Abstract
The last decade has seen great strides in the field of cancer immunotherapy, especially the treatment of melanoma. Beginning with the identification of cancer antigens, followed by the clinical application of anti-cancer peptide vaccination, it has now been proven that adoptive T-cell therapy (ACT) using cancer antigen-specific T cells is the most effective option. Despite the apparent clinical efficacy of ACT, the timely preparation of a sufficient number of cancer antigen-specific T cells for each patient has been recognized as its biggest limitation. Currently, therefore, attention is being focused on ACT with engineered T cells produced using cancer antigen-specific T-cell receptor (TCR) gene transfer. With regard to human leukemia, ACT using engineered T cells bearing the leukemia antigen-specific TCR gene still remains in its infancy. However, several reports have provided preclinical data on TCR gene transfer using Wilms' tumor gene product 1 (WT1), and also preclinical and clinical data on TCR gene transfer involving minor histocompatibility antigen, both of which have been suggested to provide additional clinical benefit. In this review, we examine the current status of anti-leukemia ACT with engineered T cells carrying the leukemia antigen-specific TCR gene, and discuss the existing barriers to progress in this area.
1. Introduction
Adoptive T-cell therapy (ACT) employing autologous engineered T cells produced using cancer antigen-specific T-cell receptor (TCR) gene transfer is currently a focus of intense interest in the field of cancer treatment. In combination with chemoradiation to reduce the immunoregulatory factors that hamper the efficacy of cancer therapy, Rosenberg and colleagues have recently achieved great success in improving the clinical efficacy of ACT using in vitro-expanded autologous cancer-specific T cells [1]. Unfortunately, application of such ACT to daily clinical practice is largely impeded by the labor-intensive nature of the procedure, and the difficulty involved in timely preparation of a sufficient number of such T cells for each patient. One recent advance in ACT is the use of engineered T cells bearing a cancer antigen-specific TCR obtained beforehand from an established cytotoxic T-cell (CTL) clone specific to a well-established cancer antigen. Recently, Johnson and colleagues have obtained successful results with antimelanoma ACT using engineered autologous T cells that have been expanded in vitro after melanoma antigen-specific TCR gene transfer [2]. Despite its feasibility and safety, ACT with such TCR gene-transferred peripheral blood lymphocytes (PBL) is less effective than that with in vitro-expanded autologous tumor-infiltrating T cells (TIL), largely because of the resulting low frequency of target TCR-expressing lymphocytes among infused cells, in the absence of selection. Furthermore, preclinical in vivo studies have suggested that contaminating nontransduced cells might actively impair the efficacy of such redirected cells [3–5].
On the other hand, the use of ACT with such engineered T cells against hematological malignancies still remains in its infancy. Over the last decade, allogeneic hematological stem cell transplantation (HSCT), which is a form of adoptive immuno-cell therapy, has successfully prolonged the survival time of patients with hematological malignancies. However, because its immune reaction is not solely directed against tumor cells, allogeneic HSCT is associated with substantial adverse effects, rendering it impractical for elderly patients and those with comorbidity. On the other hand, following the identification of a series of leukemia antigens, peptide vaccination is now the main protocol being investigated in clinical trials, rather than ACT with leukemia-specific T cells, and this has been shown to confer some additional clinical benefit [6, 7]. Against this background, several groups, including our own, have studied the feasibility of applying engineered T cells bearing Wilms' tumor gene product 1 (WT1) and minor histocompatibility antigen (HA-1,2)-specific TCR genes for the treatment of leukemia [8–11]. Our group has been focusing on ACT with WT1-specific TCR gene transfer for leukemia [11]. Here, we overview the current status of ACT using TCR-gene-transferred T cells, and discuss the technical issues presently confronting us.
2. Current Status of Cellular Immunotherapy against Hematological Malignancies
To develop a strategy that is less toxic, but with an efficacy equal to or greater than that of current therapeutic options against hematological malignances, cellular immunotherapy has attracted attention in recent years. The antitumor effect observed in patients treated successfully with allo-HSCT (graft versus tumor effect) has underscored the importance of tumor-specific CTL, and encouraged the development of cellular immunotherapy against hematological malignancy [12, 13]. Furthermore, this need for immunotherapy has also been emphasized by the fact that even the most successful molecular target drug for chronic myelogeneous leukemia (CML), imatinib, cannot induce a cure [14, 15].
Advanced molecular and immunobiological approaches have allowed the identification of various types of tumor-specific antigens (TSAs), which discriminate tumor cells from normal cells, or tumor-associated antigens (TAAs), which are overexpressed by tumor cells in comparison to normal cells. CTLs can exert cytotoxicity against tumor cells via TCR-dependent recognition of human leukocyte antigen (HLA)/TSA or TAA-derived peptide complex [16, 17]. As a corollary, it is anticipated that therapeutically increased TSA- or TAA-specific CTL in vivo would selectively and durably eradicate tumor cells within a tolerable and limited range of adverse effects against normal cells. Clinical vaccination trials against leukemia using peptides derived from TSA/TAA have already been conducted. In particular, such trials involving RP1 (the determined epitope of proteinase 3), WT1126–134, and WT1235–243 peptides have been well described [6, 7, 18]. As expected, no serious adverse events were observed in those clinical trials, and a positive correlation between the in vivo increase of TSA/TAA-specific CTLs and clinical response was demonstrated. However, the overall degree of clinical responsive still remains low, as is the case with peptide vaccination against solid tumors (total response rate, 2.9%) [19]. To overcome this clinical insufficiency, the feasibility of adoptive transfer with ex vivo-expanded TSA/TAA-specific CTLs or CD4+ T cells has been investigated, and successful results have also been demonstrated [20–23]. Technical advances with this treatment have been achieved mainly against melanoma; successful results have been obtained using ex-vivo-expanded autologous TIL [20], and by prior immunodepletion to eradicate immunoregulatory cells [1]. Despite these successes, broader application of this treatment modality is often largely hampered by lack of success with the isolation and expansion of TSA/TAA-specific T cells ex vivo from individual patients [23]. In an attempt to solve this critical problem, the feasibility of obtaining engineered T cells harboring the cancer antigen-specific TCR gene from a well-characterized TSA/TAA-specific CTL clone has been investigated, and recently success was reported in a pioneering clinical trial using ACT with autologous T cells harboring a melanoma antigen-specific TCR gene [2]. In parallel with these endeavors for the treatment of solid tumors, ACT, involving mainly donor lymphocyte infusion (DLI), has been introduced for the treatment of relapsed hematological malignancy after allo-HSCT [24]. Although DLI can provide substantial clinical benefit, it is limited to patients who have received allo-HSCT and carries a risk of deterioration to graft-versus host disease (GVHD) because the infused donor lymphocytes are not characterized beforehand as being leukemia antigen-specific [24]. To overcome such limitations, ACT using ex vivo-expanded autologous or allogeneic leukemia-specific T cells has been attempted. ACT with HLA-identical allogeneic minor antigen (HA-1)-specific T cells has been conducted for patients with relapsed CML after allo-HSCT [25]. As this modality suffers from the same limitations as those associated with the treatment of solid tumors, the feasibility of using engineered T cells harboring the tumor antigen-specific TCR gene has also been investigated for the treatment of hematological malignancies. At present, the potential use of a WT1-specific TCR gene [8, 11, 26] and a minor antigen HA-1/2-specific TCR gene [9, 10] is being actively examined, but studies are still at the preclinical stage.
3. Application of Engineered T Cells Created by TCR Gene Transfer to Hematological Malignancy
Steinmetz and colleagues were the first to demonstrate that target antigen specificity was transferable by transduction of the TCR gene obtained from a well-characterized CTL clone [30]. Subsequent studies of TSA/TAA and the establishment of TSA/TAA-specific CTL clones with high-affinity TCRs have led to the belief that for clinical application to ACT, it would be possible to obtain ex vivo-expanded tumor-specific CTLs with high efficacy by transfer of TSA/TAA-specific TCR genes to normal T cells. Based on this concept, the feasibility of transferring TCR genes specific to well-characterized TSA/TAA to normal T cells has been examined. These TCRs were specific to MART-1, MAGE-A1, MAGE-A4, gp100, WT1, NY-ESO-1, and minor antigens [8–11, 31–35]. With the aim of wider application, target TSA/TAA-specific TCRs have been centered on HLA-A*0201 and −A*2402 molecule restriction, both of which are the most prevalent HLA molecules in both Western and Japanese populations [36, 37]. Target TCR gene transfer has been largely performed using a retroviral system for which the transduction efficacy was about 20%, as determined by the detection of transferred V-β molecules or particular tetramers [8, 11, 32–35]. It was found that TCR gene-transferred normal T cells expressed the TCR heterodimer on the cell surface, conferring both identical antigen specificity and HLA restriction.
Table 1 lists reported attempts at TCR gene transfer for hematological malignancies. As can be seen, the use of this modality for treatment of hematological malignancies still remains in its infancy. We have determined the HLA-A*2402 restricted 9mer-epitope derived from WT1 protein (WT1235–243; CMTWNQMNL), and have demonstrated its clinical usefulness as a leukemia vaccine [38, 39]. Simultaneously, we have established the CTL clone specific to this epitope by HLA-A*2402 restriction [38], and subsequently described the in vitro feasibility of this WT1-specific TCR gene transfer using a lentiviral vector [11]. For the purpose of clinical application, we recently established a GMP-grade bicistronic retroviral vector carrying the full-length sequence of these TCR-α and β genes, and used it to confirm the specificity of the transferable HLA-A*2402-restricted epitope. Normal CD8+ T cells transferred with this TCR gene successfully displayed an antileukemia effect via recognition of the target epitope on leukemia cells, and normal CD4+ T cells harboring this transferred TCR gene successfully displayed antileukemia Th1 helper function also via recognition of the HLA-A*2402-restricted target epitope on leukemia cells (in preparation). Based on results from in vitro and mouse experiments, we are preparing to carry out clinical trials using these WT1-specific TCR gene-transferred T cells against hematological malignancies and several solid tumors.
Table 1.
Currently reported TCR gene transfer attempts for hematological malignancies.
| Target antigen | HLA restriction | vector | Comments | Transduction efficacy | Reference |
|---|---|---|---|---|---|
| WT1 | HLA-A*0201 | retroviral vector | WT1-specific TCR gene was transduced into PBL. CD8+ or CD4+ T cells could exert target specificity. | 40~60% in total T cells by Vb2 Ab after 2 rounds of antigen-specific stimulation | [8] |
| HLA-A*0201 | optimized retroviral vector | WT1-specific TCR gene was modified to express more efficiently (hybrid human-murine TCR, Cys-mutant TCR). Patients' PBL transduced WT1-specific TCR could reject the engraftment of autologous CML-BC cells in NOD/SCID mice model. | conventional vector: 0.6% in total CD8+ T cells by tetramer (nonstimulated) optimised vector: 6% in total CD8+ T cells by tetramer (nonstimulated) | [26, 27] | |
| HLA-A*2402 | lentiviral vector | WT1-specific TCR gene was transduced into PBL. CD8+ or CD4+ T cells could exert target specificity in an HLA class I-restricted fashion. | 60% in total T cells by Vb5.1 Ab (nonstimulated) 20% in sorted CD8+ T cells by tetramer | [11] | |
| HLA-A*2402 | optimized retroviral vector | The vector could suppress endogenous TCR by shRNA and increase transduced codon-optimized TCR simultaneously. | 44% in transduced CD8+ T cells by tetramer | [28] | |
| HA-1 | HLA-A*0201 | retroviral vector | HA-1-specific TCR gene was transduced into T cells. These transfectants could exert target specificity. | 6.6% in transduced T cells by tetrameter | [9] |
| HA-2 | HLA-A*0201 | retroviral vector | HA-2-specific TCR gene transduced CD8+ T cell clone was established. These transfectants could exert target specificity. | NA | [10] |
| HLA-A*0201 | retroviral vector | HA-2-specific TCR gene was transduced into CMV-specific CTL clone to exploit the viral specific response in vivo for the long-term persistence of target TCR transfectants. | NA | [29] |
Abbreviations: WT1, Wilms' tumor gene product 1; PBL, peripheral blood lymphocytes; CML-BC, chronic myelogenous leukemia-blastic crisis; CMV, cytomegalovirus; NA, not available; Ab, antibody.
Other preclinical attempts to investigate the feasibility of antileukemia ACT using HLA-A*0201-restricted WT1-specific TCR and HLA-A*0201-restricted HA-1/2-specific TCR [9, 10] have also been reported. With regard to HLA-A*0201-restricted WT1-specific TCR, Stauss and colleagues have technically succeeded in increasing WT1-specific TCR on transduced T lymphocytes using genetic modifications, and also in demonstrating the in vivo antileukemia effect of such engineered T cells using a murine system [8, 26].
4. Clinical Efficacy and Adverse Effects of ACT Using Engineered T Cells after TCR Gene Transfer
So far, no results of clinical trials of therapy for hematological malignancies using ACT with T cells harboring a TAA/TSA-specific TCR gene have been reported. Therefore, reference should be made to the results of pioneering clinical trials of melanoma therapy performed by Rosenberg's group, who used adoptive transfer of MART-1-specific TCR gene-transduced T cells in 17 patients with metastatic melanoma [3]. In 15 of the 17 patients, the infused MART-1-specific TCR gene-transferred T cells persisted for longer than two months, at a level of more than 10% of total peripheral lymphocytes. Two (11.8%) of the 17 patients obtained partial clinical remission, and their infused MART-1-specific T cells persisted for longer than a year in the periphery and retained their target-specific responsiveness. The same group has recently described better results in another clinical trial using newly established MART-1 and gp100-specific TCR genes with higher affinities [2]. In that study, an objective antimelanoma response, as defined by the RECIST criteria, was observed in 9 (25%) of 36 patients, and the clinical efficacy was again correlated with persistence of the infused T cells, which maintained their target-specific response in vivo. On the other hand, because MART-1 and gp100 are shared by normal melanocytes, transferred engineered T cells with high-affinity TCRs for these antigens were able to substantially damage normal melanocytes in the skin, eyes, ears, and hair. Another important phase I clinical trial using a preestablished allogeneic MART-1-specific TCR gene-transduced CTL line (C Cure 709) against melanoma was reported by Duval and colleagues [40]. The authors administered irradiated C Cure 709 cells intratumorally. No severe adverse events were observed, and 1 of 15 patients experienced a partial response. In the remaining patients, tumor reduction was observed not only at the injection site, but also in other areas.
Collectively, ACT using TSA/TAA-specific TCR gene-transferred T cells seems effective and its toxicity depends on the target antigen distribution in normal tissues and the affinity level of the transferred TCRs. If leukemia antigen-specific TCR gene-transferred T cells are used for adoptive therapy against leukemia, for example, high-affinity WT1-specific TCR gene-transferred T cells, attention should be paid to any adverse effects on WT1-expressing normal tissues, especially the hematopoietic system. Further investigation of the potential therapeutic use of TSA/TAA-specific TCR-transduced allogeneic T cells is warranted.
5. Attempts to Address Current Problems in TCR-Engineered T-Cell Therapy
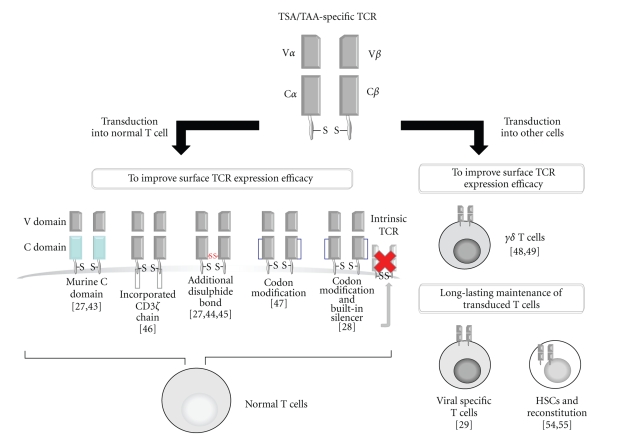
Although the engineering of T cells using TSA/TAA-specific TCR gene transfer is an attractive strategy for redirecting T cells towards malignancies, several issues still remain to be overcome, and generally these can be divided into two categories (Figure 1).
Figure 1.
Reported modifications of TSA/TAA-specific TCR transduction. With the aim of enhancing the surface expression level of the transduced TCR heterodimer, modifications are largely categorized into two options. One is to modify the target-TCR gene itself, and the other is to transduce alternative cells other than normal peripheral T lymphocytes. The former involves the murine/human hybrid, a modified two-chain TCR that encompasses total human CD3ζ, additional disulfide-bond insertion, codon modification, and insertion of built-in silencers for endogenous TCR. The latter involves TCR transduction into γδ T cells. These options aim to enhance the immediate antitumor effect of transfectants. On the other hand, TCR-gene transduction into hematopoietic stem cells is particularly aimed at achieving the prolonged presence of transfectants, which will be able to provide a durable antitumor effect.
5.1. Improvement of Surface TCR Expression Efficacy
TCR α/β heterodimer cannot be properly expressed on the cell surface when the CD3 molecule is absent [41]. The cell surface expression of transduced TCR α/β heterodimer also requires assembly with the γ, δ, ε, and ζ chains of the CD3 molecule. The competition between transduced and endogenous TCRs for association with the CD3 molecule determines the surface expression level of transduced TCR, because the total amount of available CD3 molecules on each T cell is predetermined. Recently, Heemskerk and colleagues have demonstrated that the functional activity of TCR-transduced T cells is correlated with TCR cell-surface expression, and that introduced, endogenous, and chimeric TCRs compete for cell-surface expression in favor of the TCR-CD3 complex with optimal pairing properties [42]. When considered simply, TCR assembly and expression involve two steps. Pairing of the TCR α and β chains is postulated to occur first, followed by association of the paired TCR chains with CD3 components. The latter step involves interaction between the constant region of TCR and the CD3 chains. As a corollary, manipulations to improve the efficacy of desired TCR α and β chain pairing and the CD3 assembly competition between transduced and endogenous TCRs would be expected to enhance the expression of the intended TCR heterodimer in the genetically modified T cells.
Based on this concept, there have been several attempts to enhance the expression of the target TCR. Based on the fact that murine TCR is expressed more efficiently in human T cells than human TCR, replacing the human constant region with murine sequences to yield hybrid murine/human constant region constructs leads to efficient expression of the hybrid TCR, without altering the specificity of the transduced TCR [27, 43]. In addition, it has been demonstrated that the hybrid TCR interacts with CD3 molecules with higher affinity than the human TCR, thus improving the expression of the target TCR due to strengthened CD3 assembly competition.
An alternative strategy for improving the expression of TCR is modification of the TCR α/β interface, with the aim of enhancing correct pairing between the transduced TCR α/β chains, and reducing the extent of mispairing between the transduced and endogenous TCRs. For this purpose, introduction of additional cysteine residues into the α and β chains to provide an additional disulfide bond between the modified TCR chains has been attempted. This resulted in more efficient expression of the cysteine-inserted TCR chains in comparison with the unmodified ones, accompanied by a reduced degree of mispairing between the transduced and endogenous TCRs [27, 44, 45]. To prevent this mispairing, Sebestyen and colleagues have reported an intriguing strategy by using a modified two-chain TCR that encompasses total human CD3ζ which results in highly preferred pairing between CD3ζ-modified TCR α and β chains as well as absence of TCR mispairing between TCR : CD3ζ and nonmodified TCR chains. In this article, authors demonstrated that transfer of both modified TCRα : CD3ζ and TCRβ : CD3ζ chains resulted in high surface expression, binding of peptide-MHC complexes and antigen-specific T cell functions. In addition, this genetic introduction of TCR α/β : ζ did not compromise surface expression and functions of an endogenous TCR α/β [46]. Codon-optimization to increase the production of TCR α/β proteins is another option to facilitate the surface expression of transduced TCR. Codon modification of TCR involves omission of mRNA instability motifs and cryptic splice sites, resulting in increased expression of the TCR α/β chain in the transduced CD8+ T cells, thus rendering the transfectants functionally more active against target tumor cells [47]. In addition, we have recently reported a new technique for improving the expression and reactivity of transduced tumor-specific TCR by specific silencing of endogenous TCR [28]. To reduce the degree of mispairing between transduced and endogenous TCR, which can impair the cell surface expression of the transduced TCR and result in insufficient function and potential generation of autoreactive T cells, we have developed retroviral vectors encoding both small interfering RNA (siRNA) constructs, which specifically down-regulate endogenous TCR, and a codon-optimized, siRNA-resistant TCR specific for the human tumor antigens MAGE-A4 or WT1. At a low copy number of the integrated vector, the transduced human lymphocytes exhibited high surface expression of the introduced tumor-specific TCR and reduced expression of endogenous TCRs, resulting in enhanced cytotoxic activity against antigen-expressing tumor cells. We are now planning to conduct clinical trials of antitumor adoptive therapy using T cells engineered by these vectors [28].
To avoid this mispairing between tranduced and endogenous TCR, the introduction of a TCR gene into γ/δ T cells, which originally lack an endogenous TCR, is also a promising option. Minor histocompatibility antigen HA-2-specific TCR, cytomegalovirus (CMV)-specific TCR, or MAGE-A4-specific TCR-transduced peripheral blood-derived γδ T cells have been demonstrated to exert target-specific cytotoxicity and cytokine production. Because γδ T cells do not express the CD4 or CD8 molecule, cointroduction of these coreceptor molecules sufficiently facilitates the target-specific reactivity of TCR α/β-transduced γ/δ T cells [48, 49].
5.2. Facilitation of a Long-Lasting Functional Memory Response of TCR-Transduced T Cells
The endeavors described above to improve the cell-surface expression of transduced TCR genes have been harnessed to potentiate the immediate antitumor activity of the transfectants. Although the causative factors have not been fully determined, it has been demonstrated that such engineered T cells soon lose their surface expression of transduced TCR [50]. Thus, to preserve a durable antitumor effect, especially for the prevention of disease relapse in patients with cancer, methods to maintain the introduced TCR expression should be developed. For example, exploitation of the memory T-cell response against latent cytomegalovirus (CMV) infection has been attempted for the expansion of HA-2-specific TCR-gene-transduced T cells [29]. An HA-2-specific TCR-gene-transduced CMV-specific CTL clone exerted dual cytotoxicity against both CMVpp65 antigen and HA-2-positive leukemia cells ex vivo. It was anticipated that these transfectants would exploit the virus-specific response to maintain both their survival and antitumor activity. Recently, tumor-reactive chimeric antigen receptor (CAR) gene-transduced Epstein-Barr virus (EBV)-specific CTLs applied for the treatment of individuals with neuroblastoma have displayed the anticipated persistence and antitumor activity [51]. As is observed in ACT with ex vivo-expanded tumor-specific T cells [52], for the long-term survival of TAA/TSA-specific TCR-gene-transduced T cells in vivo, the central memory fraction of such transfectants is considered to be important. However, using a murine model, Hinrichs et al. have recently revealed that naïve T cells are a better target of TSA/TAA-specific TCR gene transfer than central memory T cells for achieving long-lasting and active antitumor activity in vivo [53]. The authors reported that after repetitive stimulation, TCR-gene-transduced central memory T cells were more prone to rapid senescence than gene-modified naïve T cells, resulting in shorter and weaker antitumor activity in vivo. Therefore, isolated CD62L+CD45RO−naïve T cells from leukemia patients' leukapheresis samples could provide a better platform for T-cell engineering by TAA/TSA-specific TCR gene transfer.
For the long-term survival of TAA/TSA-specific TCR-gene-transduced T cells in vivo, TCR gene transfer to hematopoietic stem cells, which would be able to mediate continuous T-cell production through homeostatic hematopoiesis, seems attractive. To address the feasibility of this option, TCR gene-transduced hematopoietic stem cells were cultured with OP9 stromal cells expressing the Notch human ligand Delta-like 1 (OP9-DL1) in vitro, allowing stem cells to differentiate into mature T cells [54, 55]. Although several limitations still remain, the differentiated T cells obtained with this OP9-DL1 culture system, expressing transduced TCRs, were easily expandable and successfully exerted cytotoxicity against target cells in vitro. However, it still remains to be clarified whether such TAA/TSA-specific TCR gene-transduced T cells can differentiate in the circulation through the normal thymic education system. Before clinical application, it will first be necessary to address the concern about the potential risk of leukemogenesis caused by retroviral-vector-mediated uncontrolled “shotgun” gene insertion into hematopoietic stem cells. Lessons have been learned from the clinical application of suicide-gene (HVS-TK)-inserted donor lymphocyte infusion for the treatment of patients with relapsed leukemia after allo-HSCT, in whom undesirably functioning donor lymphocytes were clinically reduced in number using ganciclovir [56–58], suggesting that this option could be considered for eradicating such undesirable cells generated from genetically manipulated hematopoietic stem cells. Furthermore, long-term follow-up genome-wide analysis of T lymphocytes from patients who have received DLI using retrovirally HSV-TK gene-inserted donor lymphocytes has revealed that the transduced T-cell populations maintained remarkably stable gene expression profiles, phenotype, biological functions, and immune repertoire in vivo, with no evidence of clonal selection up to 9 years after administration. The authors suggested that integrations interfering with normal T-cell function were more likely to lead to clonal ablation than expansion in vivo [59]. These observations suggest that as long as TAA/TSA-specific TCR genes are retrovirally inserted into the peripheral mature T-cell genome, there would be little concern regarding safety. There have been some recent articles describing the feasibility of hematopoietic stem cells as a target of gene therapy [60–62]. Although these lines of evidence suggest that hematopoietic stem cells can be considered for TCR-gene transfer, this option is still in its infancy as far as clinical application is concerned.
6. Summary and Future Directions
Substantial progress has been made in clarifying the required conditions for the achievement of efficient antitumor adoptive therapy, especially with the use of TAA/TSA-specific TCR gene-transferred T cells, although this has been largely achieved from studies of melanoma and viral infections. In other words, as far as clinical application to leukemia is concerned, and for disseminated leukemia in particular, it still remains unclear whether engineered T-cell therapy could be clinically effective. Unlike antileukemia peptide vaccination, adoptive therapy with engineered T cells is advantageous in that a sufficient number of tumor-specific CTLs can be prepared, that is, disseminated leukemia should be the good target of this option. In fact, Dossett and colleagues have recently demonstrated the in vivo efficacy of ex vivo expanded and adoptively transferred TSA-specific TCR gene-transduced T cells against disseminated leukemia in a murine model [63]. This result provides considerable encouragement for the promotion of WT1-specific TCR gene-transferred T-cell therapy for human leukemia, and indeed we are shortly to conduct a phase I clinical trial using WT1-specific TCR gene-transferred T cells for the treatment of patients with hematological malignancy.
On the other hand, leukemia is a formidable adversary, and comprehensive strategies must be developed for durable control of the disease. As it has been mentioned, therapy using engineered T cells is still in its infancy, and to achieve long-term efficacy, peptide vaccination following TCR-gene-transduced T-cell therapy is a reasonable option for restimulating such transferred engineered T cells towards “memory T cells”. Alternatively, small-molecule inhibitor therapy targeting a molecule identical to that in TCR-gene therapy, which plays an important role in tumorigenesis, also appears to have promise for achieving a less toxic and more effective strategy for durable control of leukemia. As a model for the latter option, we are investigating the feasibility of Aurora A kinase-specific TCR gene-transferred T-cell therapy followed by Aurora A kinase inhibitor therapy [64]. We have recently identified that Aurora A kinase, which is widely expressed in various types of cancer, is a novel tumor target antigen for immunotherapy against leukemia [65]. We have identified an HLA-A*0201-restricted antigenic epitope of Aurora A kinase capable of inducing leukemia-reactive CTL, and established an epitope-specific TCR gene-expressing retroviral vector for TCR-gene-transferred T-cell therapy [66]; currently we are investigating the feasibility of a combination of Aurora A kinase-specific TCR gene-transferred T-cell therapy and Aurora A kinase inhibitor therapy.
Finally, it is relevant to mention our future plan for engineered T-cell therapy using TCR gene transfer for the treatment of hematological malignancy. This strategy originally requires prior determination of the TAA/TSA-specific TCR, which means that determination of the antigenic epitope, HLA-restriction, and establishment of the CTL clone must all be done beforehand. Therefore, it is fundamentally difficult to prepare multiple TCRs for different TAA/TSA with different HLA restrictions. We have already determined the HLA-A*2402-restricted antigenic epitope from human telomerase reverse transcriptase (hTERT), which is one of the best-known tumor antigens, and also established a TCR gene from hTERT-specific CTL clones [67], in addition to WT1 and Aurora A kinase. These prepared multiple TCRs make it possible to provide more adequate TCR for each patient according to the individual's HLA type and tumor antigen expression profile, that is, so-called “tailor-made” TCR therapy for each individual. Collectively, gene immunotherapy using TSA/TAA-specific TCR is an attractive option for hematological malignancies, although multiple drawbacks still need to be overcome. In order to improve the efficacy of this strategy, it is undisputed that further clinical trials are necessary to reveal tumor-derived and/or host-derived characteristics that disable TCR gene-transduced tumor-reactive T cells. Based on these endeavors, it is anticipated that gene immunotherapy using TAA/TSA-specific TCR gene transfer to T cells will open a new door for the treatment of hematological malignancies in the near future.
Conflict Interests
None of the authors declare any competing financial interests.
Abbreviations
- C domain:
constant region
- S–S:
disulfide bond
- HSC:
hematopoietic stem cell.
References
- 1.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Current Opinion in Immunology. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abad JD, Wrzensinski C, Overwijk W, et al. T-cell receptor gene therapy of established tumors in a murine melanoma model. Journal of Immunotherapy. 2008;31(1):1–6. doi: 10.1097/CJI.0b013e31815c193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Witte MA, Jorritsma A, Kaiser A, et al. Requirements for effective antitumor responses of TCR transduced T cells. Journal of Immunology. 2008;181(7):5128–5136. doi: 10.4049/jimmunol.181.7.5128. [DOI] [PubMed] [Google Scholar]
- 6.Qazilbash MH, Wieder ED, Thall PF, et al. PR1 peptide vaccine-induced immune response is associated with better event-free survival in patients with myeloid leukemia. Blood. 2007;110:p. 90a. abstract no. 283. [Google Scholar]
- 7.Keilholz U, Letsch A, Busse A, et al. A clinical and immunological phase II trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113(26):6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 8.Xue S-A, Gao L, Hart D, et al. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 2005;106(9):3062–3067. doi: 10.1182/blood-2005-01-0146. [DOI] [PubMed] [Google Scholar]
- 9.Mommaas B, van Halteren AGS, Pool J, et al. Adult and cord blood T cells can acquire HA-1 specificity through HA-1 T-cell receptor gene transfer. Haematologica. 2005;90(10):1415–1421. [PubMed] [Google Scholar]
- 10.Heemskerk MHM, Hoogeboom M, de Paus RA, et al. Redirection of antileukemic reactivity of peripheral T lymphocytes using genetransfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood. 2003;102(10):3530–3540. doi: 10.1182/blood-2003-05-1524. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji T, Yasukawa M, Matsuzaki J, et al. Generation of tumor-specific, HLA class I-restricted human Th1 and Tc1 cells by cell engineering with tumor peptide-specific T-cell receptor genes. Blood. 2005;106(2):470–476. doi: 10.1182/blood-2004-09-3663. [DOI] [PubMed] [Google Scholar]
- 12.Weiden PL, Flournoy N, Thomas ED. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. New England Journal of Medicine. 1979;300(19):1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 13.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease. Contribution to improved survival after allogeneic marrow transplantation. New England Journal of Medicine. 1981;304(25):1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 14.Michor F, Hughes TP, Iwasa Y, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435(7046):1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 15.Shah NP, Skaggs BJ, Branford S, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. Journal of Clinical Investigation. 2007;117(9):2562–2569. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nature Medicine. 2000;6(9):1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 17.Rezvani K, Grube M, Brenchley JM, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood. 2003;102(8):2892–2900. doi: 10.1182/blood-2003-01-0150. [DOI] [PubMed] [Google Scholar]
- 18.Oka Y, Tsuboi A, Taguchi T, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(38):13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Medicine. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. New England Journal of Medicine. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marijt E, Wafelman A, van der Hoorn M, et al. Phase I/II feasibility study evaluating the generation of leukemia-reactive cytotoxic T lymphocyte lines for treatment of patients with relapsed leukemia after allogeneic stem cell transplantation. Haematologica. 2007;92(1):72–80. doi: 10.3324/haematol.10433. [DOI] [PubMed] [Google Scholar]
- 24.Kolb H-J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 25.Falkenburg JHF, Wafelman AR, Joosten P, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999;94(4):1201–1208. [PubMed] [Google Scholar]
- 26.Xue S-A, Gao L, Thomas S, et al. Development of a Wilms’ tumor antigen-specific T-cell receptor for clinical trials: engineered patient’s T cells can eliminate autologous leukemia blasts in NOD/SCID mice. Haematologica. 2010;95(1):126–134. doi: 10.3324/haematol.2009.006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas S, Xue S-A, Cesco-Gaspere M, et al. Targeting the wilms tumor antigen 1 by TCR gene transfer: TCR variants improve tetramer binding but not the function of gene modified human T cells. Journal of Immunology. 2007;179(9):5803–5810. doi: 10.4049/jimmunol.179.9.5803. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto S, Mineno J, Ikeda H, et al. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Research. 2009;69(23):9003–9011. doi: 10.1158/0008-5472.CAN-09-1450. [DOI] [PubMed] [Google Scholar]
- 29.Heemskerk MHM, Hoogeboom M, Hagedoorn R, Kester MGD, Willemze R, Falkenburg JHF. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. Journal of Experimental Medicine. 2004;199(7):885–894. doi: 10.1084/jem.20031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dembic Z, Haas W, Weiss S. Transfer of specificity by murine α and β T-cell receptor genes. Nature. 1986;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- 31.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. Journal of Immunology. 1999;163(1):507–513. [PubMed] [Google Scholar]
- 32.Willemsen RA, Weijtens MEM, Ronteltap C, et al. Grafting primary human T lymphocytes with cancer-specific chimeric single chain and two chain TCR. Gene Therapy. 2000;7(16):1369–1377. doi: 10.1038/sj.gt.3301253. [DOI] [PubMed] [Google Scholar]
- 33.Hiasa A, Hirayama M, Nishikawa H, et al. Long-term phenotypic, functional and genetic stability of cancer-specific T-cell receptor (TCR) αβ genes transduced to CD8+ T cells. Gene Therapy. 2008;15(9):695–699. doi: 10.1038/sj.gt.3303099. [DOI] [PubMed] [Google Scholar]
- 34.Morgan RA, Dudley ME, Yu YYL, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. Journal of Immunology. 2003;171(6):3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. Journal of Immunology. 2005;174(7):4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Date Y, Kimura A, Kato H, Sasazuki T. DNA typing of the HLA-A gene: population study and identification of four new alleles in Japanese. Tissue Antigens. 1996;47(2):93–101. doi: 10.1111/j.1399-0039.1996.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 37.Krausa P, Brywka M, III, Savage U, et al. Genetic polymorphism within HLA-A∗02: significant allelic variation revealed in different populations. Tissue Antigens. 1995;45(4):223–231. doi: 10.1111/j.1399-0039.1995.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 38.Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8+ cytotoxic T- lymphocyte clone specific for WT1 peptide. Blood. 2000;95(1):286–293. [PubMed] [Google Scholar]
- 39.Yasukawa M, Fujiwara H, Ochi T, et al. Clinical efficacy of WT1 peptide vaccination in patients with acute myelogenous leukemia and myelodysplastic syndrome. American Journal of Hematology. 2009;84(5):314–315. doi: 10.1002/ajh.21387. [DOI] [PubMed] [Google Scholar]
- 40.Duval L, Schmidt H, Kaltoft K, et al. Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in metastatic melanoma. Clinical Cancer Research. 2006;12(4):1229–1236. doi: 10.1158/1078-0432.CCR-05-1485. [DOI] [PubMed] [Google Scholar]
- 41.Call ME, Wucherpfennig KW. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annual Review of Immunology. 2005;23:101–125. doi: 10.1146/annurev.immunol.23.021704.115625. [DOI] [PubMed] [Google Scholar]
- 42.Heemskerk MHM, Hagedoorn RS, van der Hoorn MAWG, et al. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex. Blood. 2007;109(1):235–243. doi: 10.1182/blood-2006-03-013318. [DOI] [PubMed] [Google Scholar]
- 43.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Research. 2006;66(17):8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Research. 2007;67(8):3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuball J, Dossett ML, Wolfl M, et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109(6):2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sebestyen Z, Schooten E, Sals T, et al. Human TCR that incorporate CD3ζ induce highly preferred pairing between TCRα and β chains following gene transfer. Journal of Immunology. 2008;180(11):7736–7746. doi: 10.4049/jimmunol.180.11.7736. [DOI] [PubMed] [Google Scholar]
- 47.Scholten KBJ, Kramer D, Kueter EWM, et al. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clinical Immunology. 2006;119(2):135–145. doi: 10.1016/j.clim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 48.van der Veken LT, Hagedoorn RS, van Loenen MM, Willemze R, Falkenburg JHF, Heemskerk MHM. α β T-cell receptor engineered γδ T cells mediate effective antileukemic reactivity. Cancer Research. 2006;66(6):3331–3337. doi: 10.1158/0008-5472.CAN-05-4190. [DOI] [PubMed] [Google Scholar]
- 49.Hiasa A, Nishikawa H, Hirayama M, et al. Rapid αβ TCR-mediated responses in γδ T cells transduced with cancer-specific TCR genes. Gene Therapy. 2009;16(5):620–628. doi: 10.1038/gt.2009.6. [DOI] [PubMed] [Google Scholar]
- 50.Burns WR, Zheng Z, Rosenberg SA, Morgan RA. Lack of specific γ-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood. 2009;114(14):2888–2899. doi: 10.1182/blood-2009-01-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Medicine. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. Journal of Clinical Investigation. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naïve rather than central memory CD8+ T cells mediate superior antitumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Lent AU, Nagasawa M, van Loenen MM, et al. Functional human antigen-specific T cells produced in vitro using retroviral T cell receptor transfer into hematopoietic progenitors. Journal of Immunology. 2007;179(8):4959–4968. doi: 10.4049/jimmunol.179.8.4959. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Parkhurst MR, Zheng Z, et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via notch signaling. Cancer Research. 2007;67(6):2425–2429. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276(5319):1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 57.Ciceri F, Bonini C, Marktel S, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109(11):4698–4707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 58.Ciceri F, Bonini C, Stanghellini MTL, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. The Lancet Oncology. 2009;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 59.Recchia A, Bonini C, Magnani Z, et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 61.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. New England Journal of Medicine. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 62.Krishnan A, Zaia JA, Rossi J, et al. First in human engraftment of anti-HIV lentiviral vector gene modified CD34+ peripheral blood progenitor cells in the treatment of AIDS related lymphoma (ARL) Blood. 2008;112:p. 818a. abstract no. 2348. [Google Scholar]
- 63.Dossett ML, Teague RM, Schmitt TM, et al. Adoptive immunotherapy of disseminated leukemia with TCR-transduced, CD8+ T cells expressing a known endogenous TCR. Molecular Therapy. 2009;17(4):742–749. doi: 10.1038/mt.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ochi T, Fujiwara H, Yasukawa M. Aurora-A kinase: a novel target both for cellular immunotherapy and molecular target therapy against human leukemia. Expert Opinion on Therapeutic Targets. 2009;13(12):1399–1410. doi: 10.1517/14728220903307483. [DOI] [PubMed] [Google Scholar]
- 65.Ochi T, Fujiwara H, Suemori K, et al. Aurora-A kinase: a novel target of cellular immunotherapy for leukemia. Blood. 2009;113(1):66–74. doi: 10.1182/blood-2008-06-164889. [DOI] [PubMed] [Google Scholar]
- 66.Nagai K, Fujiwara H, Ochi T, et al. Development of a novel anti-leukemia gene immunotherapy using Aurora-A kinase-specific T-cell receptor gene transfer. Blood. 2009;114:p. 158a. abstract no. 375. [Google Scholar]
- 67.Arai J, Yasukawa M, Ohminami H, Kakimoto M, Hasegawa A, Fujita S. Identification of human telomerase reverse transcriptase-derived peptides that induce HLA-A24-restricted antileukemia cytotoxic T lymphocytes. Blood. 2001;97(9):2903–2907. doi: 10.1182/blood.v97.9.2903. [DOI] [PubMed] [Google Scholar]