Abstract
A formal [4+3] epoxide cascade protocol utilizing ambiphilic sulfonamides and a variety of epoxides (masked ambiphiles) has been developed for the generation of benzothiaoxazepine-1,1′-dioxides and oxathiazepine-1,1′-dioxides. This protocol combines an epoxide ring-opening with either an SNAr or oxa-Michael cyclization pathway.
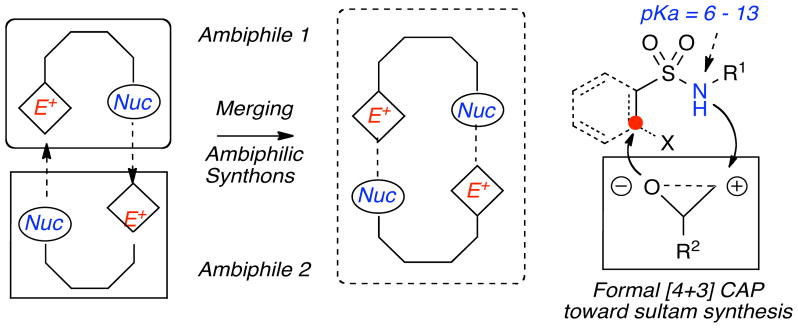
The development of cascade reactions, which couple two or more reactions together to produce heterocyclic scaffolds, is an important challenge in drug discovery and natural product synthesis.1 Cascade or domino reactions are highly efficient pathways that allow for the synthesis of complex molecules from simple substrates and encompass a variety of transformations. Many of these cascade transformations involve the utilization of synthons, which contain either a nucleophilic or an electrophilic site.2 In contrast, ambiphilic synthons possess both a nucleophilic and electrophilic site, making them ideal components for cascade protocols.3,4 Interest in the utilization of cascade reactions for the synthesis of diverse sultam scaffolds5 has led us to explore the titled protocol where ambiphilic sulfonamides are combined with an epoxide (a masked ambiphile), are combined in a cascade reaction termed complementary ambiphile pairing (CAP) (Figure 1).
Figure 1.
Generation of sultam hetrocycles utilizing complementary ampbiphile pairing (CAP)
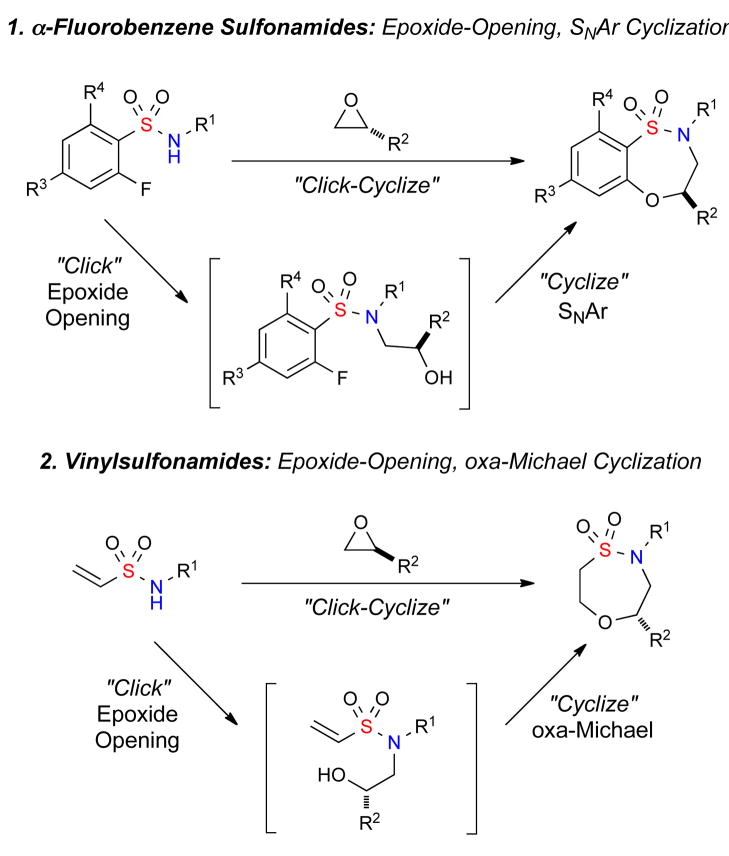
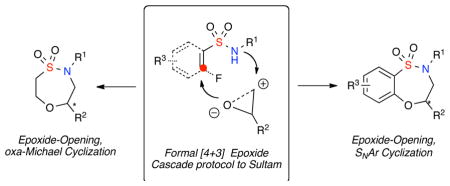
Epoxide cascade reactions have been known for over a half-century and have played a key role in the synthesis of polycyclic natural products.6 Despite the wide application of epoxide cascade reactions in natural product synthesis, application in the synthesis of complex small heterocycles has been utilized to a lesser degree. Epoxide cascades can utilize a variety of organic acids,7 or metal catalysts, to promote the cyclization. These include Au (I) cyclization with alkynes and allenes,8 SmI2 opening-iodocyclization,9 and cobalt-mediated cycloadditions.10 Notably absent from the literature are methods which combine epoxide ring-opening pathways with other pathways in a domino cascade. Towards the realization of this goal, we herein report the development of a formal [4+3] epoxide cascade protocol which combines an epoxide ring-opening with either an SNAr or oxa-Michael cyclization pathway for the generation of benzothiaoxazepine-1,1′-dioxides and oxathiazepine-1,1′-dioxides (Figure 2).
Figure 2.
Epoxide cascade protocols for the synthesis of sultams
Reports of the cyclization of an in situ generated epoxide-derived alkoxide via an intramolecular SNAr cyclization have been limited. Albanese and co-workers first reported the synthesis of piperazines utilizing an epoxide-opening, SNAr protocol.11 More recently, a key report by Cleator and co-workers at Merck demonstrated the ring-opening of epoxides with α-fluorobenzene-sulfonamides, followed by subsequent SNAr cyclization to give the corresponding piperazines and sultams.12 Reports of intramolecular Michael cyclizations with vinylsulfonamides have been utilized in seminal work by Knollmüller and Hirooka,13 to more recent applications in the area of diversity-oriented synthesis (DOS) strategies using a “Click, Click, Cyclize” approach.14 However, the opening of an epoxide and subsequent cyclization via oxa-Michael is not known.
Initial investigation into the proposed epoxide cascade focused on the development of orthogonal reaction conditions that would initiate the ring opening of the corresponding epoxide, followed by intramolecular SNAr cyclization to yield the desired sultam in a one-pot, domino process.15 It was found that both the choice of solvent and base was key to the overall reaction process with dioxane essential for the initial epoxide ring-opening step, and DMF for the SNAr ring-closing step of the cascade. After screening a wide variety of bases, anhydrous Cs2CO3 produced the best overall yield and crude purity when utilized in the cascade protocol. The utilization of microwave irradiation at 110 °C for 20 minutes was essential to obtain both high yields and crude purity in addition to a significant decrease in reaction times.16,17 After optimization of reaction conditions for the synthesis of sultam 1, the substrate scope was investigated using a variety of epoxides and α-fluorobenzenesulfonamides to yield the corresponding sultams 1–9 in good yield and crude purity (Table 1).18
Table 1.
One-pot epoxide, SNAr cascade utilizing α-fluorobenzenesulfonamides
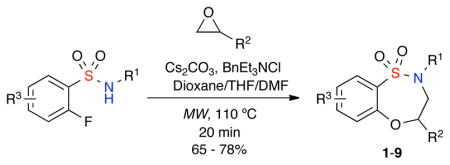 | ||||
|---|---|---|---|---|
| entry | R1 | R2 | R3 | yield |
| 1 | (CH2)3CH3 | CH2OBn | 6-F | 73 |
| 2 | (CH2)3CH3 | (CH2)2CH=CH2 | 6-F | 71 |
| 3 | 4-F-Ph(CH2)2 | CH2OPh | 6-F | 71 |
| 4 | 4-OMe-Bn | (CH2)2CH=CH2 | 6-F | 69 |
| 5 | (R)-CH(CH3)Ph | (R)-CH2CO2 (CH2)2CH3 |
6-F | 76 |
| 6 | (CH2)3CH3 | CH2OBn | 3-Br | 65 |
| 7 | Allyl | CH2O(CH2)3CH3 | 5-Cl | 74 |
| 8 | (CH2)3CH3 | CH2OPh | 3-Br | 78 |
| 9 | 2-OMe-Bn | CH2O(CH2)3CH3 | 5-Cl | 74 |
During these investigations, it was found that when utilizing tert-butyldimethylsilyl (R)-(−)-glycidyl ether under the aforementioned reactions conditions, the corresponding hydroxy-sultam 11 was isolated. This presumably occurs when the in situ-generated fluoride ion deprotects the corresponding TBDMS-protected sultam 10 yielding the free hydroxy sultam 11 in good yield (Table 2).16
Table 2.
Proposed mechanism for the generation of Sultam 11
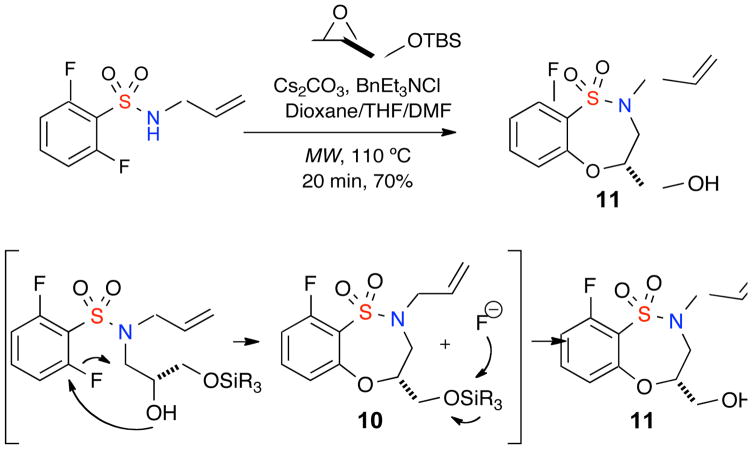 |
With sultam 11 in hand, facile derivatization of the free hydroxy was achieved via ester formation with a variety of acids. In this case, an oligomer coupling reagent, 2GOACC50, derived from ring-opening metathesis polymerization (ROMP) was utilized to yield the corresponding sultam esters 12–16 in high yield and purity without the need for conventional purification (Table 3).19
Table 3.
SNAr – intramolecular Mitsunobu route to benzothiaoxazepine-1,1-dioxides
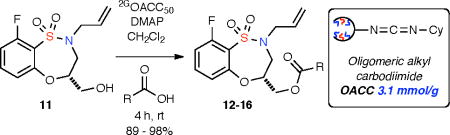 | |||
|---|---|---|---|
| entry | R1 | yield | puritya |
| 1 | 3-MePh | 94 | >95% |
| 2 | 3-OMePh | 94 | >95% |
| 3 | 3,4-MePh | 96 | >95% |
| 4 | CH2CN | 89 | >95% |
| 5 | CH2SPh | 98 | >95% |
Crude Purity determined by 1H NMR
We have previously shown that vinylsulfonamides undergo oxa- and aza-Michael additions to afford the corresponding oxathiazepine-1,1′-dioxides and oxathiazocine-1,1-dioxides.10 Therefore, the utilization of vinylsulfonamides in the aforementioned epoxy cascade reaction was investigated. In this case, it was envisioned that following the opening of an epoxide with a vinylsulfonamide, the in-situ generated hydroxy group would spontaneously undergo a oxa-Michael cyclization reaction to the corresponding sultam in a one-pot cascade protocol.
The aforementioned SNAr results showed that the utilization of dioxane was essential for quantitative epoxide opening at 110°C under microwave irradiation. Additionally, the oxa-Michael cyclization reaction proceeds efficiently in polar solvent such as THF but not DMF. Using these slightly modified conditions, a variety of vinylsulfonamides were subjected to the epoxy cascade protocol affording the corresponding sultams 17–24 in good yield and high crude purity (Table 4).
Table 4.
One-pot epoxy cascade SNAr cyclization utilizing vinylsulfonamides
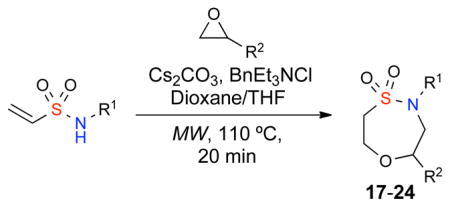 | |||
|---|---|---|---|
| entry | R1 | R2 | yield |
| 1 | Allyl | CH2OBn | 65 |
| 2 | Allyl | (S)-CH2OBn | 63 |
| 3 | (CH2)3CH3 | CH2OBn | 58 |
| 4 | Bn | 4-Me-PhOCH2 | 62 |
| 5 | Allyl | 4-Me-PhOCH2 | 57 |
| 6 | Cyclopentyl | 4-Me-PhOCH2 | 55 |
| 7 | Propargyl | CH2OPh | 53 |
| 8 | (R)-CH(CH3)Ph | (R)-CH2CO2 (CH2)2CH3 |
60 |
As demonstrated in the case of α-fluorobenzene sulfonamides (Table 1), the reaction conditions were tolerant to a variety of vinylsulfonamides and epoxides, allowing for the incorporation of functional handles and stereogenic centers into the molecule (Table 3, entry 2 and 8). Overall, the employment of ambiphilic vinylsulfonamides in comparison to α-fluorobenzene sulfonamides gives rapid access to the corresponding non-benzofused oxathiazepine-1,1′-dioxide derivatives.
In conclusion, we report the development of a facile, one-pot epoxide cascade protocol for the synthesis of benzothiaoxazepine-1,1′-dioxides and oxathiazepine-1,1′-dioxides from ambiphilic sulfonamides. Epoxide ring opening followed by either intramolecular SNAr cyclization or intramolecular oxa-Michael cyclization yields these heterocycles. In both cases, a variety of epoxides and sulfonamides were utilized to demonstrate substrate scope and utility of the method. Ongoing efforts aimed at the investigation of additional CAP strategies continue and will be reported in due course.
Supplementary Material
Acknowledgments
This publication was made possible by the Pilot-Scale Libraries Program (P41 GM076302), and the National Institutes of General Medical Sciences [The University of Kansas Center for Chemical Methodologies and Library Development (KU-CMLD) (P50 GM069663)].
Footnotes
Supporting Information Available: Experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Nicolaou KC, Chen JS. Chem Soc Rev. 2009;11:2993–3009. doi: 10.1039/b903290h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Enders D, Grndal C, Hüttl MRM. Angew Chem Int Ed. 2007;46:1570–1581. doi: 10.1002/anie.200603129. [DOI] [PubMed] [Google Scholar]; c) Tietze LF, Beifuss U. Angew Chem Int Ed Engl. 1993;32:131–163. [Google Scholar]
- 2.(a) Tietze LF, Brasche G, Gericke KM, editors. Domino Reactions In Organic Synthesis. Wiley-VCH; Weinheim, Germany: 2006. [Google Scholar]; (b) Fustero S, Jiménez D, Sánchez-Roselló M, del Pozo C. J Am Chem Soc. 2007;129:6700–6701. doi: 10.1021/ja0709829. [DOI] [PubMed] [Google Scholar]; (c) Bi HP, Liu XY, Gou FR, Guo LN, Duan XH, Shu XZ, Liang YM. Angew Chem Int Ed. 2007;46:7068–7071. doi: 10.1002/anie.200702238. [DOI] [PubMed] [Google Scholar]; (d) Rolfe A, Young K, Hanson PR. Eur J Org Chem. 2008:5254–5262. doi: 10.1002/ejoc.200800651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Bontemps S, Gornitzka H, Bouhadir G, Miqueu K, Bourissou D. Angew Chem Int Ed. 2006;45:1611–1614. doi: 10.1002/anie.200503649. [DOI] [PubMed] [Google Scholar]; (b) Bontemps S, Bouhadir G, Miqueu K, Bourissou D. J Am Chem Soc. 2006;128:12056–12057. doi: 10.1021/ja0637494. [DOI] [PubMed] [Google Scholar]; (c) Tejedor D, Méndez-Abt G, González-Platas J, Ramirez MA, García-Tellado F. Chem Comm. 2009:2368–2370. doi: 10.1039/b819613c. [DOI] [PubMed] [Google Scholar]; (d) Kozytska MV, Dudley GB. Chem Comm. 2005:3047–3049. doi: 10.1039/b503110a. [DOI] [PubMed] [Google Scholar]
- 4.(a) Molecules containing both electrophilic and nucelophilic nodes have been also defined as amphoteric molecules by Yudin, see: Hili R, Yudin AK. J Am Chem Soc. 2009;131:16404–16406. doi: 10.1021/ja9072194.Hili R, Yudin AK. Angew Chem Int Ed. 2008;47:4188–4191. doi: 10.1002/anie.200705776.Baktharaman S, Hili R, Yudin AK. Aldrichimica Acta. 2008;41:109–119.Molecules containing both electrophilic and nucleophilic nodes have also been also defined as amphiphilic molecules, see: Nakamura H, Shim JG, Yamamoto Y. J Am Chem Soc. 1997;119:8113–8114.Nakamura H, Aoyagi K, Shim JG, Yamamoto Y. J Am Chem Soc. 2001;123:372–377.Kimura M, Tamaki T, Nakata M, Tohyama K, Tamaru Y. Angew Chem Int Ed. 2008;120:5887–5889. doi: 10.1002/anie.200801252.
- 5.Sultams, the cyclic analogs of sulfonamides, although not found in nature, represent a subclass of relatively unexplored molecular space for the discovery of new therapeutic drugs. Recent reports have demonstrated that sultams possess a broad spectrum of biological activity. For an extensive list of biological sultams and methods for synthesis see: Jimenez-Hopkins M, Hanson PR. Org Lett. 2008;10:2223–2226. doi: 10.1021/ol800649n.
- 6.(a) Corey EJ, Russey WE, Ortiz de Montellano PR. J Am Chem Soc. 1966:4750–4751. doi: 10.1021/ja00972a056. [DOI] [PubMed] [Google Scholar]; (b) van Tamelen EE, Willett JD, Clayton RB, Lord KE. J Am Chem. 1966:4752–4753. doi: 10.1021/ja00972a058. [DOI] [PubMed] [Google Scholar]; (c) Vilotijevic I, Jamison TF. Angew Chem Int Ed. 2009;48:5250–5281. doi: 10.1002/anie.200900600. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Topczewski JJ, Callahan MP, Neighbors JD, Wiemer DF. J Am Chem Soc. 2009;131:14630–14631. doi: 10.1021/ja906468v. [DOI] [PubMed] [Google Scholar]; (d) Neighbors JD, Beutler JA, Wiemer DF. J Org Chem. 2005;70:925–931. doi: 10.1021/jo048444r. [DOI] [PubMed] [Google Scholar]
- 7.(a) Nicolaou KC, Prasad CVC, Somers PK, Hwang CK. J Am Chem Soc. 1989;111:5335–5340. [Google Scholar]; (b) Morimoto Y, Nishikawa Y, Ueba C, Tanaka T. Angew Chem. 2006;118:824–826. doi: 10.1002/anie.200503143. [DOI] [PubMed] [Google Scholar]; (c) Mori Y, Yaegashi K, Furukawa H. J Am Chem Soc. 1996;118:8158–8159. [Google Scholar]; (d) Heffron TP, Jamison TF. Org Lett. 2003;5:2339–2342. doi: 10.1021/ol0347040. [DOI] [PubMed] [Google Scholar]
- 8.(a) Dai LZ, Qi MJ, Shi YL, Liu XG, Shi M. Org Lett. 2007;9:3191–3194. doi: 10.1021/ol0713640. [DOI] [PubMed] [Google Scholar]; (b) Tarselli MA, Zuccarello JL, Lee SJ, Gagne MR. Org Lett. 2009;11:3490–3492. doi: 10.1021/ol901391s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Kwon DW, Cho MS, Kim YH. Synlett. 2003;7:959–962. [Google Scholar]; (b) Park HS, Kwon DW, Lee K, Kim YH. Tetrahedron Lett. 2008;49:1616–1618. [Google Scholar]
- 10.Odedra A, Lush SF, Lui RS. J Org Chem. 2007;72:567–573. doi: 10.1021/jo0620617. [DOI] [PubMed] [Google Scholar]
- 11.(a) Albanese D, Landini D, Penso M. Chem Commun. 1999:2095–2096. [Google Scholar]; (b) Albanese D, Landini D, Lupi V, Penso M. Ind Eng Chem Res. 2003;42:680–686. [Google Scholar]
- 12.After the submission of this paper for review, we became aware of the following report which appeared online on December 24, 2009: Cleator E, Baxter CA, O’Hagan M, O’Riordan TJC, Sheen FJ, Stewart GW. Tetrahedron Lett. 2010:1079–1082.
- 13.(a) Hromatka O, Binder D, Knollmüller M. Monatshfte Für Chemie. 1968;99:1062–1067. [Google Scholar]; (b) Hasegawa K, Hirooka S. Bull Soc Chem Jap. 1972;45:525–528. [Google Scholar]
- 14.(a) Zhou A, Hanson PR. Org Lett. 2008;10:2951–2954. doi: 10.1021/ol8009072. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhou A, Rayabarapu D, Hanson PR. Org Lett. 2009;11:531–534. doi: 10.1021/ol802467f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolfe A, Young K, Volp K, Schoenen F, Neuenswander B, Lushington GH, Hanson PR. J Comb Chem. 2009;11:732–738. doi: 10.1021/cc900025e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conventional heating in oil bath required 20–24 hours at 150 °C to afford the desired product, although yields were on average 10–20% lower.
- 17.2-Fluorobenzenesulfonamides bearing additional halogen functionality on the benzene ring were designed for diversification at a later stage in library format. No reduction in yield or reaction rates for the epoxide cascade protocol was observed when these were not present in the starting material.
- 18.The control experiment was carried out utilizing N-allyl-2-bromobenzenesulfonamide whereby the fluorine at the 2- and 6- position was replaced by H at the 6- and a Br at the 2-. Under the standard reaction conditions, the corresponding TBDMS protected epoxide acyclic product was produced as observed by crude 1H NMR, indicating the TBDMS group was not removed under the reaction conditions producing the corresponding free OH group.
- 19.(a) Zhang M, Vedantham P, Flynn DL, Hanson PR. J Org Chem. 2004;69:8340–8344. doi: 10.1021/jo048870c. [DOI] [PubMed] [Google Scholar]; (b) Rolfe A, Probst D, Volp KA, Omar I, Flynn D, Hanson PR. J Org Chem. 2008;73:8785–8790. doi: 10.1021/jo801578f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.