Abstract
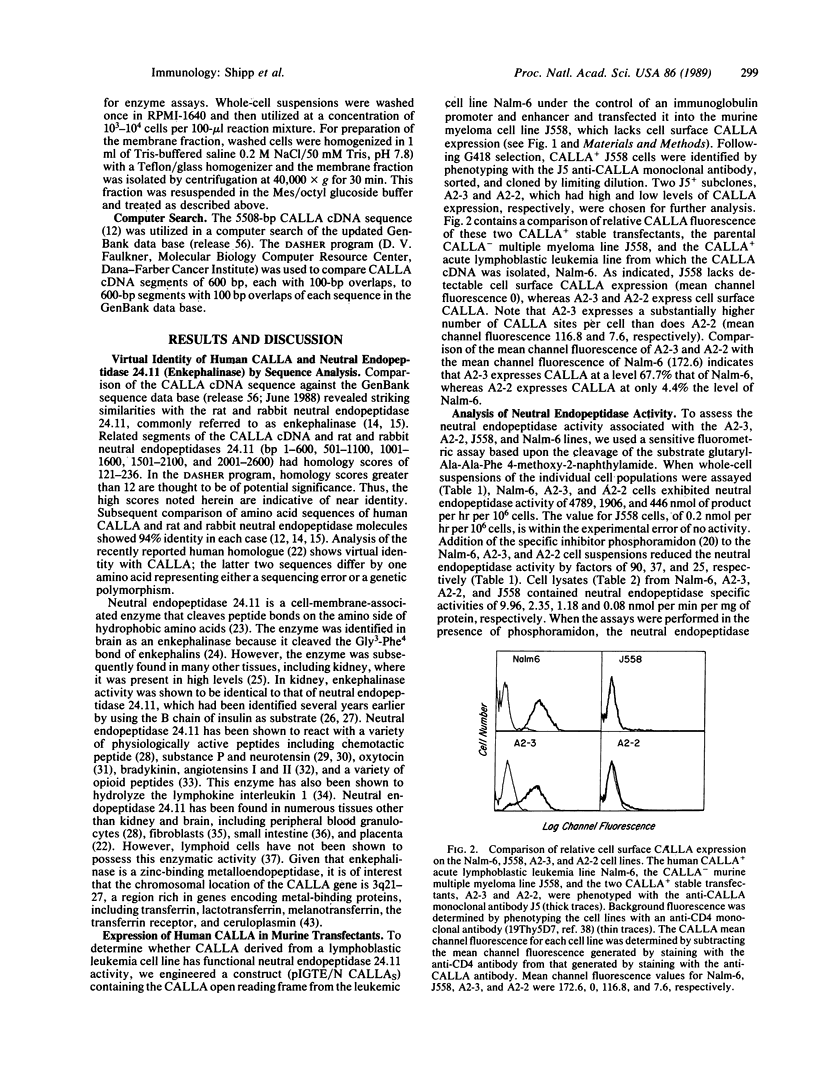
The common acute lymphoblastic leukemia antigen (CALLA) is a 749-amino acid type II integral membrane protein expressed by most acute lymphoblastic leukemias, certain other lymphoid malignancies with an immature phenotype, and normal lymphoid progenitors. A computer search against the most recent GenBank release (no. 56) indicates that human CALLA cDNA encodes a protein nearly identical to the rat and rabbit neutral endopeptidase 24.11 ("enkephalinase;" EC 3.4.24.11). This zinc metalloendopeptidase, which has been shown to inactivate a variety of peptide hormones including enkephalin, chemotactic peptide, substance P, neurotensin, oxytocin, bradykinin, and angiotensins I and II, had not been identified in lymphoid cells. To determine whether CALLA cDNA derived from human acute lymphoblastic leukemia cells (Nalm-6 cell line) encodes functional neutral endopeptidase activity, we generated CALLA+ stable transfectants in the CALLA- murine myeloma cell line J558 and analyzed them for enzymatic activity in a fluorometric assay based upon cleavage of the substrate glutaryl-Ala-Ala-Phe 4-methoxy-2-naphthylamide at the Ala-Phe bond. Total lysates as well as whole-cell suspensions of the Nalm-6 line and of the CALLA+ transfectants, but not of the CALLA- J558 cells, possessed neutral endopeptidase activity. This enzymatic activity was associated with the cellular membrane fraction and was abrogated by the specific neutral endopeptidase inhibitor phosphoramidon. The unequivocal identification of CALLA as a functional neutral endopeptidase provides insight into its potential role in both normal and malignant lymphoid function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almenoff J., Wilk S., Orlowski M. Membrane bound pituitary metalloendopeptidase: apparent identity to enkephalinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):206–214. doi: 10.1016/0006-291x(81)91508-4. [DOI] [PubMed] [Google Scholar]
- Bowes M. A., Kenny A. J. An immunohistochemical study of endopeptidase-24.11 and aminopeptidase N in lymphoid tissues. Immunology. 1987 Feb;60(2):247–253. [PMC free article] [PubMed] [Google Scholar]
- Bowes M. A., Kenny A. J. Endopeptidase-24.11 in pig lymph nodes. Purification and immunocytochemical localization in reticular cells. Biochem J. 1986 Jun 15;236(3):801–810. doi: 10.1042/bj2360801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M. P., Martin P. J., Ledbetter J. A., Hansen J. A. Granulocytes and cultured human fibroblasts express common acute lymphoblastic leukemia-associated antigens. Blood. 1983 Apr;61(4):718–725. [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Connelly J. C., Skidgel R. A., Schulz W. W., Johnson A. R., Erdös E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N. G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A. Amino acid sequence of rabbit kidney neutral endopeptidase 24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987 May;6(5):1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford J. T., Skidgel R. A., Erdös E. G., Hersh L. B. Human kidney "enkephalinase", a neutral metalloendopeptidase that cleaves active peptides. Biochemistry. 1983 Jun 21;22(13):3265–3271. doi: 10.1021/bi00282a035. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Matsas R., Kenny A. J. A monoclonal antibody to kidney endopeptidase-24.11. Its application in immunoadsorbent purification of the enzyme and immunofluorescent microscopy of kidney and intestine. Biochem J. 1983 Aug 15;214(2):377–386. doi: 10.1042/bj2140377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G., Rapson N. T., Lister T. A. Antisera to acute lymphoblastic leukemia cells. Clin Immunol Immunopathol. 1975 May;4(1):67–84. doi: 10.1016/0090-1229(75)90041-0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Hariri G., Newman R. A., Sutherland D. R., Ritter M. A., Ritz J. Selective expression of the common acute lymphoblastic leukemia (gp 100) antigen on immature lymphoid cells and their malignant counterparts. Blood. 1983 Apr;61(4):628–639. [PubMed] [Google Scholar]
- Hersh L. B. Degradation of enkephalins: the search for an enkephalinase. Mol Cell Biochem. 1982 Aug 20;47(1):35–43. doi: 10.1007/BF00241564. [DOI] [PubMed] [Google Scholar]
- Hersh L. B. Reaction of opioid peptides with neutral endopeptidase ("enkephalinase"). J Neurochem. 1984 Aug;43(2):487–493. doi: 10.1111/j.1471-4159.1984.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Fezer G., Knapp W., Thierfelder S. Anatomical distribution of call antigen expressing cells in normal lymphatic tissue and in lymphomas. Leuk Res. 1982;6(6):761–767. doi: 10.1016/0145-2126(82)90057-1. [DOI] [PubMed] [Google Scholar]
- Hokland P., Nadler L. M., Griffin J. D., Schlossman S. F., Ritz J. Purification of common acute lymphoblastic leukemia antigen positive cells from normal human bone marrow. Blood. 1984 Sep;64(3):662–666. [PubMed] [Google Scholar]
- Hokland P., Rosenthal P., Griffin J. D., Nadler L. M., Daley J., Hokland M., Schlossman S. F., Ritz J. Purification and characterization of fetal hematopoietic cells that express the common acute lymphoblastic leukemia antigen (CALLA). J Exp Med. 1983 Jan 1;157(1):114–129. doi: 10.1084/jem.157.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgin R. L., Charleson S. E., Zimmerman M., Mumford R., Wood P. L. Enkephalinase: selective peptide inhibitors. Life Sci. 1981 Dec 21;29(25):2593–2601. doi: 10.1016/0024-3205(81)90632-9. [DOI] [PubMed] [Google Scholar]
- Hussey R. E., Richardson N. E., Kowalski M., Brown N. R., Chang H. C., Siliciano R. F., Dorfman T., Walker B., Sodroski J., Reinherz E. L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988 Jan 7;331(6151):78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Skidgel R. A., Gafford J. T., Erdös E. G. Enzymes in placental microvilli: angiotensin I converting enzyme, angiotensinase A, carboxypeptidase, and neutral endopeptidase ("enkephalinase"). Peptides. 1984 Jul-Aug;5(4):789–796. doi: 10.1016/0196-9781(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Keating A., Whalen C. K., Singer J. W. Cultured marrow stromal cells express common acute lymphoblastic leukaemia antigen (CALLA): implications for marrow transplantation. Br J Haematol. 1983 Dec;55(4):623–628. doi: 10.1111/j.1365-2141.1983.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J. B., Onizuka R. J., Quackenbush E. J., Jongeneel C. V., McInnes R. R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J Exp Med. 1988 Oct 1;168(4):1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens C., Schwartz J. C. Enkephalinase activity in rat peripheral organs. Eur J Pharmacol. 1981 Jan 5;69(1):113–116. doi: 10.1016/0014-2999(81)90609-9. [DOI] [PubMed] [Google Scholar]
- Lorkowski G., Zijderhand-Bleekemolen J. E., Erdös E. G., von Figura K., Hasilik A. Neutral endopeptidase-24.11 (enkephalinase). Biosynthesis and localization in human fibroblasts. Biochem J. 1987 Dec 1;248(2):345–350. doi: 10.1042/bj2480345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfroy B., Kuang W. J., Seeburg P. H., Mason A. J., Schofield P. R. Molecular cloning and amino acid sequence of human enkephalinase (neutral endopeptidase). FEBS Lett. 1988 Feb 29;229(1):206–210. doi: 10.1016/0014-5793(88)80828-7. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schofield P. R., Kuang W. J., Seeburg P. H., Mason A. J., Henzel W. J. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun. 1987 Apr 14;144(1):59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Swerts J. P., Guyon A., Roques B. P., Schwartz J. C. High-affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature. 1978 Nov 30;276(5687):523–526. doi: 10.1038/276523a0. [DOI] [PubMed] [Google Scholar]
- McLellan S., Dyer S. H., Rodriguez G., Hersh L. B. Studies on the tissue distribution of the puromycin-sensitive enkephalin-degrading aminopeptidases. J Neurochem. 1988 Nov;51(5):1552–1559. doi: 10.1111/j.1471-4159.1988.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Metzgar R. S., Borowitz M. J., Jones N. H., Dowell B. L. Distribution of common acute lymphoblastic leukemia antigen in nonhematopoietic tissues. J Exp Med. 1981 Oct 1;154(4):1249–1254. doi: 10.1084/jem.154.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford R. A., Pierzchala P. A., Strauss A. W., Zimmerman M. Purification of a membrane-bound metalloendopeptidase from porcine kidney that degrades peptide hormones. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6623–6627. doi: 10.1073/pnas.78.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudorf S. M., LeBien T. W., Kersey J. H. Characterization of thymocytes expressing the common acute lymphoblastic leukemia antigen. Leuk Res. 1984;8(2):173–179. doi: 10.1016/0145-2126(84)90140-1. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Wilk S. Purification and specificity of a membrane-bound metalloendopeptidase from bovine pituitaries. Biochemistry. 1981 Aug 18;20(17):4942–4950. doi: 10.1021/bi00520a021. [DOI] [PubMed] [Google Scholar]
- Pesando J. M., Tomaselli K. J., Lazarus H., Schlossman S. F. Distribution and modulation of a human leukemia-associated antigen (CALLA). J Immunol. 1983 Oct;131(4):2038–2045. [PubMed] [Google Scholar]
- Pierart M. E., Najdovski T., Appelboom T. E., Deschodt-Lanckman M. M. Effect of human endopeptidase 24.11 ("enkephalinase") on IL-1-induced thymocyte proliferation activity. J Immunol. 1988 Jun 1;140(11):3808–3811. [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Lazarus H., Schlossman S. F. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980 Feb 7;283(5747):583–585. doi: 10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Schlossman S. F. Modulation of human acute lymphoblastic leukemia antigen induced by monoclonal antibody in vitro. J Immunol. 1980 Oct;125(4):1506–1514. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp M. A., Richardson N. E., Sayre P. H., Brown N. R., Masteller E. L., Clayton L. K., Ritz J., Reinherz E. L. Molecular cloning of the common acute lymphoblastic leukemia antigen (CALLA) identifies a type II integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4819–4823. doi: 10.1073/pnas.85.13.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]



