Abstract
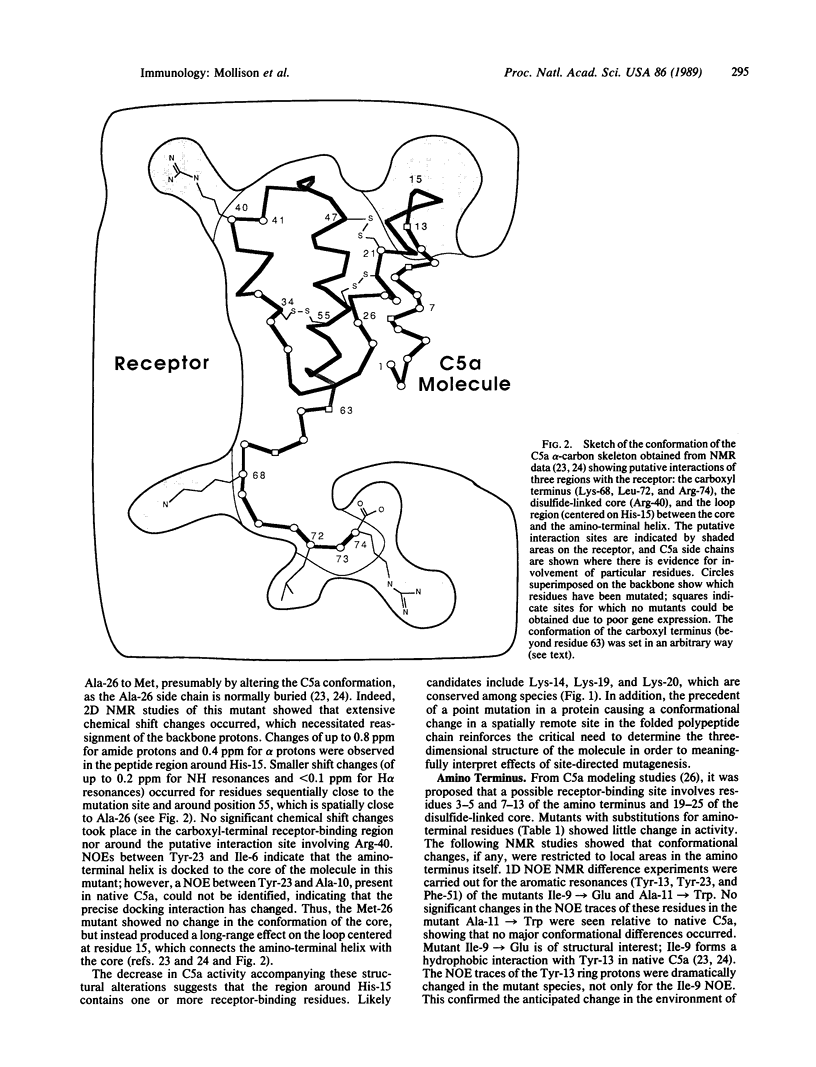
C5a is an inflammatory mediator potentially involved in a number of diseases. To help define which of its 74 residues are important for receptor binding and response triggering, changes in the amino acid sequence of C5a were introduced by site-directed mutagenesis. Synthetic C5a-encoding genes incorporating point mutations were expressed in Escherichia coli, and the mutant proteins were purified to homogeneity. Modifications of the C5a molecule causing parallel reductions in binding to polymorphonuclear leukocyte membranes and in stimulation of polymorphonuclear leukocyte locomotion (chemokinesis) suggest that carboxyl-terminal residues Lys-68, Leu-72, and Arg-74 interact with the receptor. Substitutions in the disulfide-linked core of C5a revealed involvement of Arg-40 or nearby residues, because potency losses were associated with only localized conformational changes as detected by NMR. Surprisingly, a substitution at core residue Ala-26, which did not alter C5a core structure, appeared from NMR results to reduce potency by causing a long-distance conformational change centered on residue His-15. Thus, at least three discontinuous regions of the C5a molecule appear to act in concert to achieve full potency.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale L. H., Tippett P. S., Erickson B. W., Hugli T. E. The active site of C3a anaphylatoxin. J Biol Chem. 1980 Nov 25;255(22):10758–10763. [PubMed] [Google Scholar]
- Chenoweth D. E., Goodman M. G., Weigle W. O. Demonstration of a specific receptor for human C5a anaphylatoxin on murine macrophages. J Exp Med. 1982 Jul 1;156(1):68–78. doi: 10.1084/jem.156.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerau B., Zimmermann B., Czorniak K., Wüstefeld H., Vogt W. Role of the N-terminal regions of hog C3a, C5a and C5a-desArg in their biological activities. Mol Immunol. 1986 Apr;23(4):433–440. doi: 10.1016/0161-5890(86)90141-0. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978 Oct 10;253(19):6955–6964. [PubMed] [Google Scholar]
- Frank M. M. The complement system in host defense and inflammation. Rev Infect Dis. 1979 May-Jun;1(3):483–501. doi: 10.1093/clinids/1.3.483. [DOI] [PubMed] [Google Scholar]
- Gennaro R., Simonic T., Negri A., Mottola C., Secchi C., Ronchi S., Romeo D. C5a fragment of bovine complement. Purification, bioassays, amino-acid sequence and other structural studies. Eur J Biochem. 1986 Feb 17;155(1):77–86. doi: 10.1111/j.1432-1033.1986.tb09460.x. [DOI] [PubMed] [Google Scholar]
- Gerard C., Chenoweth D. E., Hugli T. E. Molecular aspects of the serum chemotactic factors. J Reticuloendothel Soc. 1979 Dec;26(Suppl):711–718. [PubMed] [Google Scholar]
- Gerard C., Hugli T. E. Amino acid sequence of the anaphylatoxin from the fifth component of porcine complement. J Biol Chem. 1980 May 25;255(10):4710–4715. [PubMed] [Google Scholar]
- Gerard C., Showell H. J., Hoeprich P. D., Jr, Hugli T. E., Stimler N. P. Evidence for a role of the amino-terminal region in the biological activity of the classical anaphylatoxin, porcine C5a des-Arg-74. J Biol Chem. 1985 Mar 10;260(5):2613–2616. [PubMed] [Google Scholar]
- Greer J. Comparative structural anatomy of the complement anaphylatoxin proteins C3a, C4a and C5a. Enzyme. 1986;36(1-2):150–163. doi: 10.1159/000469285. [DOI] [PubMed] [Google Scholar]
- Greer J. Model structure for the inflammatory protein C5a. Science. 1985 May 31;228(4703):1055–1060. doi: 10.1126/science.3992245. [DOI] [PubMed] [Google Scholar]
- Huber R., Scholze H., Pâques E. P., Deisenhofer J. Crystal structure analysis and molecular model of human C3a anaphylatoxin. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1389–1399. doi: 10.1515/bchm2.1980.361.2.1389. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. The structural basis for anaphylatoxin and chemotactic functions of C3a, C4a, and C5a. Crit Rev Immunol. 1981 Feb;1(4):321–366. [PubMed] [Google Scholar]
- Johnson R. J., Chenoweth D. E. Structure and function of human C5a anaphylatoxin. Selective modification of tyrosine 23 alters biological activity but not antigenicity. J Biol Chem. 1985 Aug 25;260(18):10339–10345. [PubMed] [Google Scholar]
- Johnson R. J., Tamerius J. D., Chenoweth D. E. Identification of an antigenic epitope and receptor binding domain of human C5a. J Immunol. 1987 Jun 1;138(11):3856–3862. [PubMed] [Google Scholar]
- Lu Z. X., Fok K. F., Erickson B. W., Hugli T. E. Conformational analysis of COOH-terminal segments of human C3a. Evidence of ordered conformation in an active 21-residue peptide. J Biol Chem. 1984 Jun 25;259(12):7367–7370. [PubMed] [Google Scholar]
- Mandecki W., Mollison K. W., Bolling T. J., Powell B. S., Carter G. W., Fox J. L. Chemical synthesis of a gene encoding the human complement fragment C5a and its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3543–3547. doi: 10.1073/pnas.82.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandecki W. Oligonucleotide-directed double-strand break repair in plasmids of Escherichia coli: a method for site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7177–7181. doi: 10.1073/pnas.83.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandecki W., Powell B. S., Mollison K. W., Carter G. W., Fox J. L. High-level expression of a gene encoding the human complement factor C5a in Escherichia coli. Gene. 1986;43(1-2):131–138. doi: 10.1016/0378-1119(86)90016-8. [DOI] [PubMed] [Google Scholar]
- Marder S. R., Chenoweth D. E., Goldstein I. M., Perez H. D. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985 May;134(5):3325–3331. [PubMed] [Google Scholar]
- Mollison K. W., Fey T. A., Krause R. A., Mandecki W., Fox J. L., Carter G. W. High-level C5a gene expression and recovery of recombinant human C5a from Escherichia coli. Agents Actions. 1987 Aug;21(3-4):366–370. doi: 10.1007/BF01966518. [DOI] [PubMed] [Google Scholar]
- Nettesheim D. G., Edalji R. P., Mollison K. W., Greer J., Zuiderweg E. R. Secondary structure of complement component C3a anaphylatoxin in solution as determined by NMR spectroscopy: differences between crystal and solution conformations. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5036–5040. doi: 10.1073/pnas.85.14.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohura K., Katona I. M., Wahl L. M., Chenoweth D. E., Wahl S. M. Co-expression of chemotactic ligand receptors on human peripheral blood monocytes. J Immunol. 1987 Apr 15;138(8):2633–2639. [PubMed] [Google Scholar]
- Smith M. J., Walker J. R. The effects of some antirheumatic drugs on an in vitro model of human polymorphonuclear leucocyte chemokinesis. Br J Pharmacol. 1980 Jul;69(3):473–478. doi: 10.1111/j.1476-5381.1980.tb07037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unson C. G., Erickson B. W., Hugli T. E. Active site of C3a anaphylatoxin: contributions of the lipophilic and orienting residues. Biochemistry. 1984 Feb 14;23(4):585–589. doi: 10.1021/bi00299a001. [DOI] [PubMed] [Google Scholar]
- Weigle W. O., Goodman M. G., Morgan E. L., Hugli T. E. Regulation of immune response by components of the complement cascade and their activated fragments. Springer Semin Immunopathol. 1983;6(2-3):173–194. doi: 10.1007/BF00205872. [DOI] [PubMed] [Google Scholar]
- Wetsel R. A., Ogata R. T., Tack B. F. Primary structure of the fifth component of murine complement. Biochemistry. 1987 Feb 10;26(3):737–743. doi: 10.1021/bi00377a013. [DOI] [PubMed] [Google Scholar]
- Zimmermann B., Vogt W. Amino-acid sequence and disulfide linkages of the anaphylatoxin, des-Arg omega-C5a, from porcine serum. Hoppe Seylers Z Physiol Chem. 1984 Feb;365(2):151–158. doi: 10.1515/bchm2.1984.365.1.151. [DOI] [PubMed] [Google Scholar]
- Zuiderweg E. R., Henkin J., Mollison K. W., Carter G. W., Greer J. Comparison of model and nuclear magnetic resonance structures for the human inflammatory protein C5a. Proteins. 1988;3(3):139–145. doi: 10.1002/prot.340030302. [DOI] [PubMed] [Google Scholar]
- Zuiderweg E. R., Mollison K. W., Henkin J., Carter G. W. Sequence-specific assignments in the 1H NMR spectrum of the human inflammatory protein C5a. Biochemistry. 1988 May 17;27(10):3568–3580. doi: 10.1021/bi00410a007. [DOI] [PubMed] [Google Scholar]