Abstract
Objective
Both the size and diversity of an individual’s social network are strongly and prospectively linked with cardiovascular morbidity and mortality. Social relationships may influence cardiovascular outcomes, at least in part, via their impact on physiologic pathways influenced by stress, such as daytime blood pressure (BP) levels. However, scant research has examined whether social relationships influence key nocturnal pathways, such as nocturnal BP dipping.
Methods
The current study examined the degree to which social integration, as measured by participants’ reported engagement in a range of different types of social relationships, and the frequency of daily social contacts influence the ratio of night/day mean arterial pressure (MAP) in a community sample of African–American and white men and women (N = 224). In addition, we examined the degree to which observed associations persisted after statistical adjustment for factors known to covary with nocturnal BP, including objective measures of sleep, catecholamines, health behaviors, and comorbidities.
Results
In fully adjusted models, there was a significant association between both social integration and frequency of social contacts and the ratio of night/day MAP, indicating that socially isolated individuals were more likely to have blunted nocturnal BP-dipping profiles. There was also a significant interaction between social contact frequency and ethnicity, suggesting that the benefits of social relationships were particularly evident in African–Americans.
Conclusions
These findings contribute to our understanding of how social integration or conversely, social isolation, influences cardiovascular risk.
Keywords: ethnic differences, nocturnal blood pressure, psychosocial risk factors, social contacts, social networks
Introduction
Substantial prospective evidence documents the significant impact of social relationships on all-cause and disease-specific morbidity and mortality [1,2]. In particular, epidemiologic data suggest that people who have larger and more integrated social networks are at reduced risk for mortality [1], ischemic heart disease [3], and stroke [4] and have better prognoses following myocardial infarction (MI) [5] as compared to their more socially isolated counterparts. These associations are due, at least in part, to the attenuating effects of social relationships on daytime stress-related physiological pathways [6–8]. From an evolutionary perspective, social relationships may be particularly relevant for regulating physiological arousal at night-time, a period characterized by reduced responsiveness to the external environment and subsequent heightened vulnerability to potential threats. However, an important unanswered question is whether the benefits of social relationships extend into the night to influence key nocturnal risk factors for cardiovascular disease (CVD).
In particular, there is growing evidence that nocturnal blood pressure (BP) or a relatively small decline in nocturnal BP relative to daytime, is a significant independent risk factor for CVD morbidity and mortality [9] as well as target organ damage [10,11]. To our knowledge, four previous studies have examined different aspects of the social environment in relation to nocturnal BP, and their results are equivocal [12–15]. Moreover, the limited extant literature has focused exclusively on measures of close relationships, including marital status. No research in this area has evaluated the broader social networks with whom individuals may regularly come into contact, including clubs or organizations, neighbors, and religious groups. Importantly, these more extended social contacts may be particularly relevant for older populations and minority groups [16]. Moreover, evaluating the actual frequency of social contacts or lack thereof in the naturalistic environment may provide a more sensitive and ecologically valid examination of the association between social relationships and nocturnal dipping. Finally, an important limitation of the extant literature on social relationships and nocturnal BP is the failure to control for key covariates that may account for the associations, which preclude definitive conclusions regarding the independent impact of social relationships on nocturnal BP.
Therefore, the purpose of the present study was to conduct a multimodal examination of social relationships and BP dipping in a community sample of African–American and white adults, and to determine whether these relationships persist after statistically adjusting for relevant health behaviors and comorbidities, as well as a host of additional variables known to covary with nocturnal BP, including sleep characteristics [17–19], and catecholamines [20]. We incorporated a questionnaire measure of social integration comprised of both close family relationships and acquaintances, and a daily diary measure of the frequency of social interactions, to evaluate both the breadth and frequency of social contacts. Finally, given previous evidence that African–Americans are at greater risk for hypertension and are more likely to show blunted nocturnal BP declines as compared to whites [21], we explored whether there were ethnic differences in the association between social integration or frequency of social contacts and dipping status.
Methods
Participants
As described in detail in a previous publication [19], 224 participants with high/moderate or low Framingham risk scores were recruited from Heart Strategies Concentrating on Risk Evaluation (HeartSCORE), a large epidemiological study of cardiovascular risk in a community sample of African–Americans and whites. Eligibility for HeartSCORE required that participants be between the ages of 45 and 75 years, reside in the greater Pittsburgh metropolitan region, and without comorbid conditions expected to limit life expectancy to less than 5 years. Additional exclusionary criteria for the current study included pregnancy, use of continuous positive airway treatment for sleep-disordered breathing, regular use of medications for sleep problems, night-shift work, medication for diabetes, and prior diagnosis of stroke and MI, or interventional cardiology procedures. Both the parent study (HeartSCORE) and current study (SleepSCORE) protocols were approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent for both protocols.
Design overview
Participants completed sleep diaries and wore a wrist actigraph (Actiwatch 16; Philips, Bend, Oregon, USA) for 10 days and 9 nights to measure sleep and wake patterns via physical movements. On nights 1 and 2 of the study, in-home polysomnography (PSG) sleep studies were conducted. On day 3, participants were trained on how to use the ambulatory BP monitor. Ambulatory BP was monitored continuously, except while showering, bathing, or swimming, for a 48-h period (on days 4–5). On night 4, a 15-h urine collection was done for the assessment of urinary catecholamines (epinephrine and norepinephrine). Urine samples were assayed using a commercially available competitive immunoassay (2 CAT EIA) distributed by Rocky Mountain Diagnostics, Colorado Springs, Colorado, USA.
Ambulatory blood pressure assessment
The Spacelabs Monitor #90217 (Spacelabs Healthcare., Issaquah, Washington, USA) was programmed to assess BP every 30 min from 0800 to 2100 h and hourly thereafter. Nocturnal BP was averaged within the period of time that participants reported going to sleep and their reported wake time (confirmed by actigraphy and diary) across the 48-h assessment. Daytime BP was averaged for the remaining time period in which participants reported being awake. Given recent evidence attesting to the independent prognostic value of night/day BP ratios, and to reduce the number of analyses and subsequent type 1 error inflation, the primary outcome in the present analyses was the average ratio of night/day mean arterial pressure (MAP) across the 48-h assessment period.
Actigraphy
Actigraphy yields many potential outcome measures. We focused on sleep fragmentation as a potential covariate, because it has previously been correlated with nocturnal BP [19], and is a measure of movement during the inactive (i.e. sleep) period. Sleep fragmentation was averaged over two nights during which ambulatory BP recordings were also taken.
Polysomnography
Two nights of PSG recordings were conducted in the participants’ homes using an ambulatory Compumedics Siesta Monitor (Compumedics USA Inc., Charlotte, North Carolina, USA), with standard PSG montage. On the first PSG night, nasal pressure, inductance plethysomography, and fingertip oximetry were used to measure sleep-disordered breathing as indexed by the apnea–hypopnea index (AHI; number of breathing pauses or shallow breathing episodes per hour of sleep). Additional PSG variables taken from the second recording night were considered as potential covariates in the current analyses including sleep latency, wakefulness after sleep onset (WASO), sleep efficiency, and percentage of time spent in stage 1, stage 2, stages 3–4, or rapid eye movement (REM) sleep.
Social integration
The Social Network Index [22] was used to assess engagement in different types of social relationships, specifically relationships with a spouse, parents, parents-in-law, children, other close family members, close neighbors, social or religious groups, co-workers, schoolmates, and fellow volunteers. Social integration was computed as the sum of the types of relationships for which the participant indicates having had phone or in-person contact at least once every 2 weeks. This measure was administered at the time of entrance into the parent study.
Frequency of social contacts
In conjunction with the daytime ambulatory BP recordings, participants reported in electronic diaries every 30 min whether or not they had been engaged in a social interaction (in-person or via phone) in the 10 min preceding the BP recording. Frequency of social contacts was calculated as the percentage of time participants endorsed having ‘No social interactions since the last BP assessment’, reverse-coded so that a score of 100 would indicate that a participant was engaged in a social interaction during the 10 min prior to every BP daytime recordings over the 48 h assessment period. In contrast, a score of 0 would indicate that a participant was never in a social interaction within the 10 min prior to the BP assessment. The correlation between the questionnaire measure of social integration and diary measure of frequency of social contacts was Pearson’s r = 0.46 (P <0.001).
Other covariates
Age, sex, education, and race were determined by self-report. Participants reported their regular medication usage and medication use over the 2 days prior to the ambulatory study. Participants who reported a history of hypertension or had elevated BP at the research clinic visit [systolic BP (SBP) ≥130 or diastolic BP (DBP) ≥85), or were using antihypertensive medication (angiotensin-converting enzyme inhibitors, angiotensin II blockers, β-blockers, calcium channel blockers, α1-blockers, α2-agonists, and diuretics) at the time of the home study were considered hypertensive. Health behaviors including smoking status (past or current smoker versus never smoker) and alcohol consumption per week were also included as covariates. Finally, general health characteristics including body mass index (BMI; weight in kilograms/height in meters-squared) and perceived health status (single-item general health question on the SF-36; [23]) were also included as covariates.
Data analysis
Sleep variables and catecholamines were transformed as necessary (to normalize distributions) prior to analysis. Pearson’s correlations were used to determine sleep variables or catecholamines to be covaried in fully adjusted models. The primary analyses were hierarchical linear regression models which regressed average night/day MAP ratio on social integration or diary-assessed frequency of social contacts, after statistical adjustment for age, sex, education, ethnicity, hypertension status, smoking status, alcohol consumption, BMI, and perceived health, and the interaction between ethnicity and either the social integration or social contact frequency measure. For significant effects of either of the social relationships measures on night/day MAP ratios, follow-up regression analyses examined whether the association persisted after additional adjustment for sleep variables or catecholamines that showed significant (P <0.05) univariate associations with the outcome.
Results
Sample characteristics and major study variables are reported by ethnicity in Table 1. African–Americans were more likely to be hypertensive, to be a past or current smoker, have fewer years of education, higher BMI, and poorer perceived health. In addition, African–Americans had more actigraphy-assessed sleep fragmentation, and for PSG outcomes, longer sleep latencies, poorer sleep efficiency, higher percentage of stage 2, and lower percentage of stage 3–4 sleep compared to whites (P values <0.05), as previously reported in a subsample of the SleepSCORE cohort [19]. There were no ethnic differences in WASO, percentage of stage 1 or REM sleep, AHI, or catecholamines. In addition, there were no ethnic differences in social integration; however, on the diary measure, whites reported more frequent social contacts than African–Americans (P <.05).
Table 1.
Sample characteristics according to ethnicity
| Variable | White (N = 127) | African–American (N = 97) | P value |
|---|---|---|---|
| Age, mean ± SD | 60.5 ± 7.2 | 59.3 ± 7.3 | 0.28 |
| BMI, mean ± SD | 28.24 ± 4.4 | 31.2 (5.4) | <0.01 |
| Men, n (%) | 73 (60) | 37 (38) | <0.01 |
| Hypertensive statusa, n (%) | <0.01 | ||
| No | 43 (35) | 12 (12) | |
| Yes | 80 (65) | 85 (88) | |
| Years education, n (%) | <0.01 | ||
| High school or less | 15 (12) | 21 (22) | |
| Some college, vocational/technical, associate | 19 (15) | 30 (31) | |
| Four-year degree | 37 (30) | 32 (33) | |
| Advanced degree | 52 (42) | 14 (14) | |
| Current or past smoker, n (%) | 57 (46%) | 68 (54%) | |
| Consumes ≥1 alcoholic beverage/week, n (%) | 66 (68%) | 31 (32%) | |
| Perceived health | <0.01 | ||
| Excellent | 30 (71) | 12 (29) | |
| Very good | 54 (67) | 27 (33) | |
| Good | 39 (48) | 43 (52) | |
| Fair/poor | 3 (17) | 15 (83) | |
| Social integration | 5.7 ± 1.9 | 5.6 ± 1.8 | 0.75 |
| Social contact frequency | 72.2 ± 20.5 | 62.6 ± 23.0 | <0.01 |
| Ratio of night-day MAPb | .87 ± .06 | .89 ± .07 | 0.18 |
| Norepinephrine (ng/ml)c | 30.4 ± 20.3 | 39.6 ± 25.2 | 0.01 |
| Epinephrine (ng/ml)c | 3.4 ± 2.6 | 3.8 ± 2.7 | 0.10 |
| Actigraphy sleep fragmentationc | 30.9 ± 10.3 | 35.6 ± 14.6 | 0.02 |
| PSG measures | |||
| Apnea–hypopnea indexc | 13.0 ± 13.6 | 13.5 ± 16.5 | 0.91 |
| Sleep latencyc | 25.8 ± 25.5 | 37.3 ± 38.6 | 0.01 |
| WASOc | 75.6 ± 44.0 | 87.6 ± 59.5 | 0.16 |
| Sleep efficiencyc | 78.9 ± 9.6 | 74.3 ± 12. 6 | 0.003 |
| % Stage 1c | 9.8 ± 6.0 | 9.1 ± 5.9 | 0.18 |
| % Stage 2 | 61.0 ± 8.0 | 65.4 ± 7.6 | <0.01 |
| % Stage 3/4c | 6.5 ± 6.9 | 3.4 ± 4.9 | <0.01 |
| % REM | 22.7 ± 5.1 | 22.1 ± 4.9 | 0.39 |
BMI, body mass index; MAP, mean arterial pressure; PSG, polysomnography; REM, rapid eye movement; WASO, wakefulness after sleep onset.
Participants using antihypertensive medication at the time of the sleep study or had clinic systolic blood pressure (SBP) at least 130 or diastolic blood pressure (DBP) at least 85 were categorized as hypertensive.
MAP; higher ratios indicate less nocturnal dipping.
To facilitate interpretation, means (SDs) and n (%) are based on raw values; statistics are based on transformed data.
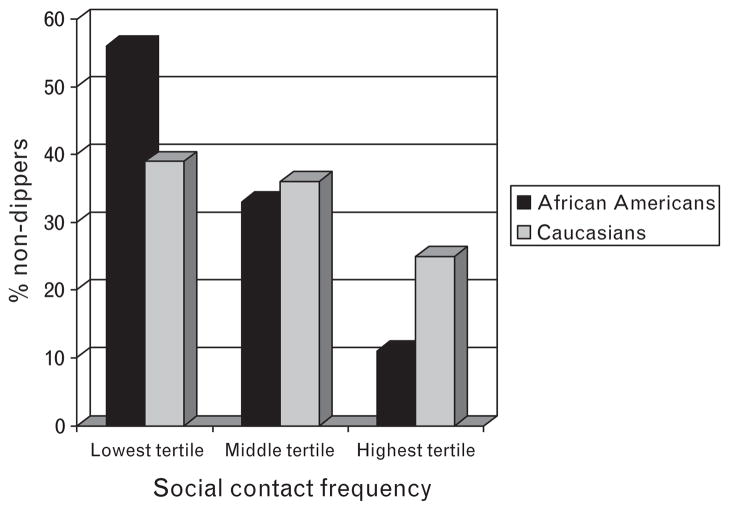
Table 2 presents the linear regression models predicting night/day MAP ratios according to the social relationship measures (social integration or social contact frequency) and the interaction between ethnicity and social relationship measures, after adjusting for age, sex, ethnicity, education, hypertension status, health behaviours, and general health characteristics. These analyses revealed a significant main effect for both social integration (β = −0.26, P <0.05) and social contact frequency (β = −0.34, P <0.001) indicating that less socially integrated individuals and those with fewer daily contacts had higher night/day MAP ratios. The interaction between ethnicity and social integration was nonsignificant (β = 0.36, P >0.10). However, there was a significant interaction between frequency of social contacts and ethnicity on MAP ratios (β = 0.50, P <0.05), indicating that the effects of social contact frequency on MAP ratio were stronger among African–Americans. For clarity of presentation, Fig. 1 shows the proportion of ‘nondippers’ (<10% decline in night–day BP) according to tertiles of social contact frequency in African–Americans versus whites.
Table 2.
Linear regression model regressing night/day MAP ratio on social integration or contact frequency and the interaction between ethnicity and social integration or social contact frequency, with adjustment for demographics and hypertension status
| Variables predicting MAP ratio | Social integration |
Social contact frequency |
||
|---|---|---|---|---|
| Covariate | β (SE) | ΔR2 for step | β (SE) | ΔR2 for step |
| Step 1 | 0.09** | 0.09** | ||
| Age (years) | 0.06 (0.00) | 0.03 (0.00) | ||
| Sex | 0.21 (0.01)*** | 0.19 (0.01)** | ||
| Ethnicity | −0.32 (0.03) | −0.43 (0.03)* | ||
| Education | −0.12 (0.01) | −0.10 (0.01) | ||
| Hypertensive risk group | 0.11 (0.01) | 0.11 (0.01) | ||
| BMI | 0.01 (0.00) | −0.01 (0.00) | ||
| Smoking statusa | 0.09 (0.01) | 0.09 (0.01) | ||
| Alcohol useb | −0.07 (0.01) | −0.07 (0.01) | ||
| General health | 0.14 (0.01)* | 0.13 (0.01)* | ||
| Step 2: social integration or contact frequency | −0.26 (0.00)** | 0.02** | −0.33 (0.00)**** | 0.03**** |
| Step 3: Ethnicity × social integration or contact frequency | 0.34 (0.01) | 0.01 | 0.53 (0.00) | 0.02** |
BMI, body mass index; MAP, mean arterial pressure (higher ratios indicate less dipping).
Smoking status coded as nonsmoker (0) versus current or past smoker (1).
Alcohol consumption coded as at least one drink per week versus fewer.
P <0.10.
P <0.05.
P <0.01.
P ≤0.001.
Fig. 1.
Percentage of ‘nondippers’ (<10% decline in night–day MAP) according to tertile of social contact frequency, for African–Americans and whites. MAP, mean arterial pressure.
Follow-up analyses, presented in Table 3, additionally controlled for actigraphy-assessed sleep fragmentation, PSG-assessed sleep efficiency, and the percentage of time spent in stage 1, stage 3–4, REM sleep, and AHI. In these models, the only covariates that remained statistically significant correlates of MAP ratio were AHI and sleep fragmentation. In addition, both the social integration and social contact frequency measures, remained significant correlates (β = −0.13, P = 0.05 and β = −0.34, P = 0.001, respectively), suggesting an independent relationship between these social relationship measures and night/day MAP ratios. The magnitude of the effect for social contact frequency (ΔR2 = 0.03) was stronger than that for social integration (ΔR2 = 0.02). In addition, the significant social contact frequency × ethni-ethnicity interaction remained statistically significant (β = 0.52, P <0.05) and in the same direction.
Table 3.
Linear regression model regressing night/day MAP ratio on social integration or contact frequency and the interaction between ethnicity and social integration or social contact frequency, with adjustment for demographics, hypertension status, and sleep covariates
| Covariate | Social integration |
Social contact frequency |
||
|---|---|---|---|---|
| β (SE) | ΔR2 for step | β (SE) | ΔR2 for step | |
| Step 1 | 0.18*** | 0.19*** | ||
| Age (years) | −0.04 (0.00) | −0.07 (0.00) | ||
| Sex | 0.02 (0.01) | 0.01 (0.01) | ||
| Ethnicity | 0.08 (0.01) | −0.34 (0.03) | ||
| Education | −0.07 (0.01) | −0.08 (0.01) | ||
| Hypertensive risk group | 0.08 (0.01) | 0.08 (0.01) | ||
| BMI | −0.01 (0.00) | −0.04 (0.00) | ||
| Smoking status | 0.11 (0.01) | 0.12 (0.01)* | ||
| Alcohol use/week | −0.10 (0.01) | −0.10 (0.01) | ||
| General health | 0.10 (0.01) | 0.08 (0.01) | ||
| AHIa | 0.16 (0.01)** | 0.19 (0.01)*** | ||
| Sleep fragmentationa | 0.16 (0.02)** | 0.14 (0.02)* | ||
| Average REM PERCENTAGE | −0.09 (0.00) | −0.11 (0.00) | ||
| Average sleep efficiencya | 0.08 (0.01) | 0.05 (0.01) | ||
| Average % stage 1a | 0.04 (0.01) | 0.06 (0.01) | ||
| Average % deltaa | −0.10 (0.00) | −0.10 (0.00) | ||
| Step 2: social integration or contact frequency | −0.13 (0.00)** | 0.02** | −0.34 (0.00)*** | 0.03*** |
| Step 3: social contact frequency × ethnicity | N/A | N/A | 0.52 (0.00)** | 0.02** |
AHI, apnea–hypopnea index; BMI, body mass index; MAP, mean arterial pressure (higher ratios indicate less dipping); REM, rapid eye movement. Interaction term is not entered for social integration model given that it was nonsignificant in the demographics adjusted model.
Variables transformed prior to analysis.
P <0.10.
P ≤0.05.
P <0.01.
P ≤0.001.
Discussion
The present study used a multimodal assessment of engagement in social relationships, and nocturnal BP profiles in a community-based sample of African–American and white men and women. We found that more socially isolated individuals, as assessed via questionnaire and daily diary methods, were more likely to show higher nocturnal/day BP ratios as compared to their more socially engaged counterparts and these results persisted even after statistically controlling for a host of demographic and general health characteristics (i.e. hypertension diagnosis, use of cardiac medications, elevated clinic BP, BMI, perceived health, health behaviors), and PSG-assessed and actigraphy-assessed sleep variables that had the potential to account for the associations.
In addition, ours was the first study to examine the association between nocturnal BP and a diary measure of social contact frequency assessed simultaneously with the BP assessment, as well as a questionnaire measure of social integration, assessed more than a year prior to the ambulatory BP assessment. Despite the difference in timing of these measures and the fact that they measure distinct, though related aspects of social networks, they showed a modest correlation with each other, and were both independently associated with nocturnal BP profiles. However, the diary measure of social contact frequency was a stronger correlate of nocturnal BP than social integration, which may reflect both the ecologic validity of the measure as well as the fact that it was measured concurrently with the BP assessment. Moreover, measuring ‘in-vivo’ social contacts may indeed provide a more sensitive measure of how social relationships influence cardiovascular morbidity and mortality.
We found inconsistent evidence regarding the degree to which ethnicity moderates the association between social relationships and nocturnal BP. Specifically, the ethnicity interaction term was nonsignificant in the social integration model; however, for the diary measure, the association between frequency of social contacts and nocturnal BP was stronger among African–Americans compared to whites. African–Americans also reported less frequent social interactions on the diary measure but not on the integration measure. Thus, these findings may reflect greater variability in social contact frequency among African–Americans and/or greater susceptibility to the health benefits of social contacts, or alternatively, the health risks of social isolation. Indeed, among African–Americans, the most socially isolated individuals (lowest tertile) were roughly five times more likely to be categorized as nondippers (according to the frequently used cut-point of <10% day-to-night BP decline) as compared to the most engaged individuals (highest tertile).
Although there was a similar trend among whites, the effects were less pronounced. Interestingly, a recent study by Cooper and colleagues [12] on perceived social support and nocturnal dipping found virtually the opposite effect of ethnicity. Specifically, their results showed a significant relationship between higher levels of social support and greater nocturnal BP dipping in whites, but in African–Americans, higher social support was associated with less dipping. These contradictory findings may be due to the fact that the former study measured the perception of social support, whereas our study measured the scope and variety of social contacts, which are related but distinct constructs. Taken together, these findings highlight the need to consider multiple aspects of the social environment as they relate to cardiovascular outcomes in diverse sociocultural contexts.
These results must be interpreted in the context of the study’s limitations and strengths. On the basis of the study recruitment criteria the sample included older African–American and white men and women, and we excluded individuals with a previously diagnosed sleep disorder. Thus, our findings may not generalize to sleep clinic patients, younger populations, or other minority groups. Limitations associated with our social relationship measures include the timing of the assessment for social integration and the fact that we did not assess the quality of those relationships or the functional aspects of support (e.g. emotional, tangible). In addition, the frequency of ambulatory BP monitoring was lower than is common in clinical practice; however, given that our study included 48 h of recording versus the traditional 24 h, these are likely to be representative estimates of day and night BPs [24]. Whereas this is the first study to show an independent association between social relationships and nocturnal BP after controlling for a host of potential nocturnal pathways, we did not include measures of all possible confounders, including measures of hypothalamic-pituitary-adrenal activity, inflammatory, hemostatic markers, or other psychosocial risk factors. Given previous associations between these factors and both nocturnal BP [25–27] and social relationships [28–30], it is plausible that these or other unmeasured variables may account for the findings. The effect sizes for social integration and social contact frequency were relatively small, accounting for 2–4% of the unique variance in night/day MAP ratio; however, these effects were roughly comparable to the effect size for all of the demographic, clinic, and health behavior covariates combined (9%). Finally, the cross-sectional and observational nature of the study precludes inferences regarding causality.
Strengths of the study include the fact that this was a diverse sample from a community-based epidemiological study of African–American and white men and women, increasing the generalizability of the study findings to the nonpatient population at large. In addition, the study’s inclusion of multiple methods for assessing social relationships and sleep, and statistical adjustment for other factors that may account for observed associations, strengthens the contention that there is an independent association between social relationships and nocturnal BP.
Ambulatory BP monitoring has become increasingly recognized as a critical component of the clinical management of hypertension, owing in part, to the growing recognition of the independent, prognostic significance of nocturnal BP. Elucidating potential, modifiable risk factors for nocturnal BP nondipping has the potential to identify novel treatment targets in populations at high-risk for CVD or in those with poorly managed manifest CVD. For the past two decades, compelling research has documented the profound effects of the social environment on cardiovascular morbidity and mortality. The current findings extend this literature by showing robust, independent relationships between social integration and frequency of social contacts and nocturnal BP in a community sample of African–American and white men and women. Incorporating a brief assessment of patients’ engagement in social relationships either in conjunction with symptom monitoring or via brief questionnaire may provide useful social data of prognostic value in a clinical setting, and this may be particularly important among African–Americans. In summary, the findings suggest that social connections or the lack thereof may influence key prognostic indicators of CVD, including nocturnal BP, which may inform our understanding of how social relationships contribute to cardiovascular health.
Acknowledgments
Supported by NIH HL076379, HL076852, HL076858, and CTSA/N-CTRC #RR024153. Support for the first author was provided by K23 HL093220. This project was funded in part under a grant with the PA Department of Health (Contract ME-02-384). The Department and the National Institutes of Health specifically disclaim responsibility for any analyses, interpretations, or conclusions. K.A.M. has had full access to the data, and takes responsibility for the integrity of the data and accuracy of the analysis.
Abbreviations
- AHI
apnea–hypopnea index
- BP
blood pressure
- CVD
cardiovascular disease
- HeartSCORE
Heart Strategies Concentrating on Risk Evaluation
- MAP
mean arterial pressure
- MI
myocardial infarction
- PSG
polysomnography
- REM
rapid eye movement sleep
- WASO
wakefulness after sleep onset
Footnotes
Portions of this research were presented at the American Psychosomatic Society, Annual Meeting, Chicago, Illinois, USA, March 2009.
There are no conflicts of interest.
References
- 1.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 2.Seeman T. Health promoting effects of friends and family on health outcomes in older adults. Am J Health Promot. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- 3.Barefoot JC, Gronbaeck M, Jensen G, Schnohr P, Prescott E. Social networks and risks of ischemic heart disease and total mortality: findings from the Copenhagen City Heart Study. Am J Epidemiol. 2005;161:960–967. doi: 10.1093/aje/kwi128. [DOI] [PubMed] [Google Scholar]
- 4.Rutledge T, Linke SE, Olson MB, Francis J, Johnson BD, Bittner V, et al. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosom Med. 2008;70:282–287. doi: 10.1097/PSY.0b013e3181656e09. [DOI] [PubMed] [Google Scholar]
- 5.Case RB, Moss AJ, Case N, McDermott M, Eberly S. Living alone after myocardial infarction: impact on prognosis. JAMA. 1992;267:515–519. [PubMed] [Google Scholar]
- 6.Piferi RL, Lawler KA. Social support and ambulatory blood pressure: an examination of both receiving and giving. Int J Psychophysiol. 2006;62:328–336. doi: 10.1016/j.ijpsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Steptoe A. Stress, social support and cardiovascular activity over the working day. Int J Psychophysiol. 2000;37:299–308. doi: 10.1016/s0167-8760(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 8.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- 9.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension [see comment] Hypertension. 2008;51:55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 10.Henskens LH, Kroon AA, van Oostenbrugge RJ, Haest RJ, Lodder J, de Leeuw PW. Different classifications of nocturnal blood pressure dipping affect the prevalence of dippers and nondippers and the relation with target-organ damage [see comment] J Hypertens. 2008;26:691–698. doi: 10.1097/HJH.0b013e3282f4225f. [DOI] [PubMed] [Google Scholar]
- 11.Routledge FS, McFetridge-Durdle JA, Dean CR. Night-time blood pressure patterns and target organ damage: a review. Can J Cardiol. 2007;23:132–138. doi: 10.1016/s0828-282x(07)70733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper DC, Ziegler MG, Nelesen RA, Dimsdale JE. Racial differences in the impact of social support on nocturnal blood pressure. Psychosom Med. 2009;71:524–531. doi: 10.1097/PSY.0b013e31819e3a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt-Lunstad J, Birmingham W, Jones BQ. Is there something unique about marriage? The relative impact of marital status, relationship quality, and network social support on ambulatory blood pressure and mental health. Ann Behav Med. 2008;35:239–244. doi: 10.1007/s12160-008-9018-y. [DOI] [PubMed] [Google Scholar]
- 14.Ituarte PH, Kamarck TW, Thompson HS, Bacanu S. Psychosocial mediators of racial differences in nighttime blood pressure dipping among normotensive adults. Health Psychol. 1999;18:393–402. doi: 10.1037//0278-6133.18.4.393. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez CJ, Burg MM, Meng J, Pickering TG, Jin Z, Sacco RL, et al. Effect of social support on nocturnal blood pressure dipping. Psychosom Med. 2008;70:7–12. doi: 10.1097/PSY.0b013e31815aab4e. [DOI] [PubMed] [Google Scholar]
- 16.Pugliesi K, Shook SL. Gender, ethnicity, and network characteristics: variation in social support resources. Sex Roles. 1998;38:215–238. [Google Scholar]
- 17.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–1218. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 18.Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004;27:1097–1103. doi: 10.1093/sleep/27.6.1097. [DOI] [PubMed] [Google Scholar]
- 19.Matthews KA, Kamarck TW, Hall M, Strollo PJ, Owens JF, Buysse DJ, et al. Blood pressure dipping and sleep disturbance in African-American and Caucasian men and women. Am J Hypertens. 2008;21:826–831. doi: 10.1038/ajh.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kario K, Mitsuhashi T, Shimada K. Neurohumoral characteristics of older hypertensive patients with abnormal nocturnal blood pressure dipping. Am J Hypertens. 2002;15:531–537. doi: 10.1016/s0895-7061(02)02266-5. [DOI] [PubMed] [Google Scholar]
- 21.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension. 1999;33:1099–1104. doi: 10.1161/01.hyp.33.5.1099. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- 23.Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 24.Llabre MM, Ironson GH, Spitzer SB, Gellman MD, Weidler DJ, Schneiderman N. How many blood pressure measurements are enough? An application of generalizability theory to the study of blood pressure reliability. Psychophysiology. 1988;25:97–106. doi: 10.1111/j.1469-8986.1988.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 25.Holt-Lunstad J, Steffen PR. Diurnal cortisol variation is associated with nocturnal blood pressure dipping. Psychosom Med. 2007;69:339–343. doi: 10.1097/PSY.0b013e318050d6cc. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Pickering TG, Shimada K, et al. Increased low-grade inflammation and plasminogen-activator inhibitor-1 level in nondippers with sleep apnea syndrome. J Hypertens. 2008;26:1181–1187. doi: 10.1097/HJH.0b013e3282fd9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Kanel R, Jain S, Mills PJ, Nelesen RA, Adler KA, Hong S, et al. Relation of nocturnal blood pressure dipping to cellular adhesion, inflammation and hemostasis. J Hypertens. 2004;22:2087–2093. doi: 10.1097/00004872-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- 29.Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci U S A. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirtz PH, Redwine LS, Ehlert U, von Kanel R. Independent association between lower level of social support and higher coagulation activity before and after acute psychosocial stress. Psychosom Med. 2009;71:30–37. doi: 10.1097/PSY.0b013e31818f6868. [DOI] [PubMed] [Google Scholar]