Abstract
Despite recent advances, stroke remains a leading cause of neurological disability with the vast majority of victims being the elderly, who exhibit more severe neurological deficits and a reduced capacity to recover from these disabilities in comparison to young stroke survivors. The objective of the present study was to develop a model of focal ischemic stroke in aged rats using endothelin-1 (ET-1) to produce low mortality rates as well as reliable, robust sensorimotor deficits that resemble functional impairments associated with stroke in humans. Here, we studied the functional and histological outcome following unilateral ET-1 infusions into the sensorimotor cortex of aged rats (20–23 months old). This procedure resulted in low mortality rates (13.3%) and no loss in body weight one week following surgery. Functional assessment was performed using a number of reliable behavioural tests: staircase test (fine motor function), horizontal ladder (skilled locomotion), bilateral tactile stimulation test (somatosensory function) and cylinder test (postural weight support). Following ET-1 induced stroke, all tests demonstrated large and sustained sensorimotor deficits in both forelimb and hindlimb function that failed to improve over the 28-day testing period. In addition, histological assessment revealed a substantial loss of retrogradely labelled corticospinal neurons in the ipsilesional hemisphere following stroke. Our results establish a model for the use of aged rats in future preclinical studies, which will enhance assessment of the long-term benefit of potential neural repair and regenerative strategies.
Keywords: Aged, Endothelin-1, Stroke, Behavioural deficits, Plasticity, Preclinical models
Introduction
Ischemic stroke represents a leading cause of long-term neurological disability that is strongly associated with age and affects the majority of the elderly population (Donnan et al., 2008; Kelly-Hayes et al., 2003). It has been reported in the United Kingdom that 98.5% of strokes occur in people over the age of 45 years, and 75% in people aged over 65 years (Carroll et al., 2001). Elderly stroke survivors are more likely to be disabled, with almost half possessing moderate to severe neurological deficits (Kelly-Hayes et al., 2003). Due to the increase in life expectancy in many countries (Kinsella, 2005), the number of individuals at risk from stroke is anticipated to rise, making the burden of stroke disability an even more serious public healthcare concern that needs to be urgently addressed.
Experimental and human studies both demonstrate that neuroplasticity, neurophysiology and neurochemistry alter during aging and that the brain's response to insults changes (Badan et al., 2003; Buga et al., 2008; Cox, 1983; Davis et al., 1995; Futrell et al., 1991; Hachinski et al., 1992; Li and Carmichael, 2006; Markus et al., 2005; Sutherland et al., 1996; Ward, 2005). Consequently, the age of the animal when modelling the clinical disorder is critical, particularly when assessing ischemic damage and the extent of recovery. At present, the majority of experimental research is still conducted on young animals, despite recommendations by the STAIR (Stroke Therapy Academic Industry Roundtable) committee and the Stroke Progress Review Group suggesting that data from aged animals might be considered more appropriate in preclinical studies (Fisher et al., 2009). This may also elucidate reasons why most strategies and treatments demonstrating effectiveness in animal models have failed to show any clinical benefits in aged humans (Fisher and Ratan, 2003; Gladstone et al., 2002; Millikan, 1992).
Preclinical stroke models in aged rats have been established, with all based on permanent or temporary occlusion of the middle cerebral artery (MCAo) or photothrombosis, yet the use of aged animals for stroke research is generally limited because of their high price and high mortality rates following surgery compared to younger animals (Futrell et al., 1991; Hachinski et al., 1992; Lindner et al., 2003; Wang et al., 1995, 2003; Zhang et al., 2000). Another method increasingly being used to induce ischemic injuries is the potent vasoconstricting peptide, endothelin-1 (ET-1) (Yanagisawa et al., 1988). Unlike other models, the use of ET-1 is diverse in that it can be applied directly to the cortical surface (Adkins et al., 2004; Fuxe et al., 1997; Hsu and Jones, 2006), applied near cerebral arteries (Sharkey et al., 1994; Sharkey and Butcher, 1995; Yager et al., 2006) or microinjected into particular regions of the brain (Frost et al., 2006; Fuxe et al., 1992; Gilmour et al., 2004; Windle et al., 2006) to cause a reduction in cerebral blood flow and consequent ischemic damage.
Topical applications of ET-1 are most common, involving direct placement onto the cortical surface to produce a localised and dose-dependent ischemic injury to the sensorimotor cortex (Windle et al., 2006). Conversely, it is difficult to ensure the exact distribution of ET-1 once applied to the surface, as well as the extent of diffusion into the underlying cortical layers. One approach to minimise this variability and provide greater accuracy is to make intracortical injections of ET-1 into specific regions of the sensorimotor cortex (Gilmour et al., 2004; Tennant and Jones, 2009; Windle et al., 2006). Functional assessment after intracortical infusions of ET-1 demonstrate long lasting impairments in skilled forelimb use (Gilmour et al., 2004; Windle et al., 2006), yet to date all have been performed on young animals. It is known that co-ordinates need to be altered in relation to animal size and age (Paxinos et al., 1985), thus to provide more clinical relevance it would be valuable to modify this technique with co-ordinates adapted for the aged rat brain and to measure the functional deficits induced by this lesion in aged rats.
Therefore, the objectives of the present study were as follows: (1) to develop a model of focal sensorimotor cortical stroke in aged rats using endothelin-1 at specific co-ordinates to ensure greater reproducibility, (2) to obtain a low mortality rate and (3) to obtain long-lasting, robust functional disabilities that resemble the sensorimotor deficits associated with stroke victims. Such a model could potentially have considerable use in assessing the functional recovery and long-term benefit of prospective neural repair strategies.
Materials and methods
Subjects
Twenty-four male Wistar rats were obtained from Harlan, UK at approximately 18 months old (480–680 g) and used in the experiment between 20 and 23 months of age. Animals were housed 2–3 to a cage in standard laboratory conditions on a 12:12 h light/dark cycle. Rats were moderately food restricted (13–15 g per rat, per day) during behavioural training and only the day before post-surgery behavioural testing sessions.
Surgery
All procedures were in accordance with guidelines from the UK Home Office and Animals (Scientific Procedures) Act of 1986. Animals were anesthetized with isoflurane (4% in O2 for induction) and then transferred to a stereotaxic frame (David Kopf Instruments, USA) where anaesthesia was maintained at 1.5–2% in O2 delivered via a facemask. Body temperature was monitored via a rectal thermometer and maintained at approximately 36 °C with a heating pad.
Prior to surgery, rats were allocated to sham or stroke group in a counterbalanced fashion ensuring that there was no difference in group mean preoperative performance of the preferred forepaw on the staircase test. Surgery was performed in a randomised block design. Unilateral lesions were performed in the hemisphere contralateral to the dominant forelimb, as determined by staircase behavioural test. In stroke rats (n=15), a midline incision was made and the sensorimotor cortex was exposed by craniotomy the following co-ordinates (defined as anterioposterior (AP), mediolateral (ML), dorsoventral (DV)): AP 4 mm to −2 mm, ML 2 mm to 4 mm, relative to bregma. The dura mater was removed using a 25-gauge needle. Due to variations in skull thickness between aged rats, a craniotomy was performed to enable accurate depth placement of ET-1 intracortical injections.
Four 2 -μl injections of ET-1 (CalBioChem; 200 pmol/μl; 0.5 μg/μl dissolved in sterile saline) was delivered via a glass micropipette connected to a syringe (Hamilton), with the first 1 μl administered at a depth of 1 mm from the brain surface and the subsequent 1 μl applied to the surface of the cortex at the following co-ordinates:
(1) AP +3.5 mm, ML 2.8 mm, DV − 1.0 mm/0 mm;
(2) AP +2 mm, ML 2.8 mm, DV − 1.0 mm/0 mm;
(3) AP +0.5 mm, ML 2.8 mm, DV − 1.0 mm/0 mm;
(4) AP −1 mm, ML 2.8 mm, DV − 1.0 mm/0 mm, from bregma, midline and brain surface respectively.
For each injection, ET-1 was delivered at a rate of 0.5 μl/min with a 1-min interval between each microlitre. The dose and delivery of ET-1 was determined from a preliminary study using aged rats (unpublished data). Prior to suturing, the animal was left undisturbed for 5 min and the skull fragment was replaced. Sham-operated rats (n = 9) received all procedures up to, but not including, craniotomy.
Animals were allowed to recover from anaesthesia in a recovery box until fully conscious and buprenorphine (0.01 mg/kg, s.c.) was administered for postoperative pain relief. During the early postoperative period, the health of each animal was carefully monitored and supplementary food (sugared-wet mash) was given when necessary.
Retrograde tracing of CST neurons
To differentiate corticospinal neurons (CSNs) from other neurons in layer V of the cortex and determine the number of surviving CSNs after injury, animals (sham n = 3; stroke n = 3) were given bilateral spinal injections of the retrogradely transported fluorescent tracer Fast Blue (FB; Sigma; 2% in PBS; 0.25 μl on each side) after all postoperative behaviour was completed. Animals were anesthetized as previously described and the cervical spinal cord at level C4 was exposed via laminectomy, a glass micropipette was positioned into the dorsal columns and FB was delivered at a rate of 0.5 μl/min. Animals were subsequently left for 10 days before being perfused.
Behavioural testing
A variety of behavioural tests previously found to be effective in assessing sensory and motor deficits were included within this study. Animals were handled every day for 3 weeks before the onset of the experiment and trained for 4 weeks on the staircase task to identify forepaw preference. All behavioural testing was carried out by an experimenter blinded to group membership by recoding animals after surgery. Baseline values were recorded 3 days before surgery on all behavioural tasks and animals were assessed every week on all tests until the fourth postoperative week. Animals that failed to meet the minimum criteria for the staircase test were excluded from behavioural analysis and used in a further study (n = 5).
Staircase test and training
The staircase test was used to assess reaching performance; this provides a sensitive measure of skilled forepaw motor function (Montoya et al., 1991). The staircase apparatus (Campden Instruments Ltd., UK) consists of a chamber with a central platform for the rat to climb onto and a set of seven steps is located on either side. Each step holds three sucrose pellets (45 mg, Research Diets Inc, New Brunswick, NJ). To reduce initial neophobic responses animals were given sucrose pellets in their home cage prior to training sessions. The animals were pre-trained twice per day on weekdays over a 4-week period; they were first introduced to the apparatus in their home cage with their cagemate, and then afterwards alone for 15 min with sucrose pellets placed along the central platform and three pellets on each step of the staircase. During each testing period the number of pellets eaten or displaced and the maximum step reached using each forepaw was recorded. Each weekly session consisted of two trials; mean scores per rat per week were calculated. The minimum criterion to be included in the study was retrieval on average 55% of the total available pellets using their dominant paw during preoperative testing.
Bilateral tactile stimulation test
To assess the magnitude of somatosensory asymmetry and sensorimotor impairments in forepaw function after stroke the bilateral tactile stimulation test (Schallert et al., 1982, 2000) was employed. For each trial, round adhesive patches (13 mm diameter, Ryman) were applied to the plantar surface of both forepaws and the animal was returned to its homecage. Two times were recorded for both forepaws: (1) contact and (2) remove; where “contact” represents the time taken for the animal to notice the adhesive patch on its forepaw and bring it to its mouth, and “remove” represents the time taken for the animal to remove the adhesive patch from its forepaw. To determine whether the rats showed bias for their affected or less-affected forelimbs, the order and side of label removal was recorded. This was repeated four times per session until a >75% preference had been found; if this was not the case a fifth trial was conducted.
The magnitude of asymmetry was established using the seven levels of stimulus pairs on both forepaws as previously described (Schallert et al., 2000; Schallert and Whishaw, 1984). During this phase, to determine the extent of ipsilateral response bias, the size of the stimulus was progressively increased on the affected forepaw and decreased on the less-affected forepaw by an equal amount (14.1 mm2), until the rat removed the stimulus on the affected forepaw first (reversal of original bias). This reversal represents the magnitude of asymmetry, where the higher the score indicates the greater the degree of somatosensory impairment.
Cylinder test
The cylinder test was used to assess forelimb use and asymmetries in postural weight support during exploratory activity (Schallert et al., 2000) within a transparent 20 cm diameter and 30 cm height cylinder. To allow movements to be recorded when the animal is turned away from the camera an angled mirror was placed behind the cylinder.
During exploration, rats rear against the vertical surface of the cylinder. The first forelimb to touch the wall was scored as an independent placement for that forelimb. Subsequent placement of the other forelimb against the wall to maintain balance was scored as “both.” If both forelimbs were simultaneously placed against the wall during rearing this was scored as “both.” Lateral movements along the wall using both forelimbs alternately were also scored as “both.” Scores were obtained from a total number of 10 full rears to control for differences in rearing between animals.
Once scores had been acquired, forelimb asymmetry was calculated using the formula: 100 × (ipsilateral forelimb use + 1/2 bilateral forelimb use)/total forelimb use observations (Hsu and Jones, 2005).
Horizontal ladder
To assess impairments in forelimb and hindlimb function after stroke, rats were required to walk along a horizontal ladder where limb placement during skilled walking can be measured (Metz and Whishaw, 2002). The apparatus consisted of Plexiglas side walls, 1.2 m long, 50 cm high and width adjusted to approximately 2 cm larger than the animal to prevent them turning around. Metal rungs were placed at a height of 20 cm; they were spaced unequally (between 1 cm and 4 cm apart) and changed weekly to avoid the animals learning the location of the rungs. To encourage animals to walk across the ladder sucrose pellets were placed at the end of the alley, along with their home cage. Animals were familiarised to the apparatus 2 weeks before baseline assessment, and within each session animals crossed the ladder twice and mean scores per rat per session were calculated.
Forelimb and hindlimb placements were rated using a 7-category scale, according to the limb position and errors that arose during limb placement: 0, total miss; 1, deep slip; 2, slight slip; 3, replacement; 4, correction; 5, partial placement; and 6, correct placement.
Histology
Animals were deeply anesthetized with sodium pentobarbital (0.4 ml, i.p.) and perfused transcardially with heparinized saline followed by 800 ml of 4% paraformaldehyde in 0.1 M phosphate buffer. Brains and spinal cords were post fixed for 2 h at 4 °C then transferred to 30% sucrose in PBS. The forebrain and spinal cord were separately embedded in 10% gelatine and blocks were post fixed for 24 h. Free-floating serial sections were then cut using a freezing stage microtome (Kryomat; Leitz, Germany). Ten series of rostral to caudal tissue sections were collected in 24 well plates containing PBS (with 0.1% sodium azide) and stored at 4 °C. Transverse C1–C4 spinal cord sections (40 μm) were cut and incubated in the following (with three PBS washes between each step): rabbit anti-protein kinase Cγ (Santa Cruz Biotechnology; 1:500; overnight) and goat anti-rabbit Alexa 488 (Molecular Probes, Invitrogen; 1:1000; 3 h). Coronal brain sections (50 μm) were cut and incubated with Neurotrace Nissl (Molecular Probes, Invitrogen; 1:100; 20 min). Free-floating sections were mounted onto slides and coverslipped with Mowiol mounting medium and visualised under a fluorescence microscope (Axioplan, Zeiss).
Quantification of ischemic damage
For assessment of tissue loss and ventricular enlargement, serial coronal sections were cut from +3.0 mm to −2.5 mm relative to bregma. Images of sections were captured using a light microscope with a high resolution digital camera (MiniVID Digital Eyepiece Camera., LW Scientific). For each section, the total area of each hemisphere or ventricle was obtained using the AxioVision LE V4.7.2 (Carl Zeiss) contour tracing software. Infarct size was calculated by subtracting the area of the ipsilesional hemisphere from the area of the contralesional hemisphere in each section. Hemisphere area measurements did not include necrotic tissue, cysts or cavities, or ventricles. Ventricle enlargement was calculated by subtracting the area of the contralesional lateral ventricle from the ipsilesional lateral ventricle in each section. Volume of injury (mm3) was calculated as the sum of the area from each section, multiplied by the distance between sections (Buchan et al., 1992).
Statistical analysis
Unless stated otherwise, statistical analysis was performed using SPSS (V16.0). Behavioural data were analysed using repeated measures analysis of covariance (RM ANCOVA), using preoperative performance as covariate, where: (1) data were normal (Kolmogorov–Smirnov test) and (2) variances were homogenous (Levene's test). Where the assumptions of sphericity were violated (Mauchly's test; p<0.05) the Greenhouse–Geisser correction was applied (degrees of freedom are reported to the nearest integer). Independent two-tailed t-tests were used to determine differences between groups on specific days. The number of Fast Blue labelled cells was compared between sham and stroke groups using repeated measures analysis of variance (RM ANOVA). Paired t-tests were performed for animal weights. p<0.05 was considered significant. Data is presented as means ± SEM and asterisks indicate significance as follows: *p≤0.05; **p≤0.01; ***p≤0.001.
G*Power 3.1 (Faul et al., 2007) was used to estimate the effect size of the difference between the stroke and sham groups for each behavioural test using the post hoc algorithm for F tests (Repeated measures, between factors). G*Power was also used to estimate the minimum sample sizes that would be required in future experiments (using two groups) to identify treatment effects of three different magnitudes (25% recovery, 50% recovery, 75% recovery) using the a priori algorithm for F tests (Repeated measures, between factors) using the following parameters; type I error threshold (α) ≤ 0.05 and power (1-β)≥0.80. The “correlation between repeated measures” parameter was derived by calculating the mean of the Pearson's product-moment correlation coefficients for each of all possible (six) pairs of (four) time points.
Results
Mortality rate and postoperative health
Prior to surgery, two of the 24 rats appeared sick and were humanely killed. Dissection revealed the presence of pituitary tumours.
After producing the ischemic lesions using ET-1, 2 of the 15 rats died after a sudden decline in respiratory function, which was not reversed with the respiratory stimulant Dopram. One animal died without awakening from anaesthesia, whereas the other animal died after exhibiting barrelrolling and other convulsive signs upon awakening from anaesthesia, which may be attributed to diffusion of ET-1 into the cerebral ventricles (Gross and Weaver, 1993).
During the first hour after ischemic lesioning, when rats had recovered from anaesthesia, some animals were observed circling though this was temporary and lasted for only a few hours. One day after surgery, there were minimal signs of stress from the animals and none experienced much reduction in body weight during the entire postoperative testing period, with mean weights of 529 ± 41.2 g before surgery and 520± 35.1 g 1 week post-surgery (paired t-test, p > 0.05).
Histological assessment of lesion
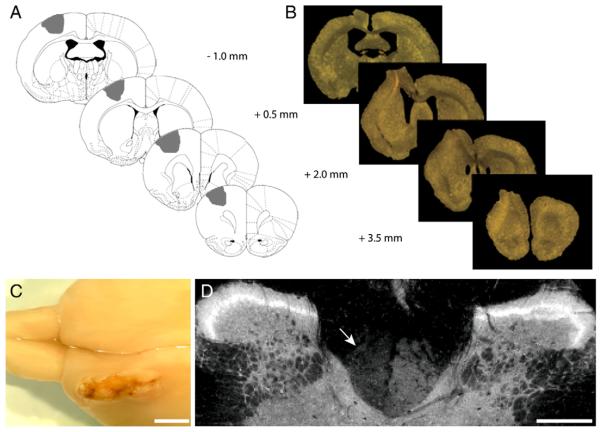
Four infusions of ET-1 were injected into the sensorimotor cortex (intended target areas are illustrated in Fig. 1A). Gross anatomy 5 weeks after stroke revealed the extent of ischemic damage in lesioned rats (Fig. 1C). The mean amount of tissue loss measured by volumetric analysis was 97.4 ± 17 mm3 in the ipsilesional hemisphere following stroke (Fig. 1B). Stroke also led to an increase in lateral ventricle size, with an enlargement of 18.1 ± 7 mm3 relative to their contralesional hemisphere.
Fig. 1. Histological assessment of lesion.
Representative illustrations of the lesion, demonstrating the AP co-ordinates of ET-1 administration relative to Bregma with the intended target area shaded in grey (A). The extent of ischemic damage through the brain can be seen in relation to the ET-1 injection sites in coronal sections viewed under a light microscope (B) and from a dorsal view of a rat brain 5 weeks post stroke in the left hemisphere (C). Immunostaining for PKCγ in transverse sections of the cervical spinal cord (C1) reveals the CST is intact on the right side and partially absent on the left side 5 weeks post stroke (D, indicated by arrow). Scale bars: 2 mm (C), 400 μm (D).
Some tissue protruded into the site of craniotomy which was revealed upon dissection to be due to displacement of the skull. This displacement has been prevented in further studies by using bone wax to seal the skull fragment into the area of craniotomy.
The extent of injury was also determined at spinal level using immunostaining for protein kinase C γ (PKCγ) to determine whether the ET-1 induced lesion led to a loss of corticospinal tract (CST) axons within the cervical spinal cord (Barritt et al., 2006; Bradbury et al., 2002; Starkey et al., 2005). ET-1 lesioned rats possessed PKCγ immunoreactivity that was present in both dorsal columns, though qualitatively it was partly reduced on the injured side (Fig. 1D). Although previous studies reveal a complete absence of PKC immuno-reactivity on the lesioned side after pyramidotomy, the presence of residual PKCγ on the injured side in this study is most likely due to sparing of CST fibres from other cortical areas that were less-affected by the lesion.
Behavioural assessment of lesion
Staircase pellet reaching test
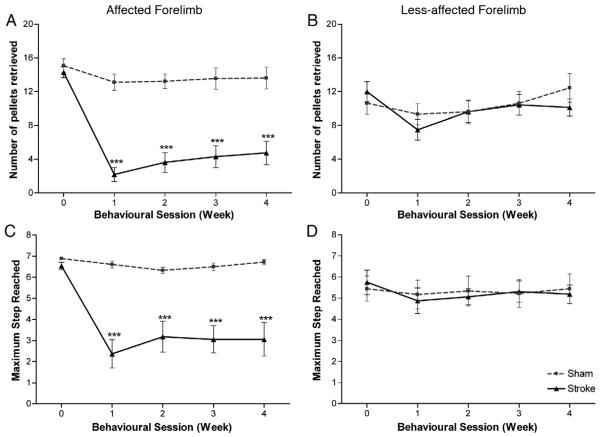
The staircase test assesses impairments in fine motor forepaw function, in terms of the number of pellets retrieved (measures the ability to grasp, retrieve and eat the sugar pellets) and the maximum step reached (measures the ability of rats to reach or displace the sugar pellets).
Statistical analysis revealed that there was a significant effect of group in both the number of pellets retrieved (F1, 14=41.6; p<0.0001) and the maximum step reached (F1, 14=23.6; p<0.0001) by the affected forepaw, with impairments persisting throughout the entire testing period (2-tailed t-tests; stroke versus sham; p<0.0001; Figs. 2A and C). Statistical analysis also revealed no effect of session or interaction of session × group in both the number of pellets retrieved (F3, 42=0.44; p=0.73 and F3, 42=0.77; p=0.52, respectively) and maximum step reached (F2, 26=0.33; p=0.71 and F2, 26=1.74; p=0.17, respectively); supporting the conclusion that stroke rats had sustained impairments in fine motor function. At 28 days post-lesion, the mean number of pellets successfully retrieved was 13.6±2.7 pellets in sham rats compared with 4.8±2.8 pellets in stroke rats. Continuous observation revealed that stroke rats tended to use their tongues to obtain the pellets from the top two steps, with animals rarely retrieving pellets from steps 3–7 and instead displacing a high number of pellets in attempts to grasp pellets on these lower steps.
Fig. 2. Staircase test.
ET-1 stroke produced considerable impairments in fine motor function with rats unable to grasp many pellets successfully with their affected forepaw (A). Impairments were also observed in the rats' ability to reach and displace the sugar pellets (C), which also persisted throughout the entire testing period. A small but significant difference in the number of pellets retrieved was observed between sham and stroke rats using their less-affected forepaw (B) but no significant difference in maximum step reached (D) was observed between sham and stroke rats for their less-affected forepaw. Results are presented as mean±SEM and were analysed using RM ANCOVA and independent two-tailed t-tests. Significance between groups is denoted as: ***p<0.001. n=9 for sham and n=8 for stroke.
The less-affected forepaw was also assessed (Figs. 2B and D). Statistical analysis showed a significant difference between the groups in the number of pellets retrieved (F1, 14=5.14; p=0.04); however, this impairment was statistically small (effect size, f=0.14) and minor in comparison to the affected forepaw. Statistical analysis revealed no effect of session or interaction of session×group (F3, 42=0.86; p=0.47 and F3, 42=1.28; p=0.29, respectively), suggesting this impairment in fine motor function by the less-affected forelimb was persistent in stroke rats. No difference was found in the maximum step reached (F1, 14=2.33; p=0.150), indicating that sham and stroke animals maintained a similar level of participation in the behavioural task.
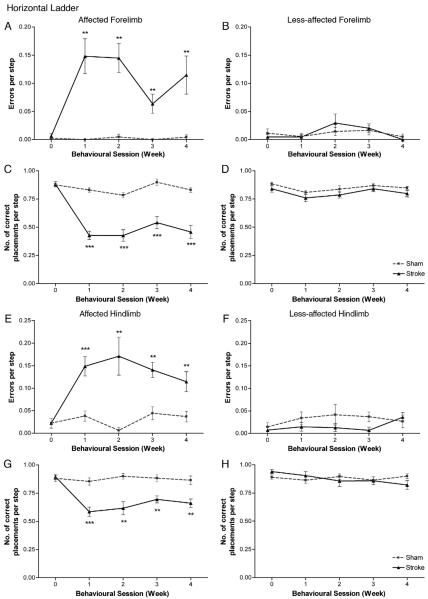
Horizontal ladder
The assessment of footslips in forelimb and hindlimb placement during skilled locomotion was measured as mean errors per step (errors were defined as scores of: 0, total miss; 1, deep slip; and 2, slight slip). One stroke rat had not learned (pre-operatively) to cross the ladder and was excluded from this part of the analysis.
The horizontal ladder revealed that stroke rats made significantly more footslips during placement of the affected limb than sham controls in both forelimb (F1, 13=23.1; p<0.0001); Fig. 3A) and hindlimb (F1, 13=64.6; p<0.0001); Fig. 3E) placement, with this inaccuracy of limb placement persisting over the 28-day testing period (2-tailed t-tests; stroke versus sham; p<0.0001). Statistical analysis also revealed no effect of session but showed an interaction of session×group in forelimb footslips (F3, 39=2.7; p=0.06 and F3, 39=3.3; p=0.03, respectively), however, a comparison of values from post week 1 with 4, revealed no significant difference in stroke rats (paired t-test, p=0.28). Analysis revealed no effect of session or interaction of session×group in the affected hindlimb footslips (F2, 24=0.48; p=0.61 and F2, 24=1.53; p=0.22, respectively). Together, these data confirm that impairments in skilled locomotion were sustained in stroke rats. After stroke, observation revealed that footslips were attributed to either: misplacement of the forepaw thus completely missing the rung; successfully grasping the rung but unable to support their weight resulting in a fall; or inaccurate placement due to unsuccessful grasping of the rung with weight support being on their wrist.
Fig. 3. Horizontal ladder test.
ET-1 stroke produced significant deficits in skilled locomotion, with animals exhibiting more footslip errors for both forelimb (A) and hindlimb (E) on the side contralateral to the stroke lesion compared to sham animals. Lesions also produced significant impairments in correct placement (score 6) with stroke animals unable to grasp the ladder rungs accurately with their affected forelimb (C) and hindlimb (G); these deficits persisted throughout the entire testing period. No significance between groups was observed in either errors per step or correct placement by the less-affected forelimb (B, D) or hindlimb (F, H). Results are presented as mean±SEM and were analysed using RM ANCOVA and independent two-tailed t-tests. Significance between groups is denoted as: **p<0.01; ***p<0.001. n=9 for sham and n=7 for stroke.
No significant difference in footslips during forelimb placement were observed between sham and stroke rats for the less-affected forelimb (F1, 13=0.46; p=0.51; Fig. 3B). Both stroke and sham rats did display footslips during hindlimb placement (Figs. 3E, F), however it was more pronounced and persistent for the affected hindlimb of stroke rats (Fig. 3E). Footslips were not significantly different for the less-affected hindlimb between groups (F1, 13=1.34; p=0.27; Fig. 3F).
We next assessed the mean number of correct placements per step (i.e., score of 6). The horizontal ladder also revealed a deficit in the ability for rats to place their affected forelimb (F1, 13=65.2; p<0.0001; Fig. 3C) and hindlimb (F1, 13=57.3; p<0.0001; Fig. 3G) accurately on the rung after stroke (score 6), with this inaccuracy of limb placement persisting over the 28-day testing period (2-tailed t-tests; stroke versus sham; p<0.0001). Statistical analysis also revealed no effect of session or interaction of session×group in both forelimb (F3, 39=2.32; p=0.09 and F3, 39=0.41; p=0.75, respectively) and hindlimb correct placement (F3, 39=0.37; p=0.77 and F3, 39=1.01; p=0.40, respectively); supporting the conclusion that impairments in skilled limb placement were sustained in stroke rats. Sham animals carried out the majority of their limb placements correctly, and grasped the rungs precisely with all four digits. Observation revealed that failures in correct placement by stroke animals were mainly attributed to an inability in placing their paw directly onto the rung and instead using their wrist, forearm or one/two digits for support (score 5). Analysis of limb placement by the less-affected fore-limb (F1, 13=4.1; p=0.07; Fig. 3D) and hindlimb (F1, 13=4.45; p=0.06; Fig. 3H) revealed no significant differences between groups.
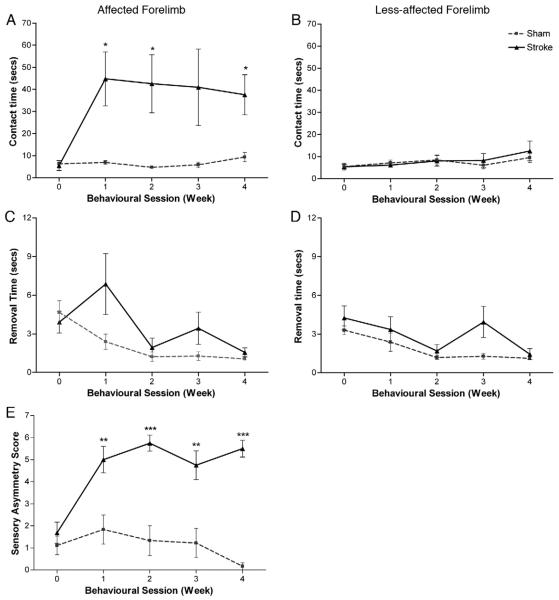
Bilateral tactile stimulation test
Statistical analysis revealed there was an increase in the amount of time taken to both contact (F1, 14=24.0; p<0.0001; Fig. 4A) and remove (F1, 14=14.01; p=0.002; Fig. 4C) the adhesive patches. Sensory responsiveness was significantly impaired in the affected forepaw following ET-1 induced lesions (Fig. 4A), with some animals failing to contact the adhesive patch even beyond the 2 min cut-off point. This sensory impairment persisted during the entire 28-day testing period. Statistical analysis revealed no effect of session or interaction of session×group in either contact (F1, 20=1.23; p=0.30 and F1, 20=0.38; p=0.61, respectively) or removal (F2, 26=0.16; p=0.83 and F2, 26=2.47; p=0.11, respectively) time; confirming that impairments were sustained in stroke rats. The mean time taken to contact the stimulus was 9.4±4.4 s in sham rats compared with 37.6±18.1 s in stroke rats. Regarding removal time the majority of animals removed the adhesive patch 5–10 s after contacting the stimulus. Observation revealed that this was occasionally aided by the support of their less-affected forepaw, by bringing the two paws simultaneously to the mouth rather than one paw by itself. Statistical analysis revealed no significance between groups in either contact (F1, 14=0.14; p=0.71) or removal (F1, 14=2.66; p=0.13) time for the less-affected forepaw, with both sham and stroke rats able to contact and remove the stimulus within similar time frames (Figs. 4B and D).
Fig. 4. Bilateral tactile stimulation test.
ET-1 stroke caused a significant impairment in somatosensory function. Lesions produced deficits in sensory awareness and sensorimotor function, with rats taking longer to contact (A) and remove (C) the adhesive patch from their affected paw compared to sham rats. No significant difference was observed to either contact (B) or remove (D) the adhesive patch from their less-affected paw. ET-1 lesions also caused a significant increase in somatosensory asymmetry (E). All deficits persisted throughout the entire testing period. Results are presented as mean±SEM and were analysed using RM ANCOVA and independent two-tailed t-tests. Significance between groups is denoted as: *p<0.05; **p<0.01; ***p<0.001. n=9 for sham, and n=8 for stroke.
Analysis also revealed a difference in the magnitude of somatosensory asymmetry between groups (F1, 14=47.5; p<0.0001; Fig. 4E). In comparison to shams, stroke animals exhibited a bias to the stimulus on the less-affected forepaw (Fig. 4E) and mostly responded in removing this stimulus first; this also persisted over the entire testing period (2-tailed t-tests; stroke versus sham; p<0.01). Statistical analysis revealed no effect of session or interaction of session×group (F3, 42=0.07; p=0.97 and F3, 42=2.3; p=0.11, respectively), supporting the conclusion that stroke rats had sustained asymmetric impairments in somatosensory function.
Cylinder test
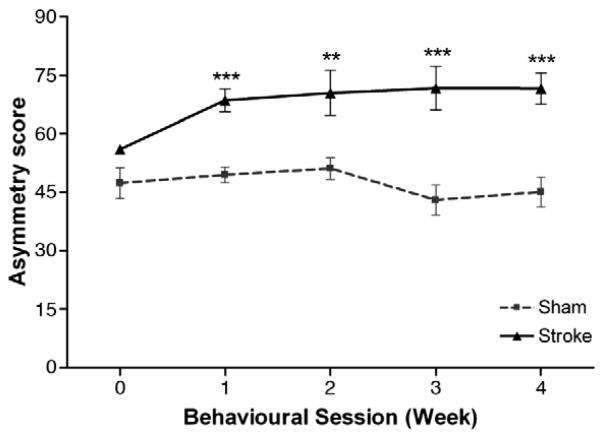
Animals were randomly allocated to groups by paw preference measured using the staircase test; whilst stroke and sham groups seemed different in preoperative performance on the cylinder test, this difference was not significant (F1, 14=2.82; p=0.12). Whilst the mean asymmetry score of sham animals ranged between 43.1±8.2 and 51.2±5.9 during the testing period, stroke animals exhibited an increased asymmetry score (F1, 14=15.7; p=0.001), indicating preferential use of the less-affected forelimb during upright support, which persisted throughout the entire postoperative testing period (2-tailed t-tests; stroke versus sham; p<0.01; Fig. 5). At 28 days post lesion, the asymmetry score was 45.1±8.1 in sham rats in contrast to 71.7±8.0 in stroke rats. Statistical analysis revealed no effect of session or interaction of session×group (F3, 42=2.51; p=0.07 and F3, 42=0.68; p=0.57, respectively), confirming that stroke rats had sustained abnormalities in postural weight support.
Fig. 5. Cylinder test.
Normal rats display equal use in forelimb weight support with an asymmetry score of approximately 50. No significant difference between groups was found prior to surgery. After stroke, rats exhibited an increase in asymmetry indicating preferential use of the less-affected forelimb for support; this persisted throughout the entire testing period. Results are presented as mean±SEM and were analysed using RM ANCOVA and independent two-tailed t-tests. Significance between groups is denoted as: **p<0.01; ***p<0.001. n=9 for sham and n=8 for stroke.
Effect sizes and sample size calculations for future experiments
The effect size of the difference between the stroke and sham groups was calculated for each behavioural test. In all cases, effect sizes were large (f>0.6, Table 1). For example, stroke in aged rats had a very large effect (f=1.7) on correct placement of the affected paw on the horizontal ladder test. We also estimated the minimum sample sizes that would be required in future experiments using two groups (control vs. treatment) to detect treatment effects of three different magnitudes (25% recovery, 50% recovery, 75% recovery) using each of these tests and allowing for a type I error rate of 0.05 and power of 0.80. For example, a relatively small total number of rats per group (4 to 8; Table 1) would be required to detect the effects of a treatment which induced a 75% recovery on a given test. The test which would require the largest numbers of rats to detect a given treatment effect is the sticky patch test (contact or removal time), although the sticky patch asymmetry test required a reasonable number of rats (Table 1). The test which would require the fewest number of rats is the horizontal ladder assessment of correct forelimb placement, which would require 4 rats per group for a treatment that induced 75% recovery, 7 rats for a 50% recovery and 20 rats for a 25% recovery.
Table 1.
Calculations of sample sizes per group for hypothetical future experiments and of effect sizes for each behavioural test.
| Behavioural test | Effect size (f) between groups (sham vs. stroke) |
Number of rats required per group to detect the following improvement in performance on the given task |
||
|---|---|---|---|---|
| 75% | 50% | 25% | ||
| Cylinder | 1.04 | 7 | 14 | 52 |
| Horizontal, ladder, affected forelimb (errors per step) | 1.26 | 5 | 10 | 35 |
| Horizontal, ladder, affected hindlimb (errors per step) | 1.03 | 6 | 11 | 38 |
| Horizontal, ladder, affected forelimb (correct placement) | 1.69 | 4 | 7 | 20 |
| Horizontal, ladder, affected hindlimb (correct placement) |
1.12 | 6 | 11 | 44 |
| Sticky patch (asymmetry) | 1.77 | 5 | 9 | 33 |
| Sticky patch (sense time) | 0.87 | 7 | 15 | 54 |
| Sticky patch (removal time) | 0.78 | 8 | 16 | 61 |
| Staircase test (pellets retrieved) | 1.48 | 5 | 8 | 28 |
| Staircase test (maximum step reached) | 1.46 | 5 | 8 | 27 |
Distribution and survival of corticospinal neurons (CSN)
To distinguish CSNs from other layer V neurons in the cortex animals were retrogradely labelled with Fast Blue, which was injected bilaterally into the corticospinal tract at mid-cervical level of the spinal cord. These injections resulted in symmetrical labelling of CSNs on both sides of the cortex in shams (data not shown). Labelled CSNs were confined to layer V and were widely distributed within motor and somatosensory cortical areas, where they typically formed a continuous band of neurons (Figs. 6A, F, K). The number and distribution of CSNs throughout the entire sensorimotor cortex in aged rats after cervical tracer injections was assessed (Fig. 6P). In sham animals, both hemispheres show an equal topographical distribution of CSNs. In contrast, stroke animals show a considerable loss of CSNs in the ipsilesional hemisphere (F3, 8=49.8; p<0.0001; Figs. 6A–O).
Fig. 6. Surviving corticospinal neurons after ET-1 stroke.
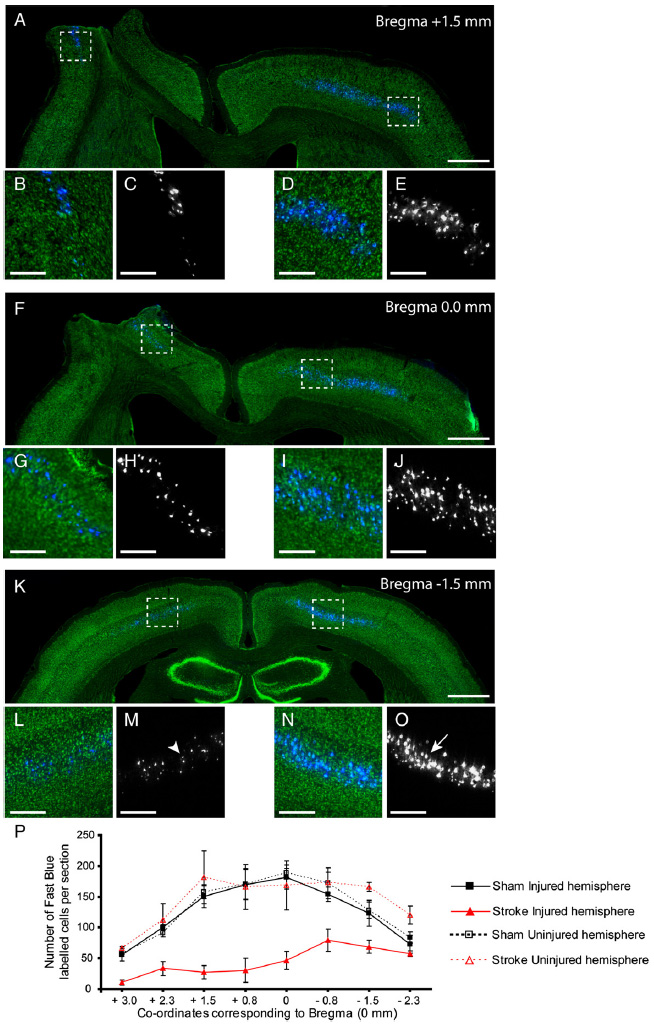
Representative low (A, F, K) and high (B–E, G–J, L–O) power images of coronal sections through the brain immunostained for Nissl substance (Green). Sections reveal a substantial loss of retrogradely labelled CSNs (blue; black and white) in the injured (left) hemisphere in comparison to the uninjured (right) hemisphere 5 weeks after stroke. High power magnification highlights the striking difference between the severely atrophied somata (L, M, indicated by arrowhead) in the ipsilesional hemisphere and large healthy somata in the contralesional hemisphere (N, O, indicated by arrow). The distribution of CSNs in both hemispheres of sham and stroke rats revealed the extent of damage within the entire sensorimotor cortex after ET-1 induced stroke (P). Scale bars: 1 mm (A, F, K), 400 μm(B–E, G–J, L–O).
Rostral to bregma, CSNs labelled in the ipsilesional hemisphere were sparse in comparison to the contralesional hemisphere, with the majority of CSNs lost from the primary (M1) and secondary (M2) motor cortex and primary somatosensory cortex (S1, particularly the forelimb (FL) and hindlimb (HL) region (Paxinos and Watson, 2005). Caudal sections possessed more surviving CSNs than rostral areas yet these cells displayed a degree of cell atrophy, with the somata of many appearing shrunken in contrast to the CSNs in the contralesional hemisphere (Figs. 6K–O).
Discussion
The objective of the present study was to characterize a unilateral stroke model which uses ET-1 to establish robust functional disabilities in aged rats. We selected a number of behavioural tests to evaluate the effects of unilateral sensorimotor cortical stroke on both motor and sensory function.
The rats included in the present study were 22 months old when stroke was induced which is in the range relevant to the clinical stroke population, as aging represents a well-known risk factor for stroke (Kelly-Hayes et al., 2003). The animals were old enough to participate in behavioural training and tasks but not so old that they possessed severe age-related functional deficits prior to surgery.
The results from the present study reveal that an alternative rodent model of stroke using ET-1 can be developed in older rats, with large and significant, sustained behavioural deficits as well as reduced mortality rates. Two major concerns with the use of aged rats are their high price and high mortality rates following surgery in comparison to younger animals. A previous study demonstrated that mortality rates after MCAo were 43.5% in 22- to 24-month-old rats in contrast to 6.3% in 3- to 4-month-old rats (Wang et al., 2003), with Lindner and colleagues (2003) effectively reducing this to 24% in 16- to 19-month-old rats. Our study has successfully provided lower mortality rates of 13.3% in 20- to 23-month-old rats, as well as providing an alternative model of stroke. Another factor leading to high mortality rates are feeding difficulties following surgery that lead to a considerable loss of body weight and deterioration of long-term health (Dittmar et al., 2003). Our study to induce stroke has also successfully displayed minor levels of stress in animals after surgery and demonstrated a consistent body weight 1 week post surgery.
The extent of ischemic damage was revealed from volumetric measures after intracortical ET-1 stroke where an 18.1±7 mm3 increase in ventricular size and a tissue loss of approximately 97.4±17 mm3 was observed in the ipsilesional hemisphere. The latter mean±SEM is comparable to infarct volumes observed in young adult rats after cortical injections of ET-1 (Windle et al., 2006), with both studies having lesions centered around the infusion sites and restricted mainly to the cortex with minor loss of the underlying white matter or striatum.
Our study involved ET-1 being injected into sensorimotor cortical regions, affecting both arterial and venous collaterals (Palacios et al., 1997) serving this area and subsequently producing a stroke to this brain circuitry. Previous studies using a similar lesion technique incorporated young animals (Gilmour et al., 2004; Windle et al., 2006), yet co-ordinates have to be altered in relation to animal size and age (Paxinos et al., 1985). Our study incorporates co-ordinates specially modified for the aged rat brain to induce the significant functional deficits observed. Due to the variations in skull thickness between aged rats (0.8–1.6 mm) and to ensure accurate depth placement of ET-1, a craniotomy was performed. Despite care when removing the bone, slight mechanical injury to the underlying tissue following craniotomy may occur. Previous studies have shown that removal of the skull can produce behavioural and neurochemical asymmetries; however, this is not permanent and persists for only a few days (Adams et al., 1994). We conclude that the sustained behavioural impairments observed in this study are a consequence of ET-1 induced damage.
Histological analysis revealed that the stroke damaged an extensive region of the sensorimotor cortex, resulting in neuronal loss involving a substantial population of CSNs as well as surviving CSNs displaying a degree of atrophy in their cell soma in the ipsilesional hemisphere. The majority of CSNs were lost from M1, M2 and S1 causing a breakdown in fine sensorimotor control, involving deterioration of motor function as well as the processing of sensory feedback (Lemon and Griffiths, 2005). However, the ET-1 lesion may not only affect CSNs but also other neurons in the somatosensory and motor cortices that contribute collaterals to other brain regions. It is known that corticothalamic projections originate from cortical neurons in layer VI (Zhang and Deschenes, 1997), and synapse upon thalamic reticular neurons (Ohara and Lieberman, 1985). The thalamo-corticothalamic network is considered to represent a functional unit for processing sensory information (Alloway et al., 2003; Bourassa et al., 1995; Williams et al., 1994), and thus disrupting this network would lead to impairments in sensory awareness. Damage to this pathway or to corticospinal input to the dorsal horn (which modulates ascending sensory information) may underlie the deficits in somatosensory function within this study. This was shown in the bilateral tactile stimulation test where animals displayed a considerable asymmetrical impairment in the ability to contact and consequently remove the adhesive patch on the forelimb contralateral to the lesion.
A major focus of the present study was to produce ET-1 lesions with good reproducibility and evaluate these impairments using a battery of sensitive behavioural tests. Whilst all models of ischemia show a degree of variability amongst animals, it is important to induce consistent reliable sensorimotor deficits particularly when assessing future therapeutic strategies. The variability of ischemic injury following MCAo is considerably high, particularly in transient focal models. Depending on the duration of MCAo, damage occurs in the frontal, parietal, temporal, and occipital cortex as well as several subcortical structures including the thalamus, striatum and hypothalamus (Garcia et al., 1995; Kanemitsu et al., 2002). Complex deficits are likely to arise due to such widespread damage to these functionally diverse regions and possibly complicate studies involving recovery of specific circuits. Photothrombosis usually induces cortical injury at a consistent region; however Zhao et al. (2005) encountered problems with variability amongst aged rats after inducing focal photothrombotic cortical injury. They reported a 10–20% increase in skull thickness in aged rats, in agreement with our findings, which may have contributed to differences in infarct size. Our model involved ET-1 being administered at specific co-ordinates and demonstrated good reproducibility, with all stroke animals exhibiting similar significant and sustained functional disabilities in the forelimb and hindlimb contralateral to the stroke lesion that failed to recover during the 4-week testing period. Our findings clearly demonstrate that all behavioural tests detected statistically significant deficits in lesioned aged rats in: (1) fine motor control, (2) forelimb and hindlimb placement in skilled locomotion, (3) somatosensory function and (4) forepaw usage for postural weight support, that were persistent throughout the entire testing period. This is in agreement with other studies incorporating this method of intracortical ET-1 infusions in young rats and who also found long lasting impairments (Gilmour et al., 2004; Windle et al., 2006). Impairments on the ipsilateral side were only detected in the staircase test which revealed subtle deficits in fine motor function (pellet retrieval); this was consistent with findings from others (Biernaskie et al., 2005; Gonzalez et al., 2004; Hsu and Jones, 2006) who also reported ipsilesional skilled reaching and movement abnormalities after cortical lesions suggesting a degree of bilateral cortical control.
Analysis of effect sizes in our study showed that each behavioural test detected a very large effect of stroke upon performance. Calculations were also performed to estimate the minimum sample sizes that would be required in future experiments (using one control and treatment group) to identify treatment effects of three different magnitudes (25% recovery, 50% recovery or 75% recovery). For example, to detect a 75% improvement, investigators would require between 4 and 8 rats per group, depending on the test selected.
As with all models of ischemia there are concerns with using ET-1. ET-1 is expressed by astrocytes in response to ischemia (Hama et al., 1997) and may play a role in the ischemic cascade. Therefore the injury may be exacerbated due to astrocytic-induced ET-1 release. Another concern with using ET-1 is that its mechanism of disrupting cerebral blood flow is vessel constriction rather than obstruction and this may make it unsuitable for studying some neuroprotective agents. However, this model could potentially have considerable use for assessing therapeutic strategies designed to promote regeneration and plasticity of spared circuits following ischemic stroke. Our model produces functional disabilities caused by cortical infarction, resembling the severe motor deficits associated with stroke in humans that fail to improve over time.
Conclusion
As far as we are aware, this is the first preclinical study to demonstrate histological and functional outcomes following ET-1 infusions into sensorimotor cortical regions in aged rats. Our model may have a number of potential advantages for studying therapeutic strategies following ET-1 induced stroke in aged rats. Firstly, our results demonstrate that this procedure can produce sustained functional deficits which can be detected using a number of reliable behavioural tests. Secondly, we have ensured low mortality rates in addition to improving animal welfare. These are two of the most important current issues with concern to the use of aged rats. Thirdly, our stroke is produced at specific co-ordinates providing good reproducibility and reliability compared to other models, with all animals exhibiting long-lasting functional deficits. Consequently, this model makes it a valuable tool for assessing therapeutic strategies to promote regeneration and plasticity following stroke in aged rats.
Acknowledgments
We thank Gary Fulcher for assistance during behavioural testing and Liz Bradbury for comments on this manuscript. This work was supported by the Medical Research Council (grant number G0600998).
References
- Adams FS, Schwarting RK, Huston JP. Behavioral and neurochemical asymmetries following unilateral trephination of the rat skull: is this control operation always appropriate? Physiol. Behav. 1994;55:947–952. doi: 10.1016/0031-9384(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Hoffer ZS, Hoover JE. Quantitative comparisons of corticothalamic topography within the ventrobasal complex and the posterior nucleus of the rodent thalamus. Brain Res. 2003;968:54–68. doi: 10.1016/s0006-8993(02)04265-8. [DOI] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J. Cereb. Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J. Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur. J. Neurosci. 2005;21:989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Pinault D, Deschenes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur. J. Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Slivka A, Xue D. The effect of the NMDA receptor antagonist MK-801 on cerebral blood flow and infarct volume in experimental focal stroke. Brain Res. 1992;574:171–177. doi: 10.1016/0006-8993(92)90814-p. [DOI] [PubMed] [Google Scholar]
- Buga AM, Sascau M, Pisoschi C, Herndon JG, Kessler C, Popa-Wagner A. The genomic response of the ipsilateral and contralateral cortex to stroke in aged rats. J. Cell. Mol. Med. 2008;12:2731–2753. doi: 10.1111/j.1582-4934.2008.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Eliahoo J, Majeed A, Murad S. Stroke incidence and risk factors in a population-based cohort study. Health Stat. Q. 2001;12:18–26. Ref Type: Online Source. [Google Scholar]
- Cox RH. Age-related changes in arterial wall mechanics and composition of NIA Fischer rats. Mech. Ageing Dev. 1983;23:21–36. doi: 10.1016/0047-6374(83)90096-9. [DOI] [PubMed] [Google Scholar]
- Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- Dittmar M, Spruss T, Schuierer G, Horn M. External carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke. 2003;34:2252–2257. doi: 10.1161/01.STR.0000083625.54851.9A. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fisher M, Ratan R. New perspectives on developing acute stroke therapy. Ann. Neurol. 2003;53:10–20. doi: 10.1002/ana.10407. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Mumert ML, Stowe AM, Nudo RJ. An animal model of capsular infarct: endothelin-1 injections in the rat. Behav. Brain Res. 2006;169:206–211. doi: 10.1016/j.bbr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Futrell N, Garcia JH, Peterson E, Millikan C. Embolic stroke in aged rats. Stroke. 1991;22:1582–1591. doi: 10.1161/01.str.22.12.1582. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Kurosawa N, Cintra A, Hallstrom A, Goiny M, Rosen L, Agnati LF, Ungerstedt U. Involvement of local ischemia in endothelin-1 induced lesions of the neostriatum of the anaesthetized rat. Exp. Brain Res. 1992;88:131–139. doi: 10.1007/BF02259134. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Bjelke B, Andbjer B, Grahn H, Rimondini R, Agnati LF. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia. NeuroReport. 1997;8:2623–2629. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Ho KL. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke. 1995;26:636–642. doi: 10.1161/01.str.26.4.636. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Iversen SD, O'Neill MF, Bannerman DM. The effects of intracortical endothelin-1 injections on skilled forelimb use: implications for modelling recovery of function after stroke. Behav. Brain Res. 2004;150:171–183. doi: 10.1016/j.bbr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur. J. Neurosci. 2004;20:3442–3452. doi: 10.1111/j.1460-9568.2004.03751.x. [DOI] [PubMed] [Google Scholar]
- Gross PM, Weaver DF. A new experimental model of epilepsy based on the intraventricular injection of endothelin. J. Cardiovasc. Pharmacol. 1993;22(Suppl. 8):S282–S287. doi: 10.1097/00005344-199322008-00074. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Wilson JX, Smith KE, Cechetto DF. Effect of age on autonomic and cardiac responses in a rat stroke model. Arch. Neurol. 1992;49:690–696. doi: 10.1001/archneur.1992.00530310032009. [DOI] [PubMed] [Google Scholar]
- Hama H, Kasuya Y, Sakurai T, Yamada G, Suzuki N, Masaki T, Goto K. Role of endothelin-1 in astrocyte responses after acute brain damage. J. Neurosci. Res. 1997;47:590–602. doi: 10.1002/(sici)1097-4547(19970315)47:6<590::aid-jnr4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur. J. Neurosci. 2005;22:2069–2080. doi: 10.1111/j.1460-9568.2005.04370.x. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp. Neurol. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kanemitsu H, Nakagomi T, Tamura A, Tsuchiya T, Kono G, Sano K. Differences in the extent of primary ischemic damage between middle cerebral artery coagulation and intraluminal occlusion models. J. Cereb. Blood Flow Metab. 2002;22:1196–1204. doi: 10.1097/01.wcb.0000037992.07114.95. [DOI] [PubMed] [Google Scholar]
- Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J. Stroke Cerebrovasc. Dis. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Kinsella KG. Future longevity—demographic concerns and consequences. J. Am. Geriatr. Soc. 2005;53:S299–S303. doi: 10.1111/j.1532-5415.2005.53494.x. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization. Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Li S, Carmichael ST. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol. Dis. 2006;23:362–373. doi: 10.1016/j.nbd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Gribkoff VK, Donlan NA, Jones TA. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J. Neurosci. 2003;23:10913–10922. doi: 10.1523/JNEUROSCI.23-34-10913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus TM, Tsai SY, Bollnow MR, Farrer RG, O'Brien TE, Kindler-Baumann DR, Rausch M, Rudin M, Wiessner C, Mir AK, Schwab ME, Kartje GL. Recovery and brain reorganization after stroke in adult and aged rats. Ann. Neurol. 2005;58:950–953. doi: 10.1002/ana.20676. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Millikan C. Animal stroke models. Stroke. 1992;23:795–797. doi: 10.1161/01.str.23.6.795. [DOI] [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Lieberman AR. The thalamic reticular nucleus of the adult rat: experimental anatomical studies. J. Neurocytol. 1985;14:365–411. doi: 10.1007/BF01217752. [DOI] [PubMed] [Google Scholar]
- Palacios B, Lim SL, Pang CC. Effects of endothelin-1 on arterial and venous resistances in anaesthetized rats. Eur. J. Pharmacol. 1997;327:183–188. doi: 10.1016/s0014-2999(97)89659-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th Ed. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J. Neurosci. Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ. Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav. Neurosci. 1984;98:518–540. doi: 10.1037//0735-7044.98.3.518. [DOI] [PubMed] [Google Scholar]
- Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, Vaughn D, Wilcox RE. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol. Biochem. Behav. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Butcher SP. Characterisation of an experimental model of stroke produced by intracerebral microinjection of endothelin-1 adjacent to the rat middle cerebral artery. J. Neurosci. Methods. 1995;60:125–131. doi: 10.1016/0165-0270(95)00003-d. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Butcher SP, Kelly JS. Endothelin-1 induced middle cerebral artery occlusion: pathological consequences and neuroprotective effects of MK801. J. Auton. Nerv. Syst. 1994;49(Suppl):S177–S185. doi: 10.1016/0165-1838(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Starkey ML, Barritt AW, Yip PK, Davies M, Hamers FP, McMahon SB, Bradbury EJ. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp. Neurol. 2005;195:524–539. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27:1663–1667. doi: 10.1161/01.str.27.9.1663. [DOI] [PubMed] [Google Scholar]
- Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. J. Neurosci. Methods. 2009;181:18–26. doi: 10.1016/j.jneumeth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Futrell N, Wang DZ, Chen FJ, Zhai QH, Schultz LR. A reproducible model of middle cerebral infarcts, compatible with long-term survival, in aged rats. Stroke. 1995;26:2087–2090. doi: 10.1161/01.str.26.11.2087. [DOI] [PubMed] [Google Scholar]
- Wang RY, Wang PS, Yang YR. Effect of age in rats following middle cerebral artery occlusion. Gerontology. 2003;49:27–32. doi: 10.1159/000066505. [DOI] [PubMed] [Google Scholar]
- Ward NS. Plasticity and the functional reorganization of the human brain. Int. J. Psychophysiol. 2005;58:158–161. doi: 10.1016/j.ijpsycho.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Williams MN, Zahm DS, Jacquin MF. Differential foci and synaptic organization of the principal and spinal trigeminal projections to the thalamus in the rat. Eur. J. Neurosci. 1994;6:429–453. doi: 10.1111/j.1460-9568.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, Corbett D. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp. Neurol. 2006;201:324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Yager JY, Wright S, Armstrong EA, Jahraus CM, Saucier DM. The influence of aging on recovery following ischemic brain damage. Behav. Brain Res. 2006;173:171–180. doi: 10.1016/j.bbr.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J. Hypertens., Suppl. 1988;6:S188–S191. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Deschenes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J. Neurosci. 1997;17:6365–6379. doi: 10.1523/JNEUROSCI.17-16-06365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen J, Li Y, Zhang ZG, Chopp M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J. Neurol. Sci. 2000;174:141–146. doi: 10.1016/s0022-510x(00)00268-9. [DOI] [PubMed] [Google Scholar]
- Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Effect of cholinergic medication, before and after focal photothrombotic ischemic cortical injury, on histological and functional outcome in aged and young adult rats. Behav. Brain Res. 2005;156:85–94. doi: 10.1016/j.bbr.2004.05.011. [DOI] [PubMed] [Google Scholar]