Abstract
Microglia are the immune cells that reside in the central nervous system (CNS). Following the facial nerve injury in the mouse, microglia are activated in the facial motor nucleus, coincident with an increase in the proinflammatory cytokine interferon-gamma (IFN-γ). The authors have previously shown that maximal facial motoneuron (FMN) survival after injury depends on the CD4+T-cell interaction with microglia. Furthermore, it appears that the anti-inflammatory T helper (Th) 2 CD4+ T-cell subset is required in the facial nucleus, although the mechanism of CNS recruitment is not known. Pituitary adenylyl cyclase-activating polypeptide (PACAP) is a neuropeptide that has previously been demonstrated to be expressed by injured FMN. Interestingly, PACAP has been shown to act on peripheral macrophages by inducing chemokine expression capable of recruiting Th2 cells. Whether CNS-resident microglia, a related lineage to peripheral macrophages, respond to PACAP by expressing Th2-associated chemokines is not known. In this study, fluorescence-activated cell sorting was utilized to measure the frequency of microglia positive for different chemokines after exposure to various treatments. The results indicate that PACAP increases the frequency of microglia expressing Th2-associated chemokine, CCL11, and decreases the frequency of microglia expressing Th1-associated chemokine, CXCL11. In contrast, IFN-γ decreases the frequency of microglia expressing Th2-associated chemokine, CCL11, and increases the frequency of microglia expressing Th1-associated chemokine, CXCL11. Treatment with both PACAP and IFN-γ reversed the proinflammatory effect of IFN-γ. Given the recent focus on the therapeutic value of Th2 cells in the CNS during neurode-generative disease, PACAP may be a future therapeutic target for improving neuroregeneration after injury.
Keywords: neuroprotection, CCL11, CXCL11, facial nerve axotomy
INTRODUCTION
Previous work in our lab has demonstrated that the CD4+ T cell plays a positive role in facial motoneuron (FMN) survival after peripheral facial nerve transection at the stylomastoid foramen in the mouse.1,2 Furthermore, Raivich et al.3 demonstrated that T cells infiltrate the axotomized mouse facial motor nucleus at low levels 2 to 4 days after injury, followed by a much stronger increase at 14 days after injury. Corroborating the findings of Raivich et al.,3 our laboratory recently reported that central nervous system (CNS)-resident microglia are necessary to reactivate CD4+ T cells centrally,4 supporting the existence of a mechanism for immune cell-mediated neuroprotection involving CNS T-cell trafficking.
Once activated, naïve CD4+ T cells differentiate into proinflammatory [interferon-gamma (IFN-γ)-producing] or anti-inflammatory (interleukin-4-producing) T helper (Th) effector cells, Th1 or Th2, respectively.5 Accordingly, we determined that the Th2, but not the Th1, effector subset is necessary for immune-mediated neuroprotection.6 Taken together, the data suggest that following a peripheral nerve injury outside the blood-brain barrier, microglia, which are capable of antigen presentation centrally,7 interact with a Th2 cell through an undefined mechanism of recruitment.
Armstrong et al.8 recently reported that facial nerve transection induces pituitary adenylyl cyclase-activating polypeptide (PACAP) mRNA expression in wild type mouse FMN. The data showed that PACAP mRNA expression is significantly reduced in immunodeficient mice following facial nerve transection and that this reduction in PACAP was reversed upon immune system reconstitution with normal splenocytes.8 Together, these data substantiate our results, demonstrating a neuroprotective role for the immune system after injury in FMN survival, as well as indicate that immune cells are important in PACAP expression. Interestingly, exposure to PACAP results in a Th2-associated chemokine-expressing phenotype that has been described for peripheral dendritic cells (DC) and macrophages.9–11 What is not yet known, however, is whether CNS-resident microglia also express Th2-associated chemokines following PACAP activation.
Recently, PACAP was reported to have a suppressive effect on the induction of proinflammatory constituents in DC,12 macrophages,13 and microglia.14 If PACAP suppresses peripheral antigen presenting cells (APC) that promote proinflammatory responses, then PACAP might also play a role in promoting the immune-related neuroprotective mechanism following facial nerve axotomy, particularly given that IFN-γ mRNA expression occurs in the facial motor nucleus after injury.3
On the basis of the aforementioned data, we hypothesized that PACAP will affect Th2-associated chemokine expression in murine microglia, as has been shown for peripheral DCs and macrophages. Additionally, because the expression of the proinflammatory cytokine IFN-γ has been reported to occur in the facial motor nucleus following facial nerve transection, we also examined the effects of IFN-γ on Th1-associated chemokine expression in microglia.
MATERIALS AND METHODS
The BV2 immortalized murine microglial cell line was a generous gift from Dr Linda Van Eldik and originally generated by Dr Elisabetta Blasi.15 Briefly, BV2 microglia were maintained in Dulbecco’s modified Eagle’s medium with L-glutamine (ATCC, Manassas, VA) supplemented with 10 percent fetal bovine serum (Gibco, Carlsbad, CA), 0.2 mM penicillin, and 0.05 mM streptomycin (Gibco) at 37°C in a humidified incubator under 95 percent/5 percent (v/v) mixture of air and CO2. For all experiments, microglia were seeded in a six-well plate (Corning International, Corning, NY) at a density of 5 × 105 cells/well in a 2-mL media with or without treatment.
Microglia were exposed to one of the following treatments for 24 hours: (1) media, (2) 10−6 M PACAP (Sigma, St. Louis, MO), (3) 2 μg/mL IFN-γ (Peprotech, Rocky Hill, NJ), or 10−6 M PACAP and 2 μg/mL IFN-γ. Cells were exposed to 10 μg/mL brefeldin A (Sigma) for the final 2 hours of treatment exposure. Cells were fixed in 4 percent paraformaldehyde for 10 minutes, washed in staining buffer (PBS, pH 7.4 + 0.5 percent Saponin [Sigma] + 0.5 percent albumin bovine fraction V [MP Biomedicals, Solon, OH] + 5 percent Hepes buffer solution [Gibco]), and resuspended to a final concentration of 1 × 105 cells in 250 μL, aliquoted to 5-mL round bottom tubes (BD, Franklin Lakes, NJ).
Each treatment group was exposed to anti-CCL11, anti-CXCL11, or anti-isotype control Ig, followed by fluorescein isothiocyanate (FITC)-conjugated secondary anti-rabbit (for CCL11) or anti-rat (for CXCL11). The following antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA): rabbit IgG, FITC-conjugated donkey anti-rabbit, and FITC-conjugated chicken anti-rat. Antibodies for rat IgG2A were purchased from BD Pharmingen (San Diego, CA), anti-CCL11 (polyclone E011047) from Ebioscience (San Diego, CA), and anti-CXCL11 (clone 131327) from R&D Systems (Minneapolis, MN).
Microglia were incubated in staining buffer with primary antibodies for 1 hour, washed twice, and incubated with secondary antibodies for 30 minutes. Data acquisition and analysis were performed by the FACSCanto (Becton-Dickinson, San Jose, CA) and Flowjo analysis software (TreeStar, Cupertino, CA), respectively, analyzing 10,000 microglia/sample. Gates were set using the isotype control as a reference, with 0.1 percent or fewer positive cells beyond the statistical marker based on a similar method previously performed.16 Data were subjected to one-way analysis of variance (ANOVA) with a post hoc Dunnett’s test.
RESULTS
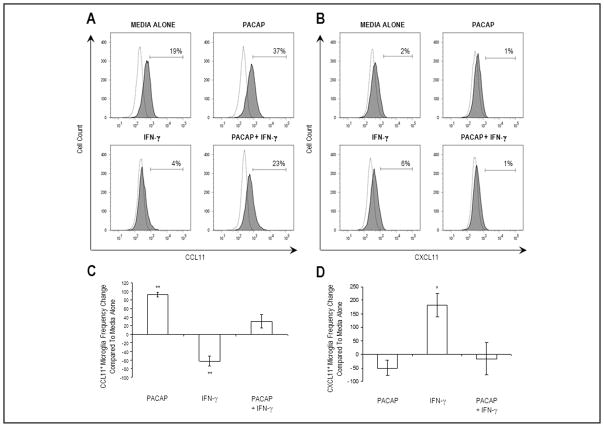
The frequency of CCL11+ microglia within the microglial cell line population was 19 percent (Figure 1A) after 24 hours of culture in media alone. The addition of PACAP to the cell culture increased the frequency of CCL11+ microglia to 37 percent. In contrast, the addition of IFN-γ decreased the frequency of CCL11+ microglia to 4 percent. When both PACAP and IFN-γ were added at the same time, the frequency of CCL11+ microglia was 23 percent. The frequency change relative to media alone for three independent experiments was 92 percent ± 6 percent, −62 percent ± 11 percent, and 30 percent ± 15 percent for microglia cultured with PACAP, IFN-γ, or PACAP and IFN-γ, respectively (Figure 1C). Thus, PACAP increased the number of cells expressing CCL11 as well as prevented the effects of IFN-γ.
Figure 1.
Effect of PACAP and/or IFN-γ on microglial expression of the Th2- and Th1-associated chemokines, CCL11, and CXCL11, respectively. Microglia (5 × 105 cells/well) were exposed to one of the following treatments: media alone, 10−6 M PACAP, 2 μg/mL IFN-γ, or 10−6 M PACAP and 2 μg/mL IFN-γ. Expression for isotype-matched control (dotted lines) and (A) anti-CCL11 or (B) anti-CXCL11 (gray shading) antibodies is shown. Numbers above bracketed lines (gates) indicate the frequency of microglia positive for each chemokine. Data from FACS analysis are represented as bar graphs to indicate the (C) CCL11+ or (D) CXCL11+ microglia frequency change compared to media alone and represent three independent experiments. Data are expressed as value ± SEM (*p < 0.05; **p < 0.01).
The frequency of CXCL11+ microglia within the microglial cell line population was 2 percent (Figure 1B) after 24 hours of culture in media alone. The addition of PACAP to the culture decreased the frequency of CXCL11+ microglia to 1 percent. In contrast, the addition of IFN-γ increased the frequency of CXCL11+ microglia to 6 percent. When both PACAP and IFN-γ were added at the same time, the frequency of CXCL11+ microglia was 1 percent. The frequency change relative to media alone for three independent experiments was −50 percent ± 29 percent, 183 percent ± 44 percent, and −17 percent ± 60 percent for microglia cultured with PACAP, IFN-γ, or PACAP and IFN-γ, respectively (Figure 1D). Thus, PACAP decreased the number of cells expressing CXCL11 as well as prevented the effects of IFN-γ.
DISCUSSION
Collectively, these data suggest that murine microglia respond to PACAP by increasing the frequency of cells expressing T helper 2 (Th2)-associated chemokine, CCL11, and decreasing expression of Th1-associated chemokine, CXCL11. Thus, PACAP regulates chemokine expression in CNS microglia and peripheral APC similarly.9 Previously, we demonstrated that mouse FMN survival following facial nerve transection outside the blood-brain barrier depended on both peripheral APC for initial CD4+ T-cell activation and centrally located microglia for CD4+ T-cell reactivation.4 We also demonstrated that the CD4+ T-cell responsible for mediating FMN survival is of the Th2 subtype.6 These results, together with evidence that mRNA for PACAP is expressed in injured mouse FMN following facial nerve transection,8 have led to a model that our lab is currently investigating to explain the mechanism for FMN survival following facial nerve injury. Key to this model is that Th2 cells are selectively recruited to the facial motor nucleus to provide brain-derived neurotrophic factor to support FMN survival, even though both Th1 and Th2 lymphocytes develop in the draining lymph node.6 This model implicates the chemokine receptor (CX3CR1)-expressing microglia, recruited by chemokine ligand (CX3CL1)-expressing rat FMN,17 as initial producers of T-cell-associated chemokines following facial nerve transection.
In summary, this in vitro study is the first to demonstrate a chemokine converting capacity of PACAP on CNS-resident microglia, consistent with its effects on peripheral APC in general. The injury-induced expression of PACAP and its effects on microglia supports the neuroprotective importance of Th2 cells in the context of facial nerve transection, the microglia that they act on, and new therapies involved in their recruitment.6,18,19 To maintain a healthy CNS, the immune system must balance the destructive effects that develop for defense against infection, disease, or malignancy with the neuroprotective effect that develops after a peripheral nerve injury. In motoneuron diseases such as amyotrophic lateral sclerosis (ALS), data from mouse disease models indicate that this balance is disturbed, resulting in microglial degeneration20 and neurotoxicity,21 as well as lymphopenia.22 To hypothesize that impaired expression of PACAP contributes to ALS23 is an intriguing proposition, in view of recent compelling studies demonstrating a relationship between PACAP expression in axotomized mouse motoneurons and the immune system.8,24 Given the important role of Th2 cells following facial nerve injury,6 future research in this area will focus on in vivo Th2- and Th1-associated chemokine expression in the facial motor nucleus following facial nerve transection.
Acknowledgments
Drs Jones and Sanders share senior authorship. This work was supported by the Les Turner ALS Foundation (DAW) and NIH grant NS40433 (KJJ and VMS).
Contributor Information
Derek A. Wainwright, Department of Cell Biology, Neurobiology, and Anatomy, Loyola University Medical Center, Maywood, Illinois; Research and Development Service, Hines VA Hospital, Hines, Illinois.
Junping Xin, Department of Cell Biology, Neurobiology, and Anatomy, Loyola University Medical Center, Maywood, Illinois; Research and Development Service, Hines VA Hospital, Hines, Illinois.
Virginia M. Sanders, Department of Molecular Virology, Immunology, and Medical Genetics, The Ohio State University, Columbus, Ohio.
Kathryn J. Jones, Department of Cell Biology, Neurobiology, and Anatomy, Loyola University Medical Center, Maywood, Illinois; Research and Development Service, Hines VA Hospital, Hines, Illinois.
References
- 1.Serpe CJ, Kohm AP, Huppenbauer CB, et al. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serpe CJ, Coers S, Sanders VM, et al. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 3.Raivich G, Jones LL, Kloss CU, et al. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byram SC, Carson MJ, DeBoy CA, et al. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stout RD, Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989;142:760–765. [PubMed] [Google Scholar]
- 6.Deboy CA, Xin J, Byram SC, et al. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exp Neurol. 2006;201:212–224. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naive T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999;55:127–134. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong BD, Hu Z, Abad C, et al. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- 9.Delgado M, Gonzalez-Rey E, Ganea D. VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. FASEB J. 2004;18:1453–1455. doi: 10.1096/fj.04-1548fje. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Jing H, Ganea D. VIP and PACAP down-regulate CXCL10 (IP-10) and up-regulate CCL22 (MDC) in spleen cells. J Neuroimmunol. 2002;133:81–94. doi: 10.1016/s0165-5728(02)00365-x. [DOI] [PubMed] [Google Scholar]
- 11.Delgado M, Leceta J, Gomariz RP, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide stimulate the induction of Th2 responses by up-regulating B7.2 expression. J Immunol. 1999;163:3629–3635. [PubMed] [Google Scholar]
- 12.Delgado M, Reduta A, Sharma V, et al. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75:1122–1130. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- 13.Delgado M, Munoz-Elias EJ, Gomariz RP, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-kappa B and IFN regulatory factor 1 activation. J Immunol. 1999;162:4685–4696. [PubMed] [Google Scholar]
- 14.Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the production of inflammatory mediators by activated microglia. J Leukoc Biol. 2003;73:155–164. doi: 10.1189/jlb.0702372. [DOI] [PubMed] [Google Scholar]
- 15.Blasi E, Barluzzi R, Bocchini V, et al. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 16.Sato W, Aranami T, Yamamura T. Cutting edge: Human Th17 cells are identified as bearing CCR2+CCR5− phenotype. J Immunol. 2007;178:7525–7529. doi: 10.4049/jimmunol.178.12.7525. [DOI] [PubMed] [Google Scholar]
- 17.Harrison JK, Jiang Y, Chen S, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix S, Nitsch R. The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol. 2007;184:100–112. doi: 10.1016/j.jneuroim.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Dhib-Jalbut S. Glatiramer acetate (Copaxone) therapy for multiple sclerosis. Pharmacol Ther. 2003;98:245–255. doi: 10.1016/s0163-7258(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 20.Fendrick SE, Xue QS, Streit WJ. Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant Cu/Zn superoxide dismutase gene. J Neuroinflammation. 2007;4:9–21. doi: 10.1186/1742-2094-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao Q, Zhao W, Beers DR, et al. Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J Neurochem. 2007;102:2008–2019. doi: 10.1111/j.1471-4159.2007.04677.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuzmenok OI, Sanberg PR, Desjarlais TG, et al. Lymphopenia and spontaneous autorosette formation in SOD1 mouse model of ALS. J Neuroimmunol. 2006;172:132–136. doi: 10.1016/j.jneuroim.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Staines DR. Are multiple sclerosis and amyotrophic lateral sclerosis autoimmune disorders of endogenous vasoactive neuropeptides? Med Hypotheses. 2008;70:413–418. doi: 10.1016/j.mehy.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Rodriguez WI, Casillas RA, et al. Axotomy-induced changes in pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP receptor gene expression in the adult rat facial motor nucleus. J Neurosci Res. 1999;57:953–961. [PubMed] [Google Scholar]