Abstract
Purpose
The purpose of this study is to test the efficacy of a culturally tailored comprehensive type 2 diabetes management intervention for Korean American immigrants (KAIs) with type 2 diabetes.
Methods
A randomized controlled pilot trial with 2 parallel arms (intervention vs control) with a delayed intervention design was used. A total of 79 KAIs, recruited from the Baltimore-Washington area, completed baseline, 18-week, and 30-week follow-ups (intervention, n = 40; control, n = 39). All participants had uncontrolled type 2 diabetes (hemoglobin A1C ≥7.5%) at baseline. The authors’ comprehensive, self-help intervention program for type 2 diabetes management (SHIP-DM) consisted of a 6-week structured psychobehavioral education, home glucose monitoring with teletransmission, and bilingual nurse telephone counseling for 24 weeks. The primary outcome of the study was A1C level, and secondary outcomes included an array of psychobehavioral variables.
Results
Using analysis of covariance, the findings support that the proposed intervention was effective in significantly lowering A1C and fasting glucose and also in improving psychosocial outcomes in the sample. Specifically, the amount of reduction in A1C among intervention group participants was 1.19% at 18 weeks and 1.31% at 30 weeks, with 10% and 15.5% of the participants achieving the suggested goal of A1C <7% at 18 and 30 weeks of follow-up, respectively.
Conclusions
The results highlight the clinical efficacy of the SHIP-DM intervention composed of a 6-week education program, self-monitoring, and follow-up counseling, in terms of maintaining the improved intervention effects obtained and in terms of glucose control.
Type 2 diabetes mellitus is a serious health problem in Asian American communities, including the Korean American immigrant (KAI) community. KAIs, one of the most underserved and understudied minority populations in the United States, are at particularly high risk of developing type 2 diabetes. This risk is compounded by the fact that Asians who have emigrated to the West tend to gain weight and develop chronic diseases, including type 2 diabetes, after immigration.1–3 Although studies of this new immigrant group are scare, available research has indicated that an overwhelming number of KAIs suffer not only from uncontrolled diabetes4 but also from a loss of self-confidence and social isolation stemming from language and cultural barriers.5 Like other immigrant ethnic minorities, they often have limited access to medical care and health information.6,7 Also, more than 50% of KAIs have no health insurance and rarely receive routine checkups. As a result, KAIs with asymptomatic chronic diseases such as type 2 diabetes often go undiagnosed and inadequately treated. These health risks are further compounded by low health literacy levels: 90% of first-generation KAI adults are monolingual (Korean only), and more than 70% report having trouble understanding medical terminology, even when the materials have been translated into Korean.8 These factors exacerbate the already high rates of undetected, undertreated, or poorly managed chronic illnesses, often leading to costly and tragic consequences.1,9–11
Researchers have found that currently available interventions for ethnic minority groups with type 2 diabetes are largely inadequate and ineffective because of insufficient integration of cultural framework and tailored strategies, which often results in suboptimal outcomes.12–16 Today’s KAIs, predominantly first-generation immigrants with strong ties to their traditional culture, are in great need of culturally relevant care. Integrating traditional diet and exercise and tailored counseling on pharmacological regimens based on their use of traditional herbal medicine and behavioral patterns are the essential components of a type 2 diabetes intervention for this population. To address the urgent need for developing a culturally sensitive and effective intervention for KAIs with type 2 diabetes that will help them achieve better glycemic control and restore their self-confidence with regard to diabetes management, a community-based self-help intervention program for type 2 diabetes (SHIP-DM) was constructed and pilot tested. The SHIP-DM is a structured, culturally tailored behavioral intervention program that focuses on empowering patients with greater knowledge, self-efficacy, and self-help skills concerning diabetes. The purpose of this article is to test the efficacy of the SHIP-DM in KAIs with type 2 diabetes.
Study Hypotheses
The main hypotheses of this project were that compared with KAIs in the control group, KAIs who received the SHIP-DM, which consisted of structured psychobehavioral education, home glucose and blood pressure (BP) telemonitoring, and individualized telephone counseling from a bilingual nurse, would have (1) greater self-help diabetes management skills including better glucose control and greater reduction in A1C, (2) better quality of life, and (3) greater levels of diabetes knowledge, self-efficacy, and self-care behaviors.
Research Design and Methods
Sample
Identification and verification processes for participant recruitment used multiple sources, including a list of participants in the current authors’ previous descriptive studies of KAIs,17 ethnic media, ethnic Korean churches, and Korean grocery stores located in the Baltimore-Washington area. During the recruitment period, the academic/community-partnered research team established a diabetes screening service area at the community-based partner site, the Korean Resource Center (KRC). The screening schedule was advertised through the ethnic news media (ie, newspapers and radio stations). At the screening, individuals who met the eligibility criteria were approached and invited to participate in the study. The eligibility criteria were (1) self-identification as KAI, (2) aged 30 years or older, (3) self-identification as having diabetes with an uncontrolled glucose level (A1C) ≥7.5% within the past 6 months, (4) resident of the Baltimore-Washington area, and (5) able to give written consent to participate in the intervention study (agreement to participate in the study data collection procedures, receive diabetes education, use the home glucose and BP monitoring with telephone transmission system, receive telephone counseling, and permit contact with the research team).
Screening/Enrollment
To minimize the participants’ burden both financially (eg, time, travel, parking) and psychologically (a large academic center research facility can be perceived as threatening by some participants), and also to minimize use of resources, a 2-step approach for participant screening was employed. Following study approval by the Institutional Review Board, potential participants were first asked to come to the community partner site, the KRC, which is conveniently located in a Korean-populated area. At the center, participants’ A1C levels were initially validated via dry blood test (A1CNOW+®).18,19 Only those whose A1C levels were 7.5% or higher were scheduled for the next confirmation blood draw within 1 to 2 weeks of the initial testing. This confirmatory blood draw was also done at the community partner site by a trained phlebotomist dispatched from the academic research center on scheduled days, as agreed on by the research team, the community partner, and the academic research center. A similar approach was also used for the follow-up blood draws.
Randomization
The 83 participants with confirmed eligibility were then randomly assigned to either the SHIP-DM intervention group (n = 41) or the control (delayed intervention) group (n = 42) by computer-automated random assignment. This method was used to ensure equivalence between groups in terms of key factors that can potentially influence the primary endpoint of A1C (eg, disease severity, age, body mass index, and gender). The software eliminated the need for a stratified sampling because it randomly assigned participants with equivalent levels across the 2 groups. Because of the nature of this intervention and the design of the study, blinding of subjects to random assignment was not feasible.
Intervention
The SHIP-DM consisted of 3 concurrent intervention components: 2-hour weekly education sessions for 6 weeks, home glucose monitoring with teletransmission (HGMT), and monthly telephone counseling by a bilingual nurse for 24 weeks. The first component, a structured education program, was delivered at a community site (the KRC) by trained bilingual nurses and a nutritionist. The education program was aimed at enhancing diabetes knowledge and promoting diabetes self-care behaviors for glucose control and was centered on the following 6 topics: (1) overview of type 2 diabetes and general diabetes management guidelines, (2) short- and long-term complications of uncontrolled type 2 diabetes, (3) healthy eating and nutrition, (4) reading food labels and exercise, (5) medications and food-drug interactions, and (6) problem solving and communication skills with a primary care physician.
The HGMT was implemented for 24 weeks following the 6-week education program. Each participant in the intervention group received a glucometer, an electronic BP monitor, and a teletransmission system. The teletransmission system allowed glucose and BP measurements to be transmitted via telephone to a contracted company; this company established a Web site for the present study that was used to store and display transmitted data for bilingual nurse counselors to review and use when providing counseling to study participants. Monthly measurement reports were generated and sent to the participants to facilitate communication between the participants and the nurse counselors. Trained bilingual nurses provided monthly counseling according to a standardized protocol. The purposes of the monthly counseling were to reinforce new knowledge learned through the education program while also discussing issues or problems experienced by participants, finding solutions to the problems or issues raised, and providing emotional support. Each counseling session lasted about 10 to 25 minutes.
Measurements at Baseline and Follow-up
Data were collected at baseline, 18 weeks, and 30 weeks. Psychobehavioral variables were assessed using a structured questionnaire modified and adapted from previously validated instruments.
Diabetes knowledge was measured using the Diabetes Knowledge Test (DKT), a knowledge test developed by Fitzgerald and colleagues.20 This test has been validated in adults with type 2 diabetes; a Korean version was used in this study. The scale has 2 components: a 14-item general test and a 9-item insulin-use subscale. The coefficient alphas for the general test and the insulin-use subscale indicated that both were reliable, with alphas ≥.70.
Self-efficacy in terms of diabetes management was measured by a self-efficacy for diabetes scale, which was adapted from the Stanford Chronic Disease Self-Efficacy scale21 and translated into Korean. The modified scale consisted of eight 10-point Likert-type items that asked how confident the individual was in managing diabetes in the areas of diet, exercise, and general self-management behaviors. These areas included making appropriate food choices, exercising regularly, and monitoring blood glucose levels. Higher scores represented higher levels of self-efficacy in managing diabetes. This self-efficacy scale has demonstrated construct validity and reliability (internal consistency alpha coefficient of .85 and a test-retest reliability of 0.80).
To assess and achieve acceptable functional, conceptual, and linguistic equivalence of the Korean version of the DKT and self-efficacy diabetes scale, a panel consensus approach was used, in addition to the back translation procedure established by Brislin.22 Specifically, 2 separate panels that consisted of professional and patient groups were formed: a panel of 4 bilingual researchers with content expertise assessed and discussed the accuracy of translation as well as the cultural relevancy of each item on the scales. Upon reaching consensus among members of the professional group, a panel of patients with type 2 diabetes assessed the conceptual clarity, readability, congruency of acculturation level, and cultural relevancy of each item. Finally, using the final version of these translated instruments, a pilot study was conducted in 70 Korean patients with type 2 diabetes, and preliminary psychometric information on the study instruments was obtained.
Diabetes self-care activities were measured by the Summary of Diabetes Self-Care Activities (SDSCA). The SDSCA determines the level at which self-care activities specific to diabetes management are being performed.23 The following activities are included: dietary information, exercise, blood glucose testing, foot care, and smoking. As reported in a recent review of 7 studies (5 randomized interventions and 2 observational studies) using the instrument in a total combined sample of 1988 people with diabetes, the average interitem correlations were high (mean = 0.47); test-retest correlations were moderate (mean = 0.40).23 Correlations with other criterion measures supported the validity of the SDSCA (mean = 0.23).
Depression was measured using the Kim Depression Scale for Korean Americans (KDSKA).24 This self-report depression scale contains 21 items divided into 4 subscales: emotional, cognitive, behavioral, and somatic. The sub-scales were developed to be consistent with cultural descriptions of the signs and symptoms of depression that KAIs perceived. Each item is presented as a declarative sentence related to one symptom of depression, followed by a set of response options that measure the frequency of depressive symptoms in the previous 1-week period. Details of the development and validation of the KDSKA have been published elsewhere,24 and the scale has been used in several previous studies. Evidence of construct validity of the KDSKA has been reported, including concurrent and discriminant validities. Its internal consistency and reliability, as assessed by Cronbach alpha, was .93 (N = 303).
Quality of life was measured by the Diabetes Quality of Life Measure (DQOL; 46 items).25 The DQOL assesses the personal experience of diabetes care and treatment, including the following 4 dimensions: worries about future effects of diabetes, worries about social and vocational issues, the impact of treatment, and personal satisfaction with treatment. The DQOL and its 4 scales have demonstrated a high degree of internal consistency (Cronbach alpha = .66–.92) and excellent test-retest reliability (r = 0.78–0.92). The convergent validity of the DQOL has also been demonstrated using conceptually relevant measures of psychiatric symptoms, perceived well-being, and adjustment to illness.25 A translated and modified version reflecting cultural values was administered in the present study.
Physiologic outcomes (A1C, fasting glucose, and lipid batteries) were also determined, with the samples being collected and processed by a single certified research laboratory. Blood pressure, height, and weight were measured by trained research staff.
Statistical Analyses
A sample size of 30 per group was calculated based on an effect size of a 0.5% reduction in A1C with a type I error of 0.05 and 90% power, with an assumed correlation of 0.80 between measurement points. A conservative rate of attrition of about 30%, and therefore enrollment of a total of 80 subjects in this project (40 subjects for each group), was considered. Since the actual retention rate was extremely high, the authors opted to use a complete set of data for 79 patients, all of whom had completed all data collection visits. Descriptive statistics were used to summarize sample characteristics and study variables. A general linear model, including baseline values as covariates, was used to compare differences in primary and secondary outcomes at weeks 18 and 30. Statistical significance was determined at α = .05.
RESULTS
Recruitment and Retention
Using a 2-step recruitment process, a total of 97 KAIs positive for diabetes via dry blood test were identified, 83 of whom were confirmed as being eligible for the study on the basis of their lab results (85.6% agreement rate). This 2-step screening process was effective in identifying KAIs with uncontrolled diabetes and was well regarded by the study participants. Of the 83 KAIs who were assigned to the intervention or control group, 79 completed the baseline, 18-week, and 30-week follow-ups (intervention, n = 40; control, n = 39). One participant from the intervention group and 3 from the control group withdrew because of a lack of time (retention rate = 95.2%). The intervention group’s participation rates for the 6-week education program were consistently high, ranging from 83% to 94% for each session, with an overall satisfaction rating of 9.7 on a 1 to 10 scale. In addition, most participants in the intervention group successfully used home glucose monitoring and provided continuous glucose data via teletransmission for the 24-week follow-up period.
Sample Characteristics
Of the 79 patients who completed the study, 44.3% were female, and the mean ± SD age at baseline was 56.4 ± 7.9 years; 87.3% were married, 70.3% were employed, 48.1% had received a higher level of education, 53.2% had been in the United States for more than 20 years, and 59.2% had a family income of >$40 000 per year (Table 1). There were no significant differences in baseline characteristics between the 2 groups.
Table 1.
Baseline Demographics and Changes in Physiological Outcomes (N = 79)
| Baseline |
Difference From Baseline at 18 Weeksa |
Difference From Baseline at 30 Weeksa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention, Mean (SD) | Control, Mean (SD) | Pb | Intervention, Mean (SD) | Control, Mean (SD) | Pb | Intervention, Mean (SD) | Control, Mean (SD) | Pb | |
| Female, % | 37.5 | 51.3 | .22 | ||||||
| Age, y | 56.2 (8.4) | 56.6 (7.6) | .83 | ||||||
| Married, % | 90.0 | 84.6 | .29 | ||||||
| Employed, % | 61.5 | 80.0 | .08 | ||||||
| High education level, %c | 52.5 | 43.6 | .43 | ||||||
| In the United States for >20 y | 52.5 | 53.8 | .91 | ||||||
| Family income >$40 000, % | 63.2 | 55.3 | .48 | ||||||
| A1C, % | 9.4 (1.5) | 9.1 (1.3) | .25 | −1.2 (1.3) | 0.1 (1.7) | .00 | −1.3 (1.3) | −0.4 (1.4) | .01 |
| Fasting glucose, mg/dL | 188.3 (56.8) | 169.3 (61.6) | .16 | −42.4 (66.6) | 0.8 (66.4) | .01 | −42.3 (61.6) | −7.0 (57.2) | .06 |
| Fasting glucose, mmol/L | 10.5 (3.2) | 9.4 (3.4) | −2.4 (3.7) | 0.0 (3.7) | −2.4 (3.4) | −0.4 (3.2) | |||
| Body mass index, kg/m2 | 25.9 | 25.7 (3.1) (3.4) | .81 | −0.2 (1.01) | −0.3 (1.2) | .76 | −0.3 (1.2) | −0.3 (1.2) | .94 |
| Systolic blood pressure, mm Hg | 131.5 (14.1) | 132.7 (17.2) | .73 | −1.4 (13.7) | −2.1 (17.0) | .95 | −0.2 (19.7) | −3.6 (16.6) | .78 |
| Diastolic blood pressure, mm Hg | 80.8 (8.8) | 77.7 (9.1) | .13 | −2.2 (10.7) | −1.1 (7.7) | .85 | −0.3 (12.3) | 0.7 (10.8) | .86 |
| Cholesterol, mg/dL | 207.0 (36.3) | 179.8 (36.6) | .00 | −19.5 (41.2) | 6.3 (42.8) | .18 | −24.7 (41.9) | 7.2 (37.2) | .03 |
| Cholesterol, mmol/L | 11.5 (2.0) | 10.0 (2.0) | −1.1 (2.3) | 0.4 (2.4) | −1.4 (2.3) | 0.4 (2.1) | |||
| HDL, mg/dL | 47.6 (10.1) | 49.7 (13.7) | .17 | 1.1 (9.0) | 1.2 (8.2) | .81 | −2.5 (6.5) | 0.6 (10.3) | .06 |
| HDL, mmol/L | 2.6 (0.6) | 2.8 (0.8) | 0.1 (0.5) | 0.1 (0.5) | −0.1 (0.4) | 0.0 (0.6) | |||
| LDL mg/dL | 124.1 (37.7) | 71.8 (181.2) | .44 | −14.2 (38.9) | 29.4 (188.4) | .31 | −15.9 (38.7) | 35.6 (185.7) | .80 |
| LDL, mmol/L | 6.9 (2.1) | 4.0 (10.1) | −0.8 (2.2) | 1.6 (10.5) | −0.9 (2.2) | 2.0 (10.3) | |||
| Triglyceride, mg/dL | 246.6 (366.8) | 160.9 (108.0) | .09 | −93.0 (376.7) | 5.3 (122.0) | .51 | −84.6 (384.4) | −4.2 (115.8) | .00 |
| Triglyceride, mmol/L | 13.7 (20.4) | 8.9 (6.0) | −5.2 (20.9) | 0.3 (6.8) | −4.7 (21.4) | −0.2 (6.4) | |||
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Difference between variables at each time point and baseline values.
Within-group difference in the intervention group minus within-group difference in the control group.
High education level: technical/vocational school and/or university.
Primary Outcomes
The final analysis using a series of analyses of covariance showed significant differences in physiologic outcomes between the 2 groups (Table 1). In particular, the intervention group experienced a greater than 1% reduction in A1C at both 18 and 30 weeks, with 10% and 15%, respectively, of the intervention group achieving the suggested goal of A1C <7% at the 2 follow-up data collection points. There was also a significant drop in fasting glucose at 18 weeks, although the reduction was no longer significant at 30 weeks (P = .062). At 30 weeks, the intervention group showed significantly lower levels of total cholesterol and triglyceride when compared with the control group. The intervention group also showed a trend toward a lower high-density lipoprotein when compared with the control group, but this difference was not statistically significant (P = .059).
Secondary Outcomes
An analysis of the psychobehavioral outcomes also revealed positive results: as compared with the control group, the intervention group showed significant improvements in diabetes knowledge, self-care activities, self-efficacy, attitudes toward diabetes, depression, and quality of life. For the diabetes knowledge items that were applicable only to insulin-injecting participants (n = 12), the intervention group tended to score better than the control group, although the difference was not statistically significant (Table 2).
Table 2.
Changes in Psychobehavioral Outcomes (N = 79)
| Baseline |
Change From Baseline at 18 Weeksa |
Change From Baseline at 30 Weeksa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention, Mean (SD) | Control, Mean (SD) | Pb | Intervention, Mean (SD) | Control, Mean (SD) | Pb | Intervention, Mean (SD) | Control, Mean (SD) | Pb | |
| Knowledge (part 1) | 7.7 (2.8) | 7.8 (3.1) | .95 | 2.2 (2.4) | 0.1 (3.2) | .00 | 2.4 (2.3) | 0.7 (2.4) | .00 |
| Knowledge (part 2c) | 3.6 (2.2) | 4.9 (1.2) | .21 | 1.6 (1.3) | 0.7 (1.6) | .77 | 0.3 (3.7) | 0.4 (0.8) | .27 |
| Self-care activities | 50.6 (15.9) | 54.1 (16.1) | .33 | 18.5 (17.0) | −1.1 (13.9) | .00 | 17.5 (16.9) | 2.5 (15.4) | .00 |
| Self-efficacy | 48.5 (12.2) | 47.3 (13.6) | .68 | 8.7 (11.4) | 2.6 (15.0) | .02 | 6.6 (14.4) | −0.9 (15.1) | .01 |
| Attitudes toward diabetes | 25.9 (7.0) | 28.1 (8.7) | .26 | −3.5 (6.5) | −0.9 (6.6) | .01 | −3.7 (7.1) | −1.4 (6.5) | .04 |
| Depression | 4.8 (4.4) | 6.4 (5.8) | .17 | −1.4 (4.0) | −0.4 (4.1) | .02 | −0.5 (4.5) | −1.0 (4.3) | .70 |
| Quality of life | 88.6 (19.2) | 96.5 (24.1) | .11 | −7.5 (17.5) | −1.9 (16.5) | .01 | −4.6 (17.3) | 0.3 (16.4) | .03 |
Difference between variables at each time point and baseline values.
Within-group difference in the intervention group minus within-group difference in the control group.
Assessed only for those injecting insulin (intervention, n = 5; control, n = 7).
Conclusions and Implications
This study has demonstrated the effectiveness of a community based, culturally tailored behavioral intervention in achieving better control of glucose and improving type 2 diabetes self-efficacy, knowledge, and quality of life in an underserved, recent immigrant group, KAIs. In particular, the reduction in A1C level in the intervention group (1.19% at 18 weeks and 1.31% at 30 weeks) was not only statistically significant but also clinically meaningful. This result highlights the clinical efficacy of the 6-week education session period, the self-monitoring, and the follow-up counseling in terms of maintaining the improvements in glucose control. The significant improvements in both the physiological and psychobehavioral outcomes are particularly encouraging in that the patients not only achieved better glucose control but also succeeded in increasing their type 2 diabetes management skills and knowledge. They also reported greater satisfaction in terms of their self-management of the disease in general. These positive results are likely to have important implications in terms of the long-term outcome of the disease. It will be important to design a study with a longer follow-up to determine whether the positive outcomes of the SHIP-DM can be sustained over a longer period of time.
Despite the common perception that it is particularly difficult to recruit members of minority (ie, hard-to-reach) populations into clinical trials, this study demonstrated that with careful planning and culturally appropriate strategies, it is possible to recruit and retain KAIs with type 2 diabetes in a randomized clinical trial. In fact, the recruitment goal (n = 83) was achieved within 3 months. One of the key factors in this successful recruitment and retention was the use of a delayed intervention design that provided the same intervention to participants in the control group. Other community-based participatory research strategies, such as partnering with community organizations (eg, the KRC, Korean ethnic news media, and churches), were also important.17 In addition, the use of a 2-step screening for A1C eligibility testing proved to be an effective, economical, and sensitive recruitment/screening strategy for this target population that yielded a clinically acceptable level of specificity. Participants particularly appreciated the fact that they could avoid going to an academic research facility, which was perceived by many as threatening (located in a large, inner-city hospital), inconvenient (traffic, no bilingual staff), and expensive (parking and travel costs, longer absence from work required). Furthermore, trained bilingual research staff members conducted the initial screenings and were available throughout the data collection process to address any questions or concerns from study participants. The retention rate of 95% also demonstrates the authors’ ability to successfully follow these participants over a 6-month period. Reminder calls and a newsletter were helpful strategies to maintain connections with participants.
These results are consistent with studies26–28 targeting ethnic minority populations that have shown improved psychosocial behaviors, greater physical activity, healthier eating, and/or improved A1C levels as a result of using intervention strategies such as education, self-management classes, and support groups. However, these studies have been hampered by the lack of a comparison group, a lack of clinical indicators, or a high attrition rate, which have limited researchers’ ability to draw broad inferences from the results in terms of identifying effective behavioral interventions for type 2 diabetes.26–28 The present study has addressed some of these design limitations by including a control group and a clear set of clinical indicators and by achieving high retention rates in both the control and intervention groups. Given the scarcity of community-based behavioral interventions for diabetes control that have specifically targeted Asian populations, this study provides much-needed evidence to support the potential usefulness of future community-based behavioral interventions for patients with type 2 diabetes among the Asian population.
It is important to note that the follow-up period (30 weeks) in the present study was relatively short, and the long-term efficacy of this type of intervention is as yet unknown. Future studies should address the longer-term efficacy of this intervention in diverse populations.
Also, this study was not designed to answer specific questions related to the relative importance of one component of the intervention versus another in terms of improving glucose control. Similarly, the relatively small sample size does not allow stronger inferences to be made regarding the precise mechanism of the intervention, such as the complex interplay among psychobehavioral factors and physiological outcomes.
In summary, the targeted intervention in KAIs with diabetes has demonstrated that a community-based, culturally tailored intervention using trained bilingual nurse counselors and self-monitoring of glucose can be effective in improving clinical indicators of diabetes and in increasing participants’ level of diabetes-related knowledge and self-efficacy. It also suggests that this approach may have high applicability to other immigrant groups with similar challenges. Future research with a larger sample size and longer follow-up period is warranted to extend and further validate these promising results.
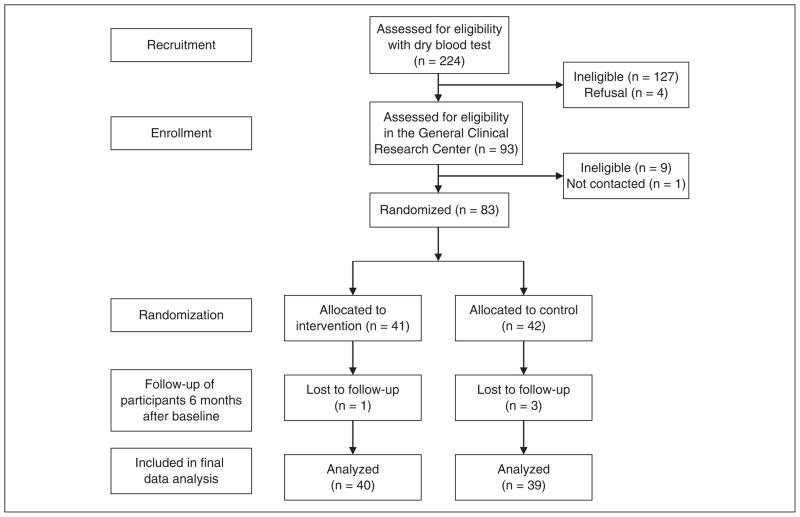
Figure 1.
Flow diagram of the behavioral intervention study in Korean American immigrants with diabetes.
Acknowledgments
This research is supported by a grant from the National Institutes of Health (NIDDK R34 DK071957), LifeScan, Inc (HCC002154), and the Johns Hopkins University School of Medicine General Clinical Research Center (M01-RR00052), from the National Center for Research Resources/National Institutes of Health (NCT00505960).
Footnotes
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- 1.Kim MT, Juon HS, Hill MN, Post W, Kim KB. Cardiovascular disease risk factors in Korean American elderly. West J Nurs Res. 2001;23:269–282. doi: 10.1177/01939450122045140. [DOI] [PubMed] [Google Scholar]
- 2.Lee SK, Sobal J, Frongillo EA., Jr Acculturation and health in Korean Americans. Soc Sci Med. 2000;51:159–173. doi: 10.1016/s0277-9536(99)00446-3. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y, Suh YK, Choi H. BMI and metabolic disorders in south Korean adults: 1998 Korea national health and nutrition survey. Obes Res. 2004;12:445–453. doi: 10.1038/oby.2004.50. [DOI] [PubMed] [Google Scholar]
- 4.Choi KM, Kim SM, Kim YE, Choi DS, Baik SH, Lee J International Diabetes Federation. Prevalence and cardiovascular disease risk of the metabolic syndrome using National Cholesterol Education Program and International Diabetes Federation definitions in the Korean population. Metabolism. 2007;56(4):552–558. doi: 10.1016/j.metabol.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Sohn L. The health and health status of older Korean Americans at the 100-year anniversary of Korean immigration. J Cross Cult Gerontol. 2004;19(3):203–219. doi: 10.1023/B:JCCG.0000034219.97686.69. [DOI] [PubMed] [Google Scholar]
- 6.Brown ER, Ojeda VD, Wyn R, Levan R. Racial and ethnic disparities in access to health insurance and health care. [Accessed December 12, 2008];2008 http://www.kff.org/uninsured/loader.cfm?url=/commonspot/security/getfile.cfm&PageID=13340.
- 7.Kandula NR, Kersey M, Lurie N. Assuring the health of immigrants: what the leading health indicators tell us. Annu Rev Public Health. 2004;25:357–376. doi: 10.1146/annurev.publhealth.25.101802.123107. [DOI] [PubMed] [Google Scholar]
- 8.Shin H, Bruno R. Language use and English-speaking ability. [Accessed October 7, 2008];2003 http://www.census.gov/prod/2003pubs/c2kbr-29.pdf.
- 9.Kim MT, Kim KB, Juon HS, Hill MN. Prevalence and factors associated with high blood pressure in Korean Americans. Ethn Dis. 2000;10:364–374. [PubMed] [Google Scholar]
- 10.Kang JH, Han HR, Kim KB, Kim MT. Barriers to care and control of high blood pressure in Korean-American elderly. Ethn Dis. 2006;16:145–151. [PubMed] [Google Scholar]
- 11.Han HR, Kim KB, Kang J, Jeong S, Kim EY, Kim MT. Knowledge, beliefs, and behaviors about hypertension control among middle-aged Korean Americans with hypertension. J Community Health. 2007;32:324–342. doi: 10.1007/s10900-007-9051-y. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SK. Diabetes in the among refugee population [unpublished] University of California; 1995. [Google Scholar]
- 13.Waldram JB, Whiting J, Kornder N, Habbick MB. Cultural understandings and the use of traditional medicine. Can J Diabetes Care. 2000;24(2):31–38. [Google Scholar]
- 14.Kao HF, Hsu MT, Clark L. Conceptualizing and critiquing culture in health research. J Transcult Nurs. 2004;15(4):269–277. doi: 10.1177/1043659604268963. [DOI] [PubMed] [Google Scholar]
- 15.Liang W, Yuan E, Mandelblatt JS, Pasick RJ. How do older Chinese women view health and cancer screening? Results from focus groups and implications for interventions. Ethn Health. 2004;9(3):283–304. doi: 10.1080/1355785042000250111. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academy Press; 2002. [PMC free article] [PubMed] [Google Scholar]
- 17.Han HR, Kang J, Kim KB, Ryu JP, Kim MT. Barriers to and strategies for recruiting Korean Americans for community-partnered health promotion research. J Immigr Minor Health. 2007;9(2):137–146. doi: 10.1007/s10903-006-9022-x. [DOI] [PubMed] [Google Scholar]
- 18.Bode B, Irvin B, Pierce J, Allen M, Clark A. Advances in hemoglobin A1c point of care technology. J Diabetes Sci Technol. 2007;1:405–411. doi: 10.1177/193229680700100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy L, Herman WH, Strange P, Harris A GOAL AIC Team. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabetes Care. 2006;29:1–8. doi: 10.2337/diacare.29.01.06.dc05-1058. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald JT, Funnell MM, Hess GE, et al. The reliability and validity of a brief diabetes knowledge test. Diabetes Care. 1998;21(5):706–710. doi: 10.2337/diacare.21.5.706. [DOI] [PubMed] [Google Scholar]
- 21.Lorig K, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program for patients with chronic disease. Effect Clin Pract. 2001;4:256–262. [PubMed] [Google Scholar]
- 22.Brislin RW. Comparative research methodology: cross-cultural studies. Int J Psychol. 1976;11:215–229. [Google Scholar]
- 23.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 24.Kim MT. Measuring depression in Korean Americans: development of the Kim depression scale for Korean Americans. J Transcult Nurs. 2002;13:109–117. doi: 10.1177/104365960201300203. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson A, Barofsky I, Cleary P, Rand L. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT) Diabetes Care. 1988;11:725–732. doi: 10.2337/diacare.11.9.725. [DOI] [PubMed] [Google Scholar]
- 26.Garvin CC, Cheadle A, Chrisman N, Chen R, Brunson E. A community-based approach to diabetes control in multiple cultural groups. Ethn Dis. 2004;14(3):83–92. [PubMed] [Google Scholar]
- 27.Goldfinger JZ, Arniella G, Wylie-Rosett J, Horowitz CR. Project HEAL: peer education leads to weight loss in Harlem. J Health Care Poor Underserved. 2008;19(1):180–192. doi: 10.1353/hpu.2008.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown SA, Hanis CL. A community-based, culturally sensitive education and group-support intervention for Mexican Americans with NIDDM: a pilot study of efficacy. Diabetes Educ. 1995;21(3):203–210. doi: 10.1177/014572179502100307. [DOI] [PubMed] [Google Scholar]