasma of control animals. Correspondingly, in patients, the TNFα concentrationwas equal in platelets before (76.16 ± 0.85 pg/108 platelets) and after surgery (78.17 ± 0.99 pg/108 platelets), suggesting that the host tissue may upregulate production to maintain a homeostasis of TNFα in the platelets after tumor removal. Interestingly, similar to our model, the plasma TNFα concentration was 1.72-fold higher in patients before surgery (146.08 ± 10.05 pg/ml) compared with 3 months postoperatively (85.05 ± 3.19 pg/ml; Figure 5B). These data support the notion that the tumor substantially contributes to the levels of MCP-1 and TNFα in blood. Thus, MCP-1 and TNFα might serve as indicators of the tumor presence and/or progression. To improve detection methods, not only plasma but also activated platelet contents should be used for diagnostic tests.
Discussion
In this study, we reveal the origin of various cancer-related cytokines and growth factors and establish their methods of circulation during tumor growth. Using species-specific protein arrays, we quantified the concentrations of tumor- and host-derived factors in the plasma and in the platelet compartment. Our main conclusions are the following: 1) Tumors secrete a number of factors that circulate in the plasma and/or that are sequestered by platelets and can be released on platelet activation. Among these many factors, tumor-derived TGF-β1 is present at the highest concentration in platelet releasate. 2) Several tumor-derived factors are present exclusively in platelets: MCP-1, MMP-2, RANK, RANKL, and TIMP-1. 3) Host tissues respond to the tumor presence by upregulating their secretion of a number of factors. The most dramatic increases stimulated by tumor were observed for HGF and IL-1β. Several factors, namely, IL-6, M-CSF, and SDF-1α, are present in the plasma of tumor-bearing animals but not control animals. These factors, however, can be found in the platelet compartment of both control and tumor-bearing mice. 4) Tumor-derived factors are more likely to be accumulated in platelets compared with host-derived factors. For example, during tumor growth,M-CSF of host origin is equally distributed between the plasma and platelets; however, tumor-derived M-CSF is 12-fold higher in platelets than in plasma (Table 2). Conversely, host-derived proteins remain mainly in the plasma. 5) It seems that accumulation of tumor-derived factors in platelets is not driven by the gradient of their concentration in plasma because there are factors absent in the plasma but present in platelets (tumor-derived MCP-1, MMP-2, RANK, RANKL, and TIMP-1; and host-derived TNFα and TPO), and there are factors abundant in the plasma but not in platelets (host-derived G-CSF, HGF, IL-1β, MMP-9, and OPG).
Our results demonstrate that the tumor produces high levels of cytokines and growth factors known to promote tumor growth and metastasis and that these factors can be sequestered in and released by platelets. Many of these factors secreted by tumors or their microenvironment might serve as new biomarkers of cancer progression or as targets for cancer treatment and, therefore, warrant further study. The correlations between the plasma concentrations of certain cytokines with cancer severity in patients have been documented, as summarized in Table W1. For example, plasma IL-6 was increased in patients with a high-stage cancer and correlated with the metastatic potential of the tumor (Table W1) [20,21]. Our study shows that the presence of tumor increases both tumor- and host-derived IL-6 in plasma. In addition, significant amounts of IL-6 are stored in platelets. Another study demonstrated that MCP-1 levels in plasma are higher in cancer patients compared with healthy individuals (Table W1) [22].We show that MCP-1 is entirely produced by tumor and is stored in platelets, which makes this chemokine a very attractive biomarker of and therapeutic target for cancer. In addition, there were multiple reports implicating MMPs in cancer progression. For example, MMP-2 was shown to serve as a marker of prostate and thyroid cancers [23]. Our study shows that a majority of tumor-secreted MMP-2 is stored in platelets; therefore, measurements should be performed using the platelet releasate not plasma as a source of MMP-2. Of interest are factors involved in bone metabolism and, potentially, in tumor metastasis to the bone, such as soluble RANK and RANKL [24–26]. Indeed, a previous study reported that, in neuroblastoma patients, RANKL is increased with stage, metastasis, and unfavorable tissue pathohistology [24]. We show that in plasma, the presence of tumor results in upregulation of RANKL, which is exclusively produced by host tissues and not by tumor. Conversely, tumor-derived RANKL is stored in platelets. Along with RANKL, platelet-stored soluble RANK seems to serve as a promising biomarker. RANKL and soluble RANK are believed to target bone tissues and control tumor metastasis [27,28]; thus, it seems that communication between tumor and bone may be mediated by platelets as carriers of factors stored in their granules. Thus, we demonstrate for the first time that many factors believed to be produced by tumor are, in fact, secreted by the host tissues in response to the tumor presence. For example, less than half of total VEGF is tumor-derived; thus, host tissues serve as a main source of this growth factor in tumorbearing animals.
In many cases, the concentrations we report here are several fold higher than those reported for humans (Table W1), which is due to the overall ratio of tumor to body volume in our animal model and, possibly, to the sensitivity of our method. The concentration of TNFα measured in our clinical samples was higher than the previously reported value of 3.95 pg/ml in the serum of prostate cancer patients [21]. Conversely, MCP-1 measured in our study was slightly lower than the reported 365.26 pg/ml in the serum of patients with acute myeloid leukemia [22]. These discrepancies may be due to differences in antibodies, collection methods, the blood component studied, patient demographics, or type of cancer studied. However, overall, the values are comparable. For example, MMP-1 concentration in plasma is 15,000 and 13,000 pg/ml on the basis of literature and our xenograft measurements, respectively. For many other factors, such as M-CSF, MMP-9, TNFα, and SDF-1α, host- and tumorderived proteins combine to produce similar concentrations to those seen in humans. Thus, our system seems to be equally or more sensitive for a number of potential biomarkers. Most importantly, we show that platelet releasate represents a better source for many of these proteins.
Our study identifies several tumor-derived factors that are dramatically enriched in platelets compared with plasma. Platelet sequestered proteins likely play a role in the interactions between the tumor and distant organs, including future metastatic sites, because sequestered proteins can be released at higher concentrations at specific locations, whereas proteins in the plasma could be degraded, used, or neutralized anywhere in the body. Intriguingly, we found that tumorderived proteins were preferentially sequestered within platelets and released on platelet activation. Thus, platelets, as specific carriers of the tumor secretome, seem to be crucial mediators of interactions between the tumor and host tissues. In summary, our study identified several potential biomarkers of cancer progression and therapeutic targets for cancer treatments. Many of these proteins are not present in plasma and must be measured in the platelet compartment or in plateletrich plasma.
Supplementary Material
Acknowledgements
The authors thank Pieter Faber of the LRI Genomics Core for assistance with the microarray analysis, Angela Leschinsky for her assistance in clinical sample collection, and Miroslava Tischenko for her technical assistance.
Abbreviations
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HGF
hepatocyte growth factor
- IL
interleukin
- MCP-1
monocyte chemotactic protein 1
- MMP
matrix metalloproteinase
- OPG
osteoprotegrin
- OPN
osteopontin
- RANK
receptor activator of NF-κB
- RANKL
RANK ligand
- SDF-1α
stromal-derived factor-1β
- TGF-β1
transforming growth factor-β1
- TIMP
tissue inhibitor of metalloproteinase
- TNFα
tumor necrosis factor α
- TPO
thrombopoietin
- uPAR
urokinase-type plasminogen activator receptor
- VEGF
vascular endothelial growth factor
Footnotes
This study was supported by research funding from the National Institutes of Health/National Cancer Institute (grant no. CA126847) to T.V.B. The authors declare no competing financial interests.
This article refers to a supplementary material, which is designated by Table W1 and is available online at www.neoplasia.com.
References
- 1.Manenti L, Paganoni P, Floriani I, Landoni F, Torri V, Buda A, Taraboletti G, Labianca R, Belotti D, Giavazzi R. Expression levels of vascular endothelial growth factor, matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 and 2 in the plasma of patients with ovarian carcinoma. Eur J Cancer. 2003;39:1948–1956. doi: 10.1016/s0959-8049(03)00427-1. [DOI] [PubMed] [Google Scholar]
- 2.Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res. 1999;5:487–491. [PubMed] [Google Scholar]
- 3.Zhong W-d, Han Z-d, He H-c, Bi X-c, Dai Q-S, Zhu G, Ye Y-k, Liang Y-x, Qin W-j, Zhang Z, et al. CD147, MMP-1, MMP-2 and MMP-9 protein expression as significant prognostic factors in human prostate cancer. Oncology. 2008;75:230–236. doi: 10.1159/000163852. [DOI] [PubMed] [Google Scholar]
- 4.Stellos K, Gawaz M. Platelet interaction with progenitor cells: potential implications for regenerative medicine. Thromb Haemost. 2007;98:922–929. doi: 10.1160/th07-02-0147. [DOI] [PubMed] [Google Scholar]
- 5.Méndez-Ferrer S, Frenette PS. Hematopoietic stem cell trafficking: regulated adhesion and attraction to bone marrow microenvironment. Ann N Y Acad Sci. 2007;1116:392–413. doi: 10.1196/annals.1402.086. [DOI] [PubMed] [Google Scholar]
- 6.Baselga J, Rothenberg ML, Tabernero J, Seoane J, Daly T, Cleverly A, Berry B, Farrington DL, Wallace LA, Yingling JM, et al. TGF-β signalling-related markers in cancer patients with bone metastasis. Biomarkers. 2008;13:217–236. doi: 10.1080/13547500701676019. [DOI] [PubMed] [Google Scholar]
- 7.Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, Slawin KM. Preoperative plasma levels of transforming growth factor beta1 (TGF-β1) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19:2856–2864. doi: 10.1200/JCO.2001.19.11.2856. [DOI] [PubMed] [Google Scholar]
- 8.Klement GL, Yip T-T, Cassiola F, Kikuchi L, Cervi D, Podust VN, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113:2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho-Tin-Noé B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68:6851–6858. doi: 10.1158/0008-5472.CAN-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–1019. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byzova TV, Plow EF. Networking in the hematostatic system. Integrin αIIbβ3 binds prothrombin and influences its activation. J Biol Chem. 1997;272:27183–27188. doi: 10.1074/jbc.272.43.27183. [DOI] [PubMed] [Google Scholar]
- 13.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Massberg S, Konrad I, Schürzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, et al. Platelets secrete stromal cell-derived factor 1α and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminski A, Hahne JC, Haddoutiel M, Florin A, Wellmann A, Wernert N. Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int J Mol Med. 2006;18:941–950. [PubMed] [Google Scholar]
- 17.Ara T, Song L, Keshelava N, Russell HV, Metelista LS, Groshen SG, Seeger RC, DeClerck YA. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Haile S, Comuzzi B, Tien AH, Wang J, Yong TMK, Jelescu-Bodos AE, Blaszczyk N, Vessella RL, Masri BA, et al. Osteoblast-derived factors induce an expression signature that identifies prostate cancer metastasis and hormonal progression. Cancer Res. 2009;69:3433–3442. doi: 10.1158/0008-5472.CAN-08-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDowell G, Temple I, Kirwan CC, Bundred NJ, McCollum CN, Burton IE, Kumar S, Byrne GJ. Alteration in platelet function in patients with early breast cancer. Anticancer Res. 2005;25:3963–3966. [PubMed] [Google Scholar]
- 20.Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. Circulating levels of inflammatory cytokines and risk of colorectal carcinomas. Cancer Res. 2008;68:323–328. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-β1 in patients with metastatic prostate carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 22.Mazur G, Wrobel T, Butrym A, Kapelko-Slowik K, Poreba R, Kuliczkowski K. Increased monocyte chemoattractant protein 1, (MCP-1/CCL-2) serum level in acute myeloid leukemia. Neoplasma. 2007;54:285–289. [PubMed] [Google Scholar]
- 23.Morgia G, Falsaperla M, Malaponte G, Madonia M, Indelicato M, Travali S, Mazzarino MC. Matrix metalloproteinase as diagnostic, (MMP-13) and prognostic, (MMP-2, MMP-9) markers of prostate cancer. Urol Res. 2005;33:44–50. doi: 10.1007/s00240-004-0440-8. [DOI] [PubMed] [Google Scholar]
- 24.Granchi D, Garaventa A, Amato I, Paolucci P, Baldini N. Plasma levels of receptor activator of nuclear factor-κB ligand and osteoprotegerin in patients with neuroblastoma. Int J Cancer. 2006;119:146–151. doi: 10.1002/ijc.21783. [DOI] [PubMed] [Google Scholar]
- 25.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 26.Baud'huin M, Duplomb L, Ruiz Velasco C, Fortun Y, Heymann D, Padrines M. Key roles of the OPG-RANK-RANKL system in bone oncology. Expert Rev Anticancer Ther. 2007;7:221–232. doi: 10.1586/14737140.7.2.221. [DOI] [PubMed] [Google Scholar]
- 27.Blouin S, Baslé MF, Chappard D. Interactions between microenvironment and cancer cells in two animal models of bone metastasis. Br J Cancer. 2008;98:809–815. doi: 10.1038/sj.bjc.6604238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.