Abstract
Aim of this study was to evaluate the usefulness of a Multidimensional Prognostic Index (MPI) based on a Comprehensive Geriatric Assessment (CGA) for predicting mortality risk in older patients with dementia. The present was a retrospective study with a year of follow-up that included 262 patients aged 65 years and older with a diagnosis of dementia. A standardized CGA that included information on clinical, cognitive, functional, and nutritional aspects, as well as comorbidity, medications, and social support network, was used to calculate MPI. The predictive value of the MPI for all-cause mortality over 1 month, 6 months, and 12 months of follow-up was evaluated. Higher MPI values were significantly associated with higher mortality at 1 month (MPI-1, low risk = 0%, MPI-2, moderate risk = 5.2%, MPI-3, severe risk = 13.7%; p < 0.002), 6-months (MPI-1 = 2.7%, MPI-2 = 11.2%, MPI-3 = 28.8%; p < 0.001), and 12-months (MPI-1 = 2.7%, MPI-2 = 18.2%, MPI-3 = 35.6%; p < 0.001) of follow-up. The discrimination of the MPI was also good, with areas under the ROC curves of 0.77 (sensitivity = 82.9%, specificity = 66.0%, with a cut off value > 0.16) at 12-months of follow up. In conclusion, the MPI, calculated from information collected in a standardized CGA, accurately stratified hospitalized elderly patients with dementia into groups at varying risk of short- and long-term mortality.
Keywords: Comprehensive Geriatric Assessment (CGA), dementia, mortality, Multidimensional Prognostic Index (MPI), prognosis, survival
INTRODUCTION
Dementia is one of the most common and significant health problems in the elderly. Its prevalence in Western countries increases from 1% in individuals aged less than 64 years to 24% in people 85 years or older [1]. Dementia is one of the main causes of disability in older age [2] with very high economic costs. In the United States, costs related to dementia have been estimated at US$100 billion per year including direct and indirect care costs. Thus, the care of these patients is a serious challenge for health care systems in Western countries [3].
The prognostic evaluation of these patients plays a key role in the decision analyses of care processes including the organization of social health care system, the support to families, caregivers, and patients as well as the choice of appropriate treatment [4]. Since in older subjects mortality results from a combination of biological, functional, psychological, and environmental factors, tools that effectively identify patients with different life expectancies should be multidimensional in nature [5,6]. Some prognostic methods have been previously described for predicting mortality in patients with dementia. Most of these tools, however, were validated in specific populations such as patients living in nursing homes [7], or required laboratory data [8], or investigated only a single functional component of the patients [9]. Recently, a Multidimensional Prognostic Index (MPI) for 1-year mortality derived from a standardized Comprehensive Geriatric Assessment (CGA) was developed and validated in two independent cohorts of elderly patients hospitalized for acute diseases or relapse of chronic diseases [10]. In both cohorts, a close agreement was found between the estimated and the observed mortality after both 1 month and 1 year of follow-up independently from the main diagnosis of the patients.
The aim of the study was to evaluate the prognostic accuracy of the MPI, derived from a standardized CGA, on evaluating short- (1 month) and long-term (6 months and 12 months) mortality rates in hospitalized elderly patients with dementia.
METHODS
Subjects
Patients aged 65 years and older admitted from January 2004 to December 2006 to the Geriatrics Unit of the Casa Sollievo della Sofferenza Hospital (IRCCS, San Giovanni Rotondo, Italy) due to acute disease or relapse of a chronic disease were screened for eligibility. Inclusion criteria were: 1) age ≥ 65 years; 2) diagnosis of dementia; 3) ability to provide an informed consent or availability of a proxy for informed consent; 4) a complete CGA during hospitalization; and 5) availability of mortality/survival information after one year from the hospitalization.
At baseline, the following parameters were collected by a systematic interview, clinical evaluation, and review of records from the patients’ general practitioners: date of birth, gender, clinical history, current pathologies, and medication history. All patients admitted to our Unit received a standard CGA. Vital status up to December 31, 2007 was assessed by directly contacting the participants or consulting the Registry Offices of the cities where the patients were residents at the time of hospital admission. Dates of death were identified from death certificates.
Diagnosis of dementia
Cognitive status was screened in all subjects admitted to our geriatric ward by the Short Portable Mental Status Questionnaire (SPMSQ) [11]. Subjects who had definite or suspected cognitive impairment (SPMSQ score ≤ 7) underwent the Mini-Mental State Examination (MMSE), and the Clinical Dementia Rating scale (CDR) [12,13]. Dementia was diagnosed by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DMS-IV) [14]. Diagnoses of possible/probable Alzheimer’s disease (AD) were made according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association Work Group (NINCDS-ADRDA) [15]. Diagnoses of possible/probable vascular dementia (VaD) were made according to the criteria of the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences Work Group (NINDS-AIREN) [16]. Differential diagnosis between AD, VaD, and mixed dementia (MD) was based also on the Hachinski Ischemic Score [17]. Scores ≤ 4 were considered as probable AD, scores ≥ 7 were included into the VaD group, and MD was recognized when the score was between 5 and 6. Diagnosis ofVaD was always supported by neuroimaging evidence (computed tomography scan and/or nuclear magnetic resonance). In particular, the presence of multiple cortical/subcortical infarcts or an infarct in a strategic area such as the thalamus or temporal lobe and/or lesions of the white matter indicated probable VaD. The absence of the above mentioned cerebrovascular lesions indicated AD [18]. To rule out the possibility of cognitive impairment due to medical or psychiatric conditions, subjects who had a present or past medical or psychiatric conditions or psychoactive substance use that can cause cerebral dysfunction were excluded from the study.
The Comprehensive Geriatric Assessment (CGA)
The CGA is a widely used instrument in geriatric practice. Functional status was evaluated by Activities of Daily Living (ADL) index [19], which defines the level of dependence/independence in six daily care activities including bathing, toileting, feeding, dressing, continence, and transferring and by Instrumental Activities of Daily Living (IADL) scale [20] which assess independence in eight activities that are more cognitively and physically demanding than ADL, including managing finances, taking medications, using telephone, shopping, using transportation, preparing meals, doing housework, and washing.
Cognitive status was assessed by the SPMSQ, a 10-item questionnaire that assesses orientation, memory, attention, calculation, and language [11].
Comorbidity was examined using the Cumulative Illness Rating Scale (CIRS) [21]. The CIRS uses a 5-point ordinal scale (score 1–5) to estimate the severity of pathology in each of 13 systems,including cardiac,vascular, respiratory, eye-ear-nose-throat, gastrointestinal, hepatic, renal, genitourinary, musculoskeletal, skin, nervous system, endocrine-metabolic, and psychiatric behavioral disorders. Based on the ratings, the Comorbidity Index (CIRS-CI) score which reflects the number of concomitant diseases was derived from the total number of categories in which moderate or severe levels (grade 3–5) of a chronic disease were identified (range 0–13).
Nutritional status was explored with the Mini Nutritional Assessment (MNA) [22], which includes information on anthropometric measures; lifestyle, medication and mobility; number of meals, food, and fluid intake and autonomy of feeding; and self perception of health and nutrition.
The Exton Smith Scale (ESS) was used to evaluate the risk of developing pressure sores. This 5-item questionnaire determines physical and mental condition, activity, mobility, and incontinence. For each item, a score from 1 to 4 is assigned [23].
Medication use was defined according to the Anatomical Therapeutics Chemical Classification code system and the number of drugs used by patients at admission was recorded. Social aspects included household composition, home service, and institutionalization were also noted. The approximate time required for collecting data for the CGA was 20 minutes (range from 15 to 25 minutes per person).
The Multidimensional Prognostic Index (MPI)
We used the Multidimensional Prognostic Index (MPI) developed and validated in two independent cohorts of elderly hospitalized patients as previously reported [10]. Briefly, a cluster analysis on CGA data was initially made for evaluating the independence of variables and identifying the most relevant domains of the CGA in predicting mortality outcome. Following a step-wise method, the domains of the CGA, one at a time, were progressively included in the model and Cox and logistic regression analyses performed. Thus an “eight domain” MPI was developed that included a total of 63 items and yielded the best index for predicting one-year mortality. For each domain a 3-level score was used, i.e., 0 = no problems, 0.5 = minor problems, and 1 = major problems. Thus, the sum of the calculated scores from the eight domains was divided by 8 to obtain a final MPI score between 0 to 1. As previously reported [10], three grades of severity risk to stratify the examined population were considered: low risk (MPI value ≤ 0.33), moderate risk (MPI value between 0.34 and 0.66), and severe risk (MPI > 0.66) of mortality.
Statistical analysis
Continuous variables were summarized as mean and standard deviation and categorical variables as frequencies and percentages. Comparisons between men and women were made using the Mann-Whitney U test. The Kruskall-Wallis and Chi squared tests were used to compare age, gender, education level, and mortality across the MPI groups.
The relationship between MPI score group and time to death was analyzed by age- and sex-adjusted Cox proportional hazards regression model. Time to death was calculated as the time between admission and time of death or the end of follow-up, whichever come first. The proportionality of the hazard assumption was graphically checked by plotting log [-log (survival function)].
Age- and sex-adjusted logistic model was carried out on the individual parameters of the MPI and the MPI itself to test the prognostic value for mortality after 1 month, 6 months, and 12 months of follow-up.
Age, ADL, IADL, SPMSQ, CIRS, MNA, ESS, and the number of medications were evaluated as continuous variables, while social support network and MPI were evaluated as ordinal variables, based on the assumption of equidistance between single unit values. Sex was analyzed as a dichotomous variable.
We assessed the predictive accuracy of the final model by looking at the two components of accuracy: calibration and discrimination. Calibration of the model was assessed by comparing the predicted mortality with the actual mortality in patients with different grades of MPI. The discrimination of the model was assessed by calculating the area under the receiver operating characteristics (ROC) curves for the MPI groups adjusted for age and gender. All analyses were performed using the SPSS version 15 software for Windows (SPSS Inc., Chicago, Illinois).
RESULTS
Study population characteristics
During the enrollment period, 2815 elderly patients were screened for the inclusion in the study. Of these, 262 patients with a diagnosis of dementia fulfilled the admission criteria and were included in the study. The population included 90 men (34.4%) and 172 women (65.6%) with a mean age of 80.8 years (± 6.7) and a range from 65 to 100 years.
Table 1 shows the characteristics of patients included in the study, divided according to gender. No significant differences in mean age, educational level, the mean values of all parameters of the CGA, the distribution rates of the type of dementia, and the CDR were observed between men and women. The overall mortality rates were 6.1% after 1 month, 13.7% after 6 months, and 18.7% after 12 months of follow-up, without significant differences between men and women.
Table 1.
Baseline Characteristics of the population included in the study by sex
| All | Males | Females | P value | |
|---|---|---|---|---|
| Patients, No. (%) | 262(100) | 90(34.4) | 172(65.6) | |
| Age | ||||
| Mean (SD), y | 80.8(6.7) | 80.4(6.7) | 81.0(6.6) | 0.33 |
| Range, y | 65–100 | 65–97 | 66–100 | |
| Educational level, mean (SD), y | 3.60(2.9) | 3.9(2.7) | 3.4(2.9) | 0.07 |
| ADL, mean (SD), score | 3.2(2.5) | 3.3(2.5) | 3.1(2.5) | 0.59 |
| IADL, mean (SD), score | 2.4(2.9) | 2.0(2.5) | 2.6(3.0) | 0.33 |
| SPMSQ, mean (SD), score | 5.7(3.2) | 5.9(3.3) | 5.6(3.29) | 0.54 |
| MMSE, mean (SD), score | 11.98(9.7) | 11.8(9.62) | 12,06(9.76) | 0.88 |
| Exton Smith, mean (SD), score | 14.7(3.7) | 14.9(3.8) | 14.6(3.6) | 0.51 |
| CIRS-CI, mean (SD), score | 2.7(1.8) | 2.9(1.6) | 2.7(1.8) | 0.17 |
| MNA, mean (SD), score | 19.7(5.8) | 20.2(6.2) | 19.4(5.5) | 0.18 |
| Number of Medications, mean (SD), No. | 3.7(2.6) | 3.7(2.9) | 3.7(2.5) | 0.73 |
| MPI, mean (SD), score | 0.49(0.2) | 0.48(0.2) | 0.49(0.2) | 0.63 |
| Type of dementia: | ||||
| Alzheimer, No. (%) | 103(39.3) | 34(33) | 69(67) | 0.001 |
| Vascular dementia, No. (%) | 88(33.6) | 32(36.4) | 56(63.6) | 0.01 |
| Mixed Dementia, No. (%) | 67(25.6) | 20(29.9) | 47(70.1) | 0.006 |
| Clinical Dementia Rating scale, mean (SD), score | 2.24(1.45) | 2.13(1.47) | 2.29(1.43) | 0.38 |
| Mortality, No. (%) | ||||
| at 1 month | 16(6.1) | 7(7.8) | 9(5.2) | 0.42 |
| at 6 months | 36(13.7) | 13(14.4) | 23(13.4) | 0.82 |
| at 12 months | 49(18.7) | 21(23.3) | 28(16.3) | 0.16 |
Abbreviation: ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; SPMSQ, Short-Portable Mental Status Questionnaire; CIRS-CI, Cumulative Illness Rating Scale – Comorbidity Index; MNA, Mini-nutritional assessment; MPI, Multidimensional Prognostic Index.
Multidimensional prognostic index
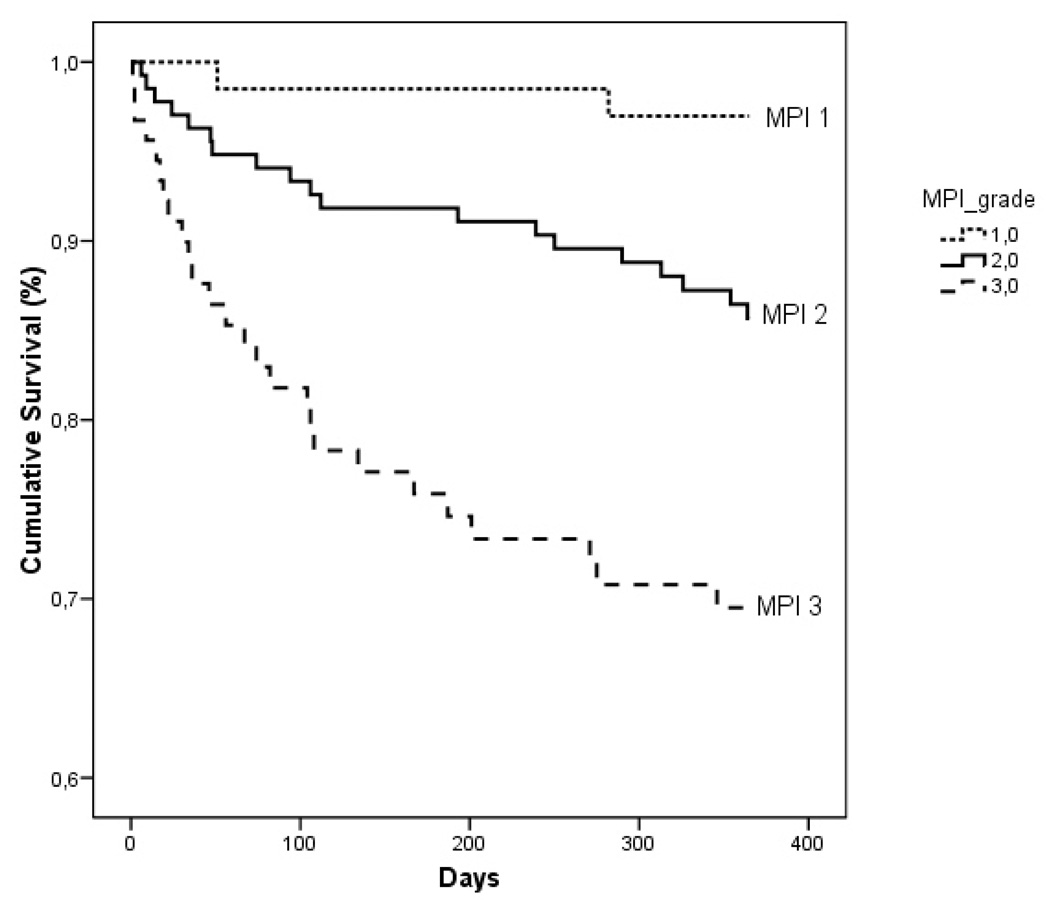
Table 2 shows the characteristics of patients divided by MPI grade. Higher MPI values were significantly associated with older age (p < 0.001), a significant decrease of the MMSE (p < 0.001), and an increase of the CDR scores (p < 0.001). With increasing MPI grade, progressively higher mortality rates were observed after 1 month (MPI 1 = 0%, MPI 2 = 5.2%, MPI 3 = 13.7%; p < 0.002), 6 months (MPI 1 = 2.7%, MPI 2 = 11.2%, MPI 3 = 28.8%; p < 0.001), and 12 months (MPI 1 = 2.7%, MPI 2 = 18.2%, MPI 3 = 35.6%; p < 0.001) of follow-up. Figure 1 shows the age-and sex-adjusted survival curve for different grades of MPI; patients with higher MPI values demonstrated a significantly higher mortality than patients with lower MPI values (p < 0.001). Table 3 reports the corresponding HR and 95% confidence intervals by different grades of MPI and the observed mortality after 1 month, 6 months, and 12 months of follow-up. Very close agreement was found between the estimated mortality by the three MPI grades and the observed mortality after 1 month, 6 months, and 12 months of follow-up.
Table 2.
Baseline Characteristics of the population according to MPI grade
| MPI 1 (Low Risk) |
MPI 2 (Moderate Risk) |
MPI 3 (Severe Risk) |
P value | |
|---|---|---|---|---|
| Patients, No. (%) | 73(27.9) | 116(44.3) | 73(27.9) | < 0.001 |
| MPI value, mean (SD) | 0.21(0.08) | 0.49(0.09) | 0.75(0.08) | < 0.001 |
| Sex | ||||
| Women, No. (%) | 47(64.4) | 79(68.1) | 46(63.0) | 0.74 |
| Age | ||||
| Mean (SD), y | 77.9(6.0) | 81.4(6.7) | 82.8(6.5) | < 0.001 |
| Range, y | 65–94 | 66–98 | 67–100 | |
| Educational levels, mean (SD) | 3.8(3.1) | 3.6(2.7) | 3.3(2.8) | 0.71 |
| MMSE, mean (SD), score | 21.7(6.3) | 11.48(7.8) | 3(5.15) | < 0.001 |
| Type of dementia: | ||||
| Alzheimer, No. (%) | 26(35.6) | 47(40.5) | 30(41.1) | 0.75 |
| Vascular dementia, No. (%) | 28(38.4) | 36(31) | 24(32.9) | 0.58 |
| Mixed dementia, No. (%) | 19(26) | 33(28.4) | 19(26) | 0.91 |
| Clinical Dementia Rating scale, mean (SD) | 1.3(0.59) | 2.1(1.26) | 3.3(1.57) | < 0.001 |
| Mortality, No. (%) | ||||
| at 1 month | 0(0) | 6(5.2) | 10(13.7) | 0.002 |
| at 6 months | 2(2.7) | 13(11.2) | 21(28.8) | < 0.001 |
| at 12 months | 2(2.7) | 21(18.2) | 26(35.6) | < 0.001 |
Abbreviation: MPI, multidimensional prognostic index.
Fig. 1.
Age- and sex-adjusted survival curve for different grades of MPI at one year.
Table 3.
Cumulative HR for mortality according to different grades of severity of MPI after 1 month, 6 months and 12 months of follow-up
| MPI grade | Time | EM HR (95% CI) | OM | Δ* |
|---|---|---|---|---|
| 1 (low risk) | 1 month | 0 (0) | 0 | 0 |
| 6 months | 0.015 (0.044–0.014) | 0.014 | 0.001 | |
| 12 months | 0.031(0.072–0.010) | 0.027 | 0.004 | |
| 2 (moderate risk) | 1 month | 0.034 (0.067–0.001) | 0.034 | 0 |
| 6 months | 0.09 (0.141–0.039) | 0.095 | −0.001 | |
| 12 months | 0.155 (0.218–0.092) | 0.164 | −0.009 | |
| 3 (high risk) | 1 month | 0.12 (0.194–0.046) | 0.123 | 0.003 |
| 6 months | 0.292 (0.394–0.190) | 0.288 | 0.004 | |
| 12 months | 0.364 (0.472–0.256) | 0.356 | 0.008 |
Abbreviation: MPI, multidimensional prognostic index; EM, estimated mortality; HR, hazard ratio; OM, observed mortality.
Delta indicates the difference between HR and OM values.
Logistic analyses adjusted for age and sex demonstrated that MPI was significantly associated with the mortality rates after 1 month (OR = 4.18, 95% CI = 1.71–10.1), 6 months (OR = 3.50, 95% CI = 1.97–6.23), and 12 months (OR = 3.40, 95% CI = 2.06–5.60) of follow-up. The odds ratio values after 1 month, 6 months, and 12 months were higher for MPI compared to the odds ratio values of the individual parameters used to construct the MPI (Table 4).
Table 4.
Mortality odds ratios of the variables utilized to constitute MPI after 1 month, 6 months and 12 months of follow-up
| 30 Days |
6 Months |
12 Months |
||||
|---|---|---|---|---|---|---|
| Risk Factors | OR (95% CI) | p | OR(95% CI) | p | OR(95% CI) | p value |
| MPI | 4.18 (1.71–10.14) | 0.001 | 3.50 (1.97–6.23) | < 0.001 | 3.40 (2.06–5.60) | < 0.001 |
| Age | 1.04 (0.96–1.12) | 0.30 | 1.08 (1.02–1.14) | 0.005 | 1.09 (1.04–1.14) | < 0.001 |
| Sex (male) | 1.52 (0.54–4.24) | 0.41 | 1.09 (0.52–2.27) | 0.81 | 1.56 (0.83–2.95) | 0.17 |
| ADL | 1.51 (1.15–1.98) | 0.002 | 1.43 (1.21–1.70) | < 0.001 | 1.38 (1.20–1.60) | < 0.001 |
| IADL | 1.74 (1.08–2.82) | 0.022 | 1.34 (1.10–1.63) | 0.002 | 1.30 (1.10–1.51) | 0.001 |
| SPMSQ | 1.19 (0.98–1.44) | 0.07 | 1.20 (1.06–1.37) | 0.004 | 1.21 (1.08–1.35) | < 0.001 |
| CIRS-CI | 1.08 (0.82– 1.43) | 0.53 | 1.27 (1.04–1.54) | 0.014 | 1.34 (1.12–1.60) | 0.001 |
| MNA | 1.12 (1.03–1.23) | 0.006 | 1.11 (1.05–1.18) | < 0.001 | 1.10 (1.04–1.16) | < 0.001 |
| Exton Smith Scale | 1.34 (1.15–1.55) | < 0.001 | 1.31 (1.18–1.46) | < 0.001 | 1.28 (1.16–1.40) | < 0.001 |
| No. of drugs | 1.12 (0.93–1.35) | 0.21 | 1.04 (0.91–1.19) | 0.54 | 1.06 (0.94–1.19) | 0.31 |
| Social Network | 0.61 (0.27–1.35) | 0.22 | 0.77 (0.48–1.23) | 0.28 | 0.90 (0.61–1.34) | 0.63 |
Abbreviation: MPI, Multidimensional-Prognostic; ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; SPMSQ, Short-Portable Mental Status Questionnaire; CIRS-CI, Cumulative Illness Rating Scale – Comorbidity Index; MNA, Mininutritional assessment.
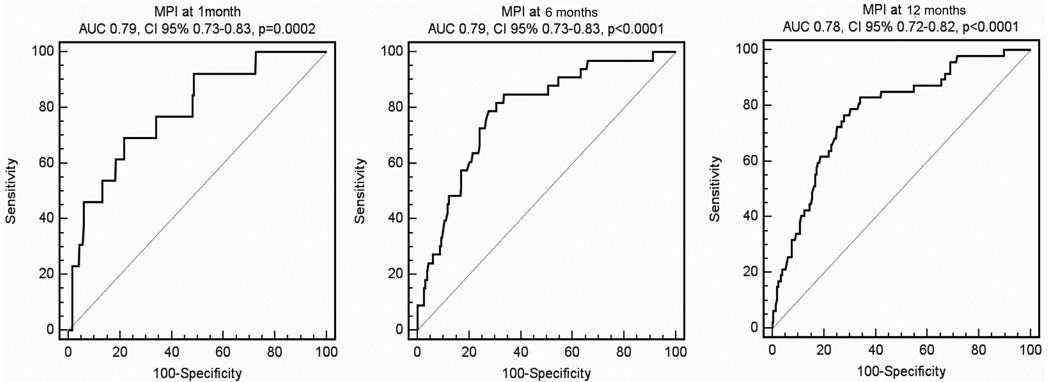
As showed in Fig. 2, the ROC curves demonstrated an area under the curve of 0.79, 95% CI = 0.73–0.84 (sensitivity = 69.2%, specificity = 78.3, cut off value > 0.06) at 1 month, of 0.79, 95% CI = 0.73–0.84 (sensitivity=78.49%,specificity=72.49, cut off value > 0.14) at 6 months and of 0.78, 95% CI = 0.72–0.83 (sensitivity = 83%, specificity = 66.05, cut off value > 0.16) at 12 months of follow-up.
Fig. 2.
Receiver Operating Curves (ROC) for MPI at 1 month (left), 6 months (center), and 12 months of follow-up (right).
DISCUSSION
In the present study we demonstrated that the MPI, derived from a standardized CGA, was effective in predicting short- and long-term mortality risk in elderly patients with dementia admitted to a geriatric hospital ward. The MPI was derived from parameters obtained from a standardized CGA and was effective in predicting 1-month, 6-month, and 12-month mortality risk as demonstrated by the close agreement between the estimated and observed mortality rates in patients divided by their MPI grades. These data are similar to the results reported in previous studies carried out in patients with acute diseases or relapse of a chronic disease [10] as well as in patients with gastrointestinal bleeding [24] or pneumonia [25]. As expected, in this population the individual parameters used to construct the MPI, i.e., ADL, IADL, SPMSQ, CIRS, ESS, and MNA were factors independently associated with mortality. Logistic regression analysis confirmed that the MPI was significantly associated with mortality and that the risk of MPI-related mortality was higher than those provided by the individual parameters used for constructing it. Overall these findings supported the concept that considering multidimensional aggregate information is very important for predicting short- and long-term mortality in older patients with dementia. Moreover, the present findings suggested that the management of dementia in elderly patients may require a multidimensional approach involving an interdisciplinary staff including specialized physicians and nurses, physiotherapists, social workers, and the familiar network. Indeed, while clinicians’ interview-based global severity scales, mainly based on cognitive domains, such as the Functional Assessment Staging (FAST) procedure [26] were used to predict 6-month survival among newly admitted nursing home residents with advanced dementia [27], this MPI appeared to predict short- and long-term mortality in demented patients regardless of the stage.
The overall 1-year mortality rate observed in this study was 17.9%. This finding was in agreement with a 13.9% of all-cause mortality reported in a previous study [28]. In the present study no significant differences were observed in the survival rates between men and women. This finding was in agreement with previous studies carried out in older patients with dementia reporting that significant differences in mortality rates between men and women only in patients under 85 years of age [29].
The median survival time of these patients is 4.5 years from the onset of the disease [30] and their evolution may rapidly lead to an early institutionalization [31]. Therefore, prognostic definition of elderly patients with dementia is a crucial point for physicians, patients, and their families that may be important for the identification of the more adequate management of patients. Previous epidemiological studies suggested that age, male sex, socio-demographic characteristics, the severity of dementia, other comorbid conditions, and genetic characteristics may be significant predictors of mortality in the elderly population with dementia [32,33]. The present study confirms that the following characteristics are associated with poorer survival in dementia, regardless of the stage: older age [34, 35], greater functional [34–36] and cognitive impairment [34], other comorbid conditions [34],the presence of pressure sores [34], and poor nutritional status [34, 35]. However, the short- and long-term mortality risk as predicted by the MPI in this population was higher in comparison with the mortality risk as predicted by the individual parameters used to construct the MPI. To the best of our knowledge, this is the first description of a prognostic index based on data available from a standard CGA for older patients hospitalized with dementia. This instrument is easy to use and the time to complete all the tests is about 20 minutes per evaluation. Moreover, all the tests included in the MPI are well known by medical staff, validated in elderly populations, and broadly used in clinical practice. The originality of the MPI is that it uses widely accepted evaluation tools to obtain a prognostic index of mortality that represent an extreme synthesis of a multidimensional process. Moreover, the different tools of the MPI may give information about different domains allowing an optimization of the care processes of the patient. As showed by the ROC curves, the discrimination of the MPI was also good with area under the ROC curves of 0.79 with a cut off of 0.06 at 1 month, of 0.79 with a cut off of 0.14 at 6 months, and of 0.78 with a cut off of 0.16 at 12 months of follow-up. These values are similar to the area under the ROC curves of 0.75 (95% CI, 0.71–0.80) at 6 months, and 0.75 (95% CI, 0.71–0.80) at 12 months of follow-up reported in a previous study carried out in 857 elderly patients hospitalized for an acute disease or a relapse of a chronic disease [10].
The present study has some limitations. Since the study population comprised selected patients admitted to a geriatric unit, it is possible that it may not be applicable to institutionalized or ambulatory demented patients. Indeed, in different groups of elderly subjects, the MPI would probably include other predictor variables that take into account the different settings. Moreover, since MPI needs a complete CGA assessment in an acute setting, it is possible that it may be an index too complex for a bedside use in all elderly patients. Finally, the study population was relatively small and the patients were recruited within a single hospital. Larger prospective multicenter studies are needed to confirm these findings. In conclusion, we have described a multidimensional prognostic index of short- and long-term mortality in hospitalized elderly patients with dementia. This index was a sensitive multidimensional risk measure that might be useful in identifying elderly demented patients at different risk of mortality who probably need a different intensity of clinical interventions.
ACKNOWLEDGMENTS
This work was fully supported by grants from Ministero della Salute,IRCCS Research Program 2006–2008, Line 2: “Malattie di rilevanza sociale”.
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=19).
REFERENCES
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M Alzheimer’s Disease International. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependance in the elderly: 3-year follow-up data from a population based study. Am J Public Health. 1998;10:1452–1456. doi: 10.2105/ajph.88.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Neurological disorders: public health challenges. [Accessed January 14, 2008];WHO. 2006 3.1, 42–54, www.who.int/mental_health/neurology/neurological_disorders_report_web.pdf.
- 4.Levine SK, Sachs GA, Jin L, Meltzer D. A prognostic model for 1 year mortality in older adults after hospital discharge. Am J Med. 2007;120:455–460. doi: 10.1016/j.amjmed.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci L, Weilan D. Multidimensional Geriatric Assessment: back to the future. J Gerontol A Biol Sci Med Sci. 2008;63:272–274. doi: 10.1093/gerona/63.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubenstein LZ. Joseph T. Freeman award lecture: Comprehensive Geriatric Assessment: from miracle to reality. J Gerontol A Biol Sci Med Sci. 2004;59:473–477. doi: 10.1093/gerona/59.5.m473. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell SL, Kiely DK, Hamel MB, Park PS, Morris JN, Fries BE. Estimating prognosis for nursing home residents with advanced dementia. JAMA. 2004;291:2734–2740. doi: 10.1001/jama.291.22.2734. [DOI] [PubMed] [Google Scholar]
- 8.Walter LC, Brand RJ, Counsell SR, Palmer RM, Landefeld CS, Fortinsky RH, Covinsky KE. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 9.Lee HB, Kasper JD, Shore AD, Yokley JL, Black BS, Rabins PV. Level of cognitive impairment predicts mortality in high risk community samples: the memory and medical care study. J Neuropsychiatry Clin Neurosci. 2006;18:543–546. doi: 10.1176/jnp.2006.18.4.543. [DOI] [PubMed] [Google Scholar]
- 10.Pilotto A, Ferrucci L, Franceschi M, D’Ambrosio LP, Scarcelli C, Cascavilla L, Paris F, Placentino G, Seripa D, Dallapiccola B, Leandro G. Development and validation of a Multidimensional Prognostic Index for 1-Year Mortality from the Comprehensive Geriatric Assessment in Hospitalized Older Patients. Rejuvenation Res. 2008;11:151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 12.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: APA; 1994. [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo J-M, Brun A, Hofman A, Moody DM, O’Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Bermejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmann A, Scheinberg P. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 17.Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, Russell RW, Symon L. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 18.Orsitto G, Seripa D, Panza F, Franceschi M, Cascavilla L, Placentino G, Matera MG, Paris F, Capurso C, Solfrizzi V, Dallapiccola B, Pilotto A. Apolipoprotein E genotypes in hospitalized elderly patients with vascular dementia. Dement Geriatr Cogn Disord. 2007;23:327–333. doi: 10.1159/000100972. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, Downs TD, Cash HR, Grotz RC. Progress in the development of an index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 21.Linn B, Linn M, Gurel L. Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 22.Guigoz Y, Vellas B. The Mini Nutritional Assessment (MNA) for grading the malnutrition states of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Performe Programme. 1999;1:3–11. doi: 10.1159/000062967. [DOI] [PubMed] [Google Scholar]
- 23.Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Mon Bull Minis Health Public Health Lab Serv. 1966;25:238–268. [PubMed] [Google Scholar]
- 24.Pilotto A, Ferrucci L, Scarcelli C, Niro V, Di Mario F, Seripa D, Andriulli A, Leandro G, Franceschi M. Usefulness of the comprehensive geriatric assessment in older patient with upper gastorintestinal bleeding: a two year follow-up study. Dig Dis. 2007;25:124–128. doi: 10.1159/000099476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilotto A, Addante F, Ferrucci L, Leandro G, D’Onofrio G, Corritore M, Niro V, Scarcelli C, Dallapiccola B, Franceschi M. The Multidimensional Prognostic Index (MPI) predicts short and long-term mortality in older patients with community-acquired pneumonia. J Gerontol A Biol Sci Med Sci. 2009;64A:880–887. doi: 10.1093/gerona/glp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisberg B. Functional assessment staging (FAST) Psychopharmacol Bull. 1988;24:653–659. [PubMed] [Google Scholar]
- 27.Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc. 1997;45:1054–1059. doi: 10.1111/j.1532-5415.1997.tb05966.x. [DOI] [PubMed] [Google Scholar]
- 28.Bianchetti A, Scuratti A, Zanetti O, Binetti G, Frisoni GB, Magni E, Trabucchi M. Predictor of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia. 1995;6:108–112. doi: 10.1159/000106930. [DOI] [PubMed] [Google Scholar]
- 29.Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Mortality from dementia in advanced age: a 5 year follow-up study of incident dementia cases. J Clin Epidemiol. 1999;52:737–743. doi: 10.1016/s0895-4356(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 30.Helmer C, Joly P, Letenneur L, Commenges D, Dartigues JF. Mortality with Dementia: Results from a French Prospective Community based Cohort. Am J Epidemiol. 2001;154:642–648. doi: 10.1093/aje/154.7.642. [DOI] [PubMed] [Google Scholar]
- 31.Cortes F, Nourhashémi F, Guérin O, Cantet C, Gillette-Guyonnet S, Andrieu S, Ousset PJ, Vellas B REAL-FR Group. Prognosis of Alzheimer’s disease today: A two-year prospective study in 686 patients from the REAL-FR Study. Alzheimers Dement. 2008;4:22–29. doi: 10.1016/j.jalz.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Xie J, Brayne C, Matthews FE Medical Research Council Cognitive Function and Ageing Study collaborators. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ. 2008;336:258–262. doi: 10.1136/bmj.39433.616678.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, Kukull WA. Survival after Initial Diagnosis of Alzheimer Disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 34.Gambassi G, Landi F, Lapane KL, Sgadari A, Mor V, Bernabei R. Predictors of mortality in patients with Alzheimer’s disease living in nursing homes. J Neurol Neurosurg Psychiatry. 1999;67:59–65. doi: 10.1136/jnnp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aquero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Prognostic factors in very old demented adults: a seven-year follow-up from a population-based survey in Stockholm. J Am Geriatr Soc. 1998;46:444–452. doi: 10.1111/j.1532-5415.1998.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 36.Carlson MC, Brandt J, Steele C, Baker A, Stern Y, Lyketsos CG. Predictor index of mortality in dementia patients upon entry into long-term care. J Gerontol A Biol Sci Med Sci. 2001;56:M567–M570. doi: 10.1093/gerona/56.9.m567. [DOI] [PubMed] [Google Scholar]