Abstract
The brain is the key organ of stress reactivity, coping, and recovery processes. Within the brain, a distributed neural circuitry determines what is threatening and thus stressful to the individual. Instrumental brain systems of this circuitry include the hippocampus, amygdala, and areas of the prefrontal cortex. Together, these systems regulate physiological and behavioral stress processes, which can be adaptive in the short-term and maladaptive in the long-term. Importantly, such stress processes arise from bidirectional patterns of communication between the brain and the autonomic, cardiovascular, and immune systems via neural and endocrine mechanisms underpinning cognition, experience, and behavior. In one respect, these bidirectional stress mechanisms are protective in that they promote short-term adaptation (allostasis). In another respect, however, these stress mechanisms can lead to a long-term dysregulation of allostasis in that they promote maladaptive wear-and-tear on the body and brain under chronically stressful conditions (allostatic load), compromising stress resiliency and health. This review focuses specifically on the links between stress-related processes embedded within the social environment and embodied within the brain, which is viewed as the central mediator and target of allostasis and allostatic load.
Keywords: allostasis, allostatic load, amygdala, autonomic nervous system, hippocampus, hypothalamic-pituitary-adrenal axis, immune system, neuroplasticity, prefrontal cortex, socioeconomic status, stress
Introduction
It is well established that life stress can presage ill health among vulnerable individuals.1 This stress-related vulnerability is determined by genetic, biobehavioral, and environmental factors that interact over the lifespan to influence individual risk trajectories, particularly through neurobiological pathways. Conventionally, stress is defined as a transactional process arising from real or perceived environmental demands that can be appraised as threatening or benign, depending on the availability of adaptive coping resources to an individual.2,3 In extension, the biological, behavioral, and social coping responses that ensue from stress perception and appraisal processes are held to specifically influence risk for and resilience against ill health.1,4,5 These stress processes impacting health can be heuristically labeled as “good,” “tolerable,” and “toxic”—depending on the degree to which an individual has control over a given stressor and has support systems and resources in place for handling a given stressor over the lifespan.6,7 For example, overcoming some stressful experiences can lead to growth, adaptation, and beneficial forms of learning that promote future resiliency. Other stressful experiences, however, can lead to a proliferation of interacting behavioral, cognitive, physiological, and neural changes that promote vulnerability to ill health.
The brain is a primary mediator and target of stress resiliency and vulnerability processes because it determines what is threatening and because it regulates the behavioral and physiological responses to a given stressor. The hippocampus, a particular brain system supporting memory and mood, was the first area besides the hypothalamus to be recognized specifically as a target of stress hormones.8 Importantly, stressful experiences and associated changes in the release of stress hormones produce both adaptive and maladaptive effects on the hippocampus, hypothalamus, and other brain regions throughout life.5 For example, the amygdala (important for detecting and responding to threats in the environment) and areas of the prefrontal cortex (important for decision making and regulating emotions, impulsivity, and autonomic and neuroendocrine function) are also targets of stress processes.
As reviewed here, early maltreatment, conflict-laden familial relationships, stressful life events, and adverse physical and social conditions—often occasioned by lower socioeconomic environments—during development and aging can influence the structural and functional plasticity of the hippocampus, amygdala, and prefrontal cortex—processes collectively referred to as neuroplasticity. In turn, alterations in the neuroplasticity of these brain systems can affect patterns of emotional expression and regulation, stress reactivity, recovery, and coping, and perhaps even the rate of bodily aging (see further).
Critically, however, the effects of stress on the brain do not necessarily constitute permanent “damage” per se and are amenable to recovery, preventative strategies, and interventions that include pharmaceutical agents and lifestyle factors (e.g., exercise, dietary changes, and social support). Hence, because stress processes—particularly those that unfold in social environments—have powerful effects through the brain on the body, all public and private sector social policies will necessarily affect mental and physical health. As such, these policies can be considered as top-down intervention efforts to affect neuroplasticity and stress resiliency. In the following sections, we review emerging translational animal and human studies explicating the neurobiological pathways potentially linking stress-related processes and health. We note that this review is presented within the context of a conceptual framework and processes emphasizing the brain as the central mediator and target of two neurobiological processes. Key concepts include:
Allostasis, defined as a dynamic regulatory process wherein homeostatic control is maintained by an active process of adaptation during exposure to physical and behavioral stressors, and
Allostatic load, defined as the consequence of allodynamic regulatory wear-and-tear on the body and brain promoting ill health, involving not only the consequences of stressful experiences themselves, but also the alterations in lifestyle that result from a state of chronic stress.
Throughout, this review emphasizes a life course perspective—wherein the effects of early caregiving, maltreatment, and stressors encountered during development and aging are viewed as holding the potential to modify neuroplasticity andstress resiliency both in the short term and over the long term. Further, we will emphasize the brain as the central mediator of stress processes, insofar as distributed brain networks encode, filter, and store environmental information according to unique personal histories and life experiences to determine what is threatening and thus “stressful” to the individual. Moreover, we will emphasize the brain as the instrumental organ for regulating biological, behavioral, and social responses that are influenced by short-term (acute) and long-term (chronic) stress processes. Finally, we will emphasize the brain as a central target of stress processes, insofar as stressful experiences affect neuroplasticity through nonlinear feedforward and feedback mechanisms linking the central and peripheral nervous systems.
Complimenting other contributions to this volume, we will review the limited, but growing, evidence on the putative neurobiological pathways possibly linking socioeconomic status (SES) and health through such stress-related processes. This evidence is largely derived from the study of animal models that permit identifying stress mechanisms at the cellular level, as well as studying stress-related processes that unfold over the entire lifespan. These animal models are critical in that they permit causal inferences and in that they inform translational human experimental, epidemiological, and clinical intervention research. In addition, we review human neurobiological and neuroimaging studies of stress reactivity and the impact of SES on brain functionality and morphology.
Socioeconomic status, health, and stress-related processes center on the brain
There is cumulative evidence reviewed elsewhere in this volume that disparities in income, education, occupation, and other dimensions of SES account for appreciable variance in all-cause and disease-specific morbidity and mortality rates, as well as the prevalence of risk factors for chronic medical conditions9–11 and prevalent psychopathologies of mood and substance abuse.12,13 That health and longevity track a socioeconomic gradient cannot be explained entirely by material deprivation, illiteracy, or restricted availability of quality health care among those occupying a lower socioeconomic position.9,14,15 Hence, several conceptual models of SES-related health disparities posit that life experiences inherent to socioeconomic position at the individual, familial, and community levels could influence well-being and disease risk through stress-related pathways.9,14,16,17 For example, the chronic experience of low SES at the individual level could involve enduring financial hardships, a sense of insecurity regarding future prosperity, and the possible demoralizing feelings of marginalization or social exclusion attributable to comparative social, occupational, or material disadvantage. Further, an individual's perception of her or his relative standing or ranking in a social hierarchy, formally termed subjective social status, may affect an individual's pattern of emotional, behavioral and physiological reactivity to and recovery from life stressors, consequently impacting risk for ill health.18–23
As reviewed further, these stress-related processes are mediated by and feedback to the brain, impacting its abilities to regulate peripheral physiology, engage in adaptive social and health behaviors, experience and control emotions, and support cognitive functioning. Hence, a person who develops, matures, and ages in a low socioeconomic position could become vulnerable to impairments in the functionality of stress regulatory systems of the brain and body important for health. Critically, such stress-related processes may unfold not only at the individual level, but also at the level of families and residential areas. For example, children who develop in lower SES households, in addition to being exposed to toxic substances and excessive noise and temperature variations, are more likely to live in unfavorable housing conditions and to be exposed to what have been termed “risky family” dynamics, characterized by conflict-laden relationships, aggressive and harsh parenting, and other forms of early life stress which may alter risk trajectories for ill health in later life.24 Finally, individuals living in low SES neighborhoods may be more frequently exposed to stressful life events25,26 in association with higher concerns over community crime, pollution, and crowding,27 as well as unstable, effortful, and unrewarding employment opportunities related to persistent economic hardship (see Diez-Roux, this volume).
Yet despite epidemiological and population-based evidence linking low SES with health via purported stress processes, little is known about the neurobiological pathways linking stress and health in the context of SES. Next, we review available animal and human studies potentially bearing on this issue, focusing specifically on those brain systems instrumental for stress regulatory processes. Importantly, from a multilevel and translational perspective, the stress-related neurobiological pathways documented by these animal and human studies may be modifiable by interventions at the individual and population levels, and some of these will be discussed at the end of this chapter.
Protective and damaging effects of neurobiological stress processes
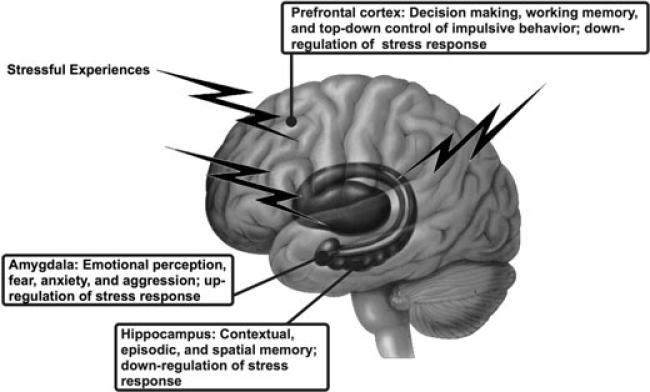
To the extent that low SES is a potential source of life stress associated with ill health, then the brain systems linking SES-related stress processes to health most plausibly include limbic brain areas that jointly (i) support social and emotional information processing; (ii) regulate neuroendocrine, immune, autonomic nervous system functions involved in both adaptation and pathophysiology, as embodied in the concepts of allostasis and allostatic load; and (iii) express well-characterized forms of neuroplasticity in association with conditions of chronic and acute stress in nonhuman animal models. Although several limbic areas meet one or more of these criteria, cumulative translational evidence from animal and human studies reviewed below implicates three in particular: the hippocampus, amygdala, and subdivisions of the prefrontal cortex (see Fig. 1). Next, we provide an overview of the role of these brain areas in their dual control of visceral, cognitive, and emotional processes after summarizing the concepts of allostasis and allostatic load.
Figure 1.
Schematic illustration of the location and key functions of limbic brain areas that play an integrated role in cognitive, emotional, and visceral control processes important for allostasis, allostatic load, and stress responding. Each of the three brain areas is discussed in detail in the text in relation to both animal model studies that focus on what happens at the cellular and molecular levels and studies on the human brain using functional and structural imaging and neuropsychological and neuroendocrine assessments.
Stress, allostasis, and allostatic load
The brain not only processes inputs from the external environment, but also controls adjustments of the body engendered by behavioral states like waking, sleeping, lying, standing, and exercising. These bodily adjustments promote adaptive activities, such as locomotion, and coping with aversive situations and discrete stimuli, such as noise, crowding, hunger, excessive heat or cold, and other threats to safety. Systems promoting adaptation include the hypothalamic-pituitary-adrenal (HPA) axis; the autonomic nervous system; the metabolic system (including the thyroid axis, insulin, other metabolic hormones); the gut; the kidneys; and the immune system (including the regulated network of cytokine producing cells throughout the body). The biomediators of these systems (e.g., cortisol, sympathetic and parasympathetic transmitters, cytokines, metabolic hormones) operate as a nonlinear, interactive network in which mediators down- and up-regulate each other, depending on such factors as concentration, location in the body, and sequential temporal patterning.28 Importantly, the activity of these mediating systems and mediators are closely coupled to the psychological and genetic make-up, developmental history, and behavioral state of the individual.
Adversity, including interpersonal conflicts, social instability, and other stressful experiences, can accelerate pathophysiological processes through adaptive systems of the body, increasing vulnerability for higher morbidity and mortality rates at the population level. For example, the cardiovascular system is one of the most susceptible systems to stress. Hence, blood pressure increases are sensitive to job stress in factory workers, in employees with repetitive jobs and time pressures,29 and in British civil servants of departments undergoing privatization.30 As further evidence, cardiovascular disease is a primary reason for the increased death rate in Eastern Europe amidst the social collapse after the fall of communism.31 Finally, it is noteworthy that otherwise adaptive and brain-mediated stressor-evoked blood pressure surges have been linked to accelerated atherosclerosis,32 as well as increased risk for myocardial infarction (MI).33,34 Besides the cardiovascular system, there are indications that metabolic disorders and abdominal obesity—contributors to cardiovascular disease—are increased at the lower end of the socioeconomic gradient in Swedish males35 and in the British Civil Service.36 Finally, there is growing epidemiological evidence that impaired immune system function is also a likely target of stress processes within the context of socioeconomic position.19,37–42
Stress-related processes impacting health within the context of SES can be viewed and understood by appreciating the marked differences individuals show in response to adverse acute and chronic stressors. In other words, individuals respond in different ways to adversity and threats (real or implied) to their safety and homeostasis. As also discussed in the chapter by Seeman et al (this volume), physiological responses of the autonomic nervous system, HPA axis, cardiovascular, metabolic and immune systems lead to protection and adaptation of the organism to these challenges. This process, referred to as allostasis,43 is an essential component of maintaining homeostasis. However, adaptation to adversity has a price, and the cost of adaptation has been labeled as allostatic load.44,45 Hence, allostatic load is the wear-and-tear on the body and brain resulting from chronic dysregulation (i.e., over-activity or inactivity) of physiological systems that are normally involved in adaptation to environmental challenge. While it is true that physiological parameters like blood oxygen and pH are maintained in a narrow range (homeostasis), the cardiovascular system, metabolic machinery, immune system and central nervous system all show a large range of activity as a function of the time of day and in response to external and internal demands (allostasis).
Mediators of allostasis, therefore, facilitate adaptation whereas the parameters associated with homeostasis do not vary as a means of promoting adaptation. Importantly, such variation in parameters associated with adaptation has long been appreciated, particularly beginning with the early work of Walter Cannon.46 Allostatic systems are involved in coping and adaptation, and generally, they are most useful when they can be rapidly mobilized and terminated when not needed. It is when they are prolonged or not terminated promptly that these systems undermine health. Moreover, the inability to engage allostatic systems when needed also produces a load on the body, because the normal protection afforded by these systems is lacking.
An important aspect of allostasis and allostatic load is the notion of anticipation. Although originally introduced in relation to explaining the reflex that prevents us from blacking out when we get out of bed in the morning,43 anticipation also implies psychological states, such as apprehension, worry, and anxiety, as well as cognitive preparation for a coming event. Because anticipation can drive the output of allostatic biomediators (this is particularly true of hormones like ACTH, cortisol, and adrenalin), it is likely that states of prolonged anxiety and anticipation can theoretically result in allostatic load.47
Other important aspects of individual responses in relation to allostasis and allostatic load are health damaging and health promoting behaviors, such as smoking, drinking, sleeping, eating a prudent diet, and regularly exercising, collectively called “lifestyle” behaviors. These may be embodied within the overall notion of allostasis—i.e., how individuals cope with a challenge – and they also contribute in some ways to allostatic load (e.g., a Western (high-fat) diet accelerates atherosclerosis and progression to Type II diabetes; smoking accelerates atherogenesis; exercise and restorative sleep promote cognitive functioning and health28).
Within the framework presented here and detailed elsewhere, there are four types of physiological response that may contribute to and reflect allostatic load. The first type is related to frequent stressors, for example, blood pressure surges that not only trigger MI in susceptible individuals, but accelerate atherosclerosis and prime the risk for MI when they are supposedly repeatedly expressed over the lifespan. Here, it is the frequency and intensity of the “hits” or events (e.g., large blood pressure surges) that determines the level of allostatic load engendered by this type. Although, frequent stress may lead into the other types described below as the body responds to repeated events by either failing to terminate neural and endocrine responses or failing to respond adequately.
The second type of allostatic load involves a failure to habituate to repetition of the same stressor, leading to a persistent elevation of mediators like cortisol. This was first described for a subset of individuals in a repeated public speaking challenge who failed to habituate their cortisol response.48 Later studies have shown that these individuals have low self esteem and a smaller hippocampus, stress-related behavioral, and neurobiological processes discussed later.49,50
The third type of allostatic load involves failure to terminate adaptive autonomic and neuroendocrine responses. Consider, for example, blood pressure elevations in repetitive, time pressured work51 and the fact that chronic, elevated levels of glucocorticoids accelerate obesity and Type II diabetes. Moreover, we note below that persistent glucocorticoid elevation and/or excitatory activity in brain systems regulating glucocorticoid secretion causes dendritic remodeling and neuronal death in the hippocampus and other limbic brain areas.
The fourth type of allostatic load is the failure to respond adequately to a challenge. Consider, for example, autoimmunity and inflammation that is associated with inadequate endogenous glucocorticoid responses, as in the Lewis rat52 and possibly also in chronic fatigue syndrome and fibromyalgia.53–55 Here, other biomediators of allostatic systems—such as inflammatory cytokines—show elevated activity, and this may increase allostatic load because of inadequate HPA regulation, which normally “constrains” their activity. Post-traumatic stress is also a form of psychopathology that is yet another example of how an acute, but traumatic event, leads to dysregulated HPA axis activity that may not respond adequately to acute challenge and promote comorbid physical disease.56
Joint roles of amygdala, hippocampus, and prefrontal cortex in visceral functions
The hippocampus and amygdala are limbic brain structures that process experiences by interfacing with lower vegetative brain areas, such as the hypothalamus and brainstem, and higher cortical areas, particularly within the prefrontal cortex. They also help to interpret, on the basis of current and past experiences, whether an event is threatening or otherwise stressful—thus influencing allostatic responses. The amygdala is an essential neural component of the memory system for fearful and emotionally laden events, whereas the hippocampus supports determining the context in which such events take place, as well as other aspects of episodic and declarative memory.57–59 For example, whereas lesions to the central or lateral amygdala abolish conditioning of the freezing response of an animal to a tone paired with a shock, hippocampal lesions have no such effects. On the other hand, hippocampal lesions abolish conditioning of the freezing response to the “context,” i.e., to the environment of a particular conditioning chamber.58
As illustrated in Figure 1, the amygdala and hippocampus are linked to each other anatomically and functionally.60–62 For example, lesions of the basolateral amygdaloid nucleus reduce long-term potentiation—a process underpinning memory—in the hippocampal dentate gyrus and stimulation of this nucleus facilitates dentate gyrus long-term potentiation.63,64 The hippocampus and amygdala also regulate the HPA axis, with the hippocampus in general being inhibitory and the amygdala being excitatory.62,65–67 However, this statement oversimplifies a great deal of complexity. For example, within the hippocampus, certain sites respond to electrical stimulation by increasing HPA activity.68 Moreover, other brain areas are involved. For example, a recent brain lesion and steroid implant study—as well as emerging neuroimaging evidence reviewed below—indicate that the medial prefrontal cortex (mPFC) plays an important role in constraining the HPA axis under stress-related conditions.69
Further, glucocorticoid implants into the mPFC reduce the magnitude of the HPA response to stress, and they reduce plasma insulin levels in rodents.69 In contrast, lesions of the dorsal and ventral areas of the prefrontal cortex differentially impair regulation of the HPA stress response via circuitry with the hypothalamus.70,71 Among other implications, these findings point to the important role of steroid feedback to the brain in the control of HPA activity, particularly to sites outside of the hippocampus and hypothalamus. It is important to note that the HPA axis is dynamically regulated, and that steroid feedback operates at several levels in relation to neural control of the turning on and shutting off of the stress response.72,73 Besides rate-sensitive and level-sensitive feedback, delayed feedback may be viewed as both a thermostat (steroid elevation turning down ACTH release) and a modulation by neural activity, which can be inhibitory (perhaps via the GABA system), as well as excitatory upon hypothalamic paraventricular nucleus (PVN) neurons.67 Further, the bed nucleus of the stria terminalis—a basal forebrain structure involved in many motivational and stress-related processes—is reported to have both inhibitory and excitatory pathways to the PVN that regulate limbic system inputs to the HPA axis.74 The demonstration that constant steroid feedback via corticosterone pellets implanted into adrenalectomized (ADX) rats normalizes ACTH levels, but allows for sustained ACTH secretion after stress, highlights the importance of neural control in the allostatic shut-off of the HPA stress response.72,73 The fact that in the same study, diurnal exposure to CORT in the drinking water also normalized ACTH levels in ADX rats but allowed for a more rapid termination of the HPA stress response, even when no steroid was present, further highlights the importance of understanding the role of diurnally varying levels of adrenal steroids in priming neural mechanisms subserving a shut-off of the HPA axis.72,73 A further aspect of feedback regulation of HPA function is the ability of energy sources, such as sucrose, to reduce ACTH secretion independently of adrenal steroids.75 We shall now examine the roles of hippocampus, amygdala and prefrontal cortex in cognitive functions and emotional regulation, particularly as they relate to allostatic processes mediated by and targeting the brain.
Brain systems mediating allostatic processes
As reviewed earlier, the hippocampus, amygdala, and prefrontal cortex are anatomically networked components of a neural circuitry that coordinates behavior with neuroendocrine, immune, and autonomic functions in the service of adaptively coping with environmental and psychosocial challenges. In the following sections, we review translational animal and human studies focusing on these areas, particularly within the context of their importance for mediating allodynamic processes important for health. To establish a context for this review, we present a conceptual model in Figure 2 that embodies the concepts of allostasis and allostatic load as mediated by and impacting brain systems important for stress regulation. Importantly, this model highlights specific stress-related dimensions of SES potentially linked to risk for ill health. The following discussion is accompanied by summary sections for those readers who do not want to read the details.
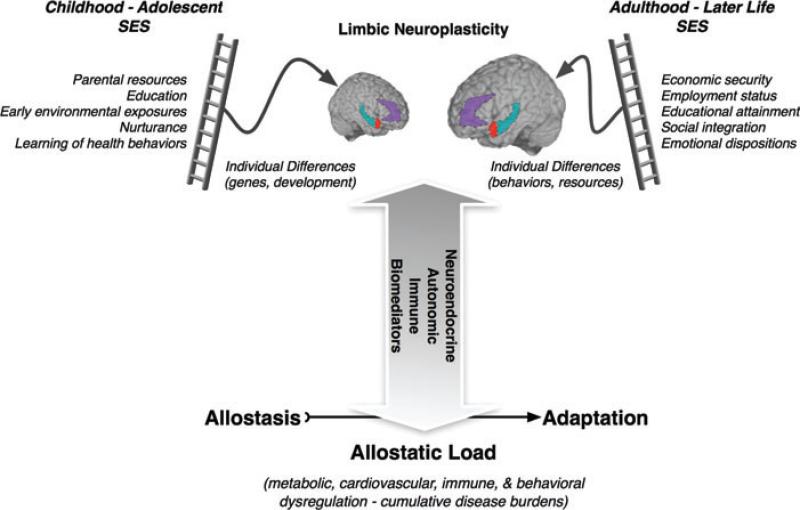
Figure 2.
Neurobiological pathways of SES and allostatic load. A heuristic schematic illustrating the potential neurobiological pathways by which psychosocial factors related to SES may impact allostatic control systems underpinning allostatic load and disease risk. In childhood and adolescence, psychosocial factors related to SES and reviewed elsewhere in this volume (e.g., parental resources and education) are likely to interact with genetic and dispositional individual differences to affect the neuroplasticity of limbic brain areas that regulate allostatic control systems. These brain areas include subdivisions of the prefrontal cortex (e.g., the anterior cingulate cortex in purple), hippocampus (in blue-green), and the amygdala (in red). Importantly, these limbic areas regulate neuroendocrine, autonomic, and immune systems, which are involved in the bidirectional allodynamic control of central and peripheral physiology. In adulthood and later life, psychosocial factors related to SES (e.g., meaningful employment and social integration) may similarly interact with individual difference and behavioral lifestyle factors to affect the neuroplasticity and aging of the same limbic systems mediating and targeted by allostatic control systems. To the extent that lower SES adversely affects limbic neuroplasticity via stress-related factors, then the regulation of key allostatic control systems may become impaired, leading to allostatic load on the body and brain and perhaps increased risk for ill health.
Hippocampus and stress processes
Functional neuroanatomy of the hippocampus
The hippocampus is located in the medial temporal lobe and—as reviewed above—plays instrumental roles in learning and remembering declarative and spatial information, processing the contextual aspects of emotional events, and regulating visceral functions, including the HPA axis. Also as summarized earlier, the hippocampus is interconnected with the amygdala and prefrontal cortex. The hippocampus contains receptors for adrenal steroids, and for major metabolic hormones that have functional effects on the hippocampus. Specifically, these biomediators can enhance cognitive processes, affect mood and motivation, and promote excitability and neuroprotection. Yet, these same biomediators can have deleterious effects on the hippocampus under conditions associated with chronic stress and allostatic load.76
Animal model studies of the hippocampus
A number of animal models demonstrate that chronic stressful experiences (e.g., prolonged immobilization, housing in dominance hierarchies, early maternal separation) can remodel hippocampal neurons and result in changes in the gross morphology of the hippocampus. Notably, the hippocampus is one of the most sensitive and malleable regions of the brain, and it is very important for cognitive function. Within the hippocampus, input from the entorhinal cortex to the dentate gyrus is ramified by connections between the dentate gyrus and the CA3 pyramidal neurons. Hence, one granule neuron innervates, on average, 12 CA3 neurons, and each CA3 neuron innervates, on average, 50 other CA3 neurons via axon collaterals, as well as 25 inhibitory cells via other axon collaterals. The net result is a 600-fold amplification of excitation, as well as a 300-fold amplification of inhibition, that provides some degree of control of the system.77
As to why this type of circuitry exists, the dentate gyrus-CA3 system is believed to play a role in the memory of event sequences, although long-term storage of memory occurs in other brain regions.78 But, because the DG-CA3 system is so delicately balanced in its function and vulnerability to damage, there is also adaptive structural plasticity: New neurons continue to be produced in the dentate gyrus throughout adult life, and CA3 pyramidal cells undergo a reversible remodeling of their dendrites in conditions such as hibernation and chronic stress.77,79–81 The role of this plasticity may be to protect against permanent damage. As a result, the hippocampus undergoes a number of allostatic or adaptive changes in response to acute and chronic stress.
One type of change involves replacement of neurons via neurogenesis. The sub-granular layer of the dentate gyrus contains cells that have some properties of astrocytes (e.g., expression of glial fibrillary acidic protein) and which give rise to granule neurons.82,83 After Bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) administration to label DNA of dividing cells, these newly born cells appear as clusters in the inner part of the granule cell layer, where a substantial number will subsequently differentiate into granule neurons within just 7 days. In the adult rat, 9000 new neurons are born per day and survive with a half-life of 28 days.84 There are many hormonal, neurochemical and behavioral modulators of neurogenesis and cell survival in the dentate gyrus, including estradiol, insulin-like growth factor 1 (IGF-1), antidepressants, voluntary exercise, and hippocampal-dependent learning.85–87 With respect to stress, certain types of acute stress and many chronic stressors suppress neurogenesis or cell survival in the dentate gyrus, and the mediators of these inhibitory effects include excitatory amino acids acting via N-methyl-d-aspartic acid (NMDA) receptors and endogenous opioids.88
Another form of neuroplasticity is the remodeling of dendrites in the hippocampus. Chronic restraint stress causes retraction and simplification of dendrites in the CA3 region of the hippocampus.77,89 Such dendritic reorganization is found in both dominant and subordinate rats undergoing adaptation of psychosocial stress in the visible burrow system, which is independent of adrenal size.90 What this particular result emphasizes is that it is not adrenal size or presumed amount of physiological stress per se that determines dendritic remodeling, but a complex set of other interacting factors that modulate neuronal structure. Indeed, in species of mammals that hibernate, dendritic remodeling is a reversible process, and it occurs within hours of the onset of hibernation in European hamsters and ground squirrels. Moreover, it is reversible within hours of wakening of the animals from torpor.79–81,91 This implies that reorganization of the cytoskeleton is taking place rapidly and reversibly and that changes in dendrite length and branching are not “damage” but a form of structural plasticity. Further, in humans, remarkable changes in hippocampal morphology—specifically volumetric changes—have been associated with the extent of expertise about the spatial layout of cities,92,93 further suggesting dynamic experience-dependent neuro-plasticity in the hippocampus.
Regarding the mechanism(s) of structural remodeling, adrenal steroids are important mediators of hippocampal neuroplasticity during repeated stress, and exogenous adrenal steroids can also mediate neuroplasticity in the absence of an external stressor. The mediating role of adrenal steroids depends on interactions with neurochemical systems, including serotonin, GABA and excitatory amino acids.77,94 Perhaps the most important interactions are those with excitatory amino acids such as glutamate. Excitatory amino acids released by the hippocampal mossy fiber pathway play a key role in remodeling the CA3 region of the hippocampus, and regulation of glutamate release by adrenal steroids may play a particularly important role.77,94
Among the consequences of chronic stress, such as prolonged restraint, is the elevation of extracellular glutamate levels, leading to induction of glial glutamate transporters, as well as increased activation of a nuclear transcription factor, the phosphorylated form of cyclic AMP response element binding protein (phosphoCREB).95 Moreover, 21d of chronic restraint stress (21d CRS) depletes clear vesicles from mossy fiber terminals and increases expression of presynaptic proteins involved in vesicle release.96–98 Taken together with the fact that vesicles that remain in the mossy fiber terminal are near active synaptic zones and that there are more mitochondria in the terminals of stressed rats, this suggests that CRS increases the release of glutamate.98
Extracellular molecules also play a role in remodeling and neuroplasticity. Neural cell adhesion molecule (NCAM) and its polysialated-NCAM (PSA-NCAM), as well as L1 are expressed in the dentate gyrus and CA3 region and the expression of both NCAM, L1, and PSA-NCAM are regulated by 21d CRS.99 Tissue plasminogen activator (tPA) is an extracellular protease and signaling molecule that is released with neural activity and is required for chronic stress-induced loss of spines and NMDA receptor subunits on CA1 neurons.100
Within the neuronal cytoskeleton, the remodeling of hippocampal neurons by chronic stress and hibernation alters the acetylation of microtubule subunits—consistent with a more stable cytoskeleton101—and alters microtubule associated proteins, including the phosphorylation of a soluble form of τ, which is increased in hibernation and reversed when hibernation is terminated.91
Neurotrophic factors also play a role in dendritic branching and length. For example, mice bred to show reduced levels of brain derived neurotrophic factor (BDNF±) show a less branched dendritic tree and do not show a further reduction of CA3 dendrite length with chronic stress, whereas wild-type mice show reduced dendritic branching (Magarinos, McEwen unpublished observations). However, there is contradictory information thus far concerning whether CRS reduces BDNF mRNA levels, with some studies reporting a decrease102 and others reporting no change.103–105 This may reflect the balance of two opposing forces, namely, that stress triggers increased BDNF synthesis to replace depletion of BDNF caused by stress.106 BDNF and corticosteroids also appear to oppose each other—with BDNF reversing reduced excitability in hippocampal neurons induced by stress levels of corticosterone.107
Corticotrophin releasing factor (CRF) is another key mediator of many aspects of neuroplasticity related to stress.108 CRF in the PVN regulates ACTH release from the anterior pituitary gland, whereas CRF in the central amygdala is involved in control of behavioral and autonomic responses to stress, including the release to tPA that is an essential part of stress-induced anxiety and structural plasticity in the medial amygdala.109 CRF in the hippocampus is expressed in a subset of γ-aminobutyric acid (GABA) neurons (Cajal-Retzius cells) in the developing hippocampus, and early life stress produces a delayed effect that reduces cognitive function and the number of CA3 neurons as well as decreased branching of hippocampal pyramidal neurons.110,111 Indeed CRH inhibits dendritic branching in hippocampal cultures in vitro.112
Summary
Animal model studies on the hippocampus have revealed a mechanism by which repeated stress causes remodeling of hippocampal circuitry; namely, shortening of dendrites, loss of spine synapses and suppression of the neurogenesis that is ongoing in the young adult dentate gyrus region of the hippocampal formation. This is a reversible process for stressors lasting a number of weeks, and it involves as mediators not only circulating glucocorticoids but also excitatory amino acid neurotransmitters and other endogenous mediators and modulators. Because of these two inter-related roles of the hippocampus—supporting aspects of memory and regulating HPA activity—impairment of hippocampal function through changes in either excitability, reversible plasticity or permanent damage may be expected to have two effects: (1) The first is to impair hippocampal involvement in episodic, declarative, contextual and spatial memory; impairments of these functions are likely to debilitate an individual's ability to process information in new situations and to make decisions about how to deal with new challenges. (2) The second effect is to impair the hippocampal role in regulating HPA activity, particularly the termination of the stress response, leading to elevated HPA activity and further exacerbating the actions of adrenal steroids in the long-term effects of repeated stress. This concept, first called the “glucocorticoid cascade hypothesis” of hippocampal aging,113 stands at the center of the notion of “allostasis” and “allostatic load” and the central role of the brain.
Human neuroimaging studies of the hippocampus
Complementing animal studies of stress-related processes mediated by and affecting neuroplasticity in the hippocampus, a growing number of human structural neuroimaging studies have begun to examine stress processes in association with aspects of gross hippocampal morphology. For example, individuals with stress-related psychiatric disorders, such as major depressive disorder and post-traumatic stress disorder, show volumetric reductions in the hippocampus.114–128 Reduced hippocampal volume has also been found in Cushing's Disease.129 Interestingly, in Cushing's, surgical correction of hypercortisolemia has been reported to at least partially reverse hippocampal volume reduction as well as mood and memory deficits.130,131 In depression, there is evidence of volumetric increase in the hippocampus after antidepressant treatment,120 suggesting that the deficits in depression are potentially reversible. Moreover, there is increasing support for the notion that targeting the plasticity of the hippocampus in depression and mood disorders may underpin pharmacological and nonpharmacological treatment efficacy.132
In addition to clinical studies, there is emerging evidence from otherwise healthy individuals for a relationship between chronic stressful experiences and changes in hippocampal morphology. Among post-menopausal women, for example, higher levels of chronic perceived stress, as measured over an approximate 20-year period of life, have been associated with reduced gray matter volume in the hippocampus in addition to the orbital prefrontal cortex.133 Further, more than 3 years after the terrorist attacks on the World Trade Center buildings on September 11, 2001, otherwise healthy adults living in close proximity to the buildings showed a reduction in gray matter volume in the hippocampus, as well as in anatomically networked areas of the amygdala and mPFC.134
Although these structural neuroimaging findings are provocative, it is important to note that it has not yet been demonstrated that putatively stress-related variation in the morphology of the hippocampus or other brain regions in humans is invariably the permanent consequence of so-called “neurotoxic” stress-related mechanisms.135–137 It is possible, for example, that pre-existing individual differences in hippocampal and regional brain morphology could partly increase vulnerability to and decrease resiliency against life stress.138 These individual differences could emerge early in life, and could result from a combination of genetic and developmental influences. In line with this notion, there is recent evidence that individual differences in self-esteem and locus of control, positive psychological attributes that emerge early in life and modify the appraisal of environmental stressors, are associated with hippocampal volume and related changes in HPA regulation in both young and elderly people.50 Further, there is evidence that birth weight itself predicts hippocampal volume in adulthood, particularly among women reporting unfavorable maternal care—suggesting that the postnatal caregiving environment may affect the neurodevelopmental consequences of prenatal risk.139
In addition to these early life processes, recent human evidence shows that carriers of the methionine (met) allele of the valine(val)66met BDNF polymorphism express lower gray matter volume in the hippocampus and prefrontal cortex compared with carriers of the val/val allele.140–142 As reviewed earlier, in animal models, chronic stress is known to down-regulate BDNF, possibly contributing to cellular remodeling in the hippocampus.143–145 Thus, given that the met allele is associated with relatively reduced activity-dependent secretion and intracellular trafficking of pro-BDNF, this allele could plausibly affect the contribution of BDNF to signaling cascades mediating synaptic plasticity and, potentially, neurogenesis in response to stress. In further support of genetically mediated plasticity of the hippocampus possibly affecting stress resiliency, a recent twin study demonstrated that smaller hippocampal volume may predict vulnerability to the development of PTSD.146 In aggregate, these structural neuroimaging studies of humans complement translational animal studies of stress processes in that they reveal both vulnerability and experience-dependent patterns of hippocampal morphology relevant to risk for and resilience against ill health.
Within the context of the allostatic load model presented in Figure 2, there are several additional immune-mediated mechanisms involving bidirectional brain–body and body–brain patterns of communication that may further account for individual differences in hippocampal morphology. More precisely, growing evidence supports an association between peripheral immune activation and behavioral, affective and cognitive disturbances. Peripheral proinflammatory cytokines, such as interleukin (IL)-6, represent plausible mediators of these effects, as they can penetrate the blood–brain barrier directly via active transport mechanisms147,148 or indirectly via the vagus nerve149,150 to stimulate the production of central proinflammatory cytokines, including IL-6, which are expressed in hippocampus along with their receptors.151,152
Moreover, this central inflammation may adversely affect learning and memory through processes related to neurodegeneration and structural remodeling of the hippocampus in particular. In humans, there is evidence for an inverse association between peripheral levels of IL-6, a relatively stable marker of systemic inflammation, and memory function in mid-life adults.153 In an extension of this particular study,154 a computational structural neuroimaging method (voxel-based morphometry) was used to test the relationship between plasma IL-6 levels and hippocampal gray matter volume. Results showed that peripheral levels of IL-6 covaried inversely with hippocampal gray matter volume. However, the exact mechanisms by which peripheral IL-6 relates to hippocampal gray matter volume and cognition in humans remain unclear, as do their implications for stress-related processes involved in mediating neuroplasticity, particularly within the hippocampus.
Interestingly, sleep disruption is associated with elevated plasma levels of IL-6.155 The hippocampus is also affected by jet lag and circadian disruption, and a study using structural brain imaging on airline crews with short turn recovery times after international flights across multiple time zones revealed smaller volumes of the temporal lobe containing the hippocampus compared to air crews with a longer time between flights.156 Related to inflammation are metabolic imbalance and oxidative stress157 and the consequences of diabetes for cognitive function and the hippocampus. Studies of Type 2 diabetes have revealed reduced hippocampal volume that is larger in those subjects with the greatest elevations of glycosylated hemoglobin, indicative of elevated blood glucose levels.158 Mild cognitive impairment in aging is also associated with hippocampal volume reduction that is also related to elevated glycosylated hemoglobin levels below the threshold for Type 2 diabetes.159 One of the treatments that can prevent Type 2 diabetes is regular physical activity and a recent study shows that fit individuals have larger left and right hippocampal volumes than unfit individuals.160
In addition to structural neuroimaging studies of chronic stress and related processes, an increasing number of functional neuroimaging studies in humans are beginning to link hippocampal activity with acute stressor-evoked changes in the HPA axis, as measured by salivary cortisol. As has been widely demonstrated in laboratory studies,161 these functional neuroimaging studies have shown that the HPA axis is reliably engaged by stressors that involve completing demanding and uncontrollable cognitive challenges with added negative social evaluation. For example, using a modified version of the Trier Social Stress Test (TSST) administered during positron emission tomography (PET) scanning, significant associations between increased salivary cortisol levels and decreased activity in the hippocampus and networked brain areas, including the amygdala and hypothalamus have been documented.162 These particular findings are notable in that the hippocampus is thought to exert an inhibitory control over the hypothalamus, and thus the HPA axis. When “deactivated” under stress, the hippocampus and other limbic areas innervating the hypothalamus may in turn disinhibit the HPA axis and the consequent release of cortisol. In a more recent study, associations between cortisol reactivity to the TSST and patterns of activation in brain areas other than the hippocampus during PET scanning have also been documented.163 The results of this study extended those of Pruessner and colleagues to show that in response to the TSST, increased activation of areas of the mPFC covaried with decreased salivary cortisol reactivity. These particular findings are broadly consistent with the notion that the mPFC plays an integrative role in cognitive and affective processing164,165 and with animal models demonstrating that subregions of the mPFC regulate the HPA axis through inhibitory control mechanisms.69,166
In view of these conceptualizations of the mPFC, Kern and colleagues interpreted their findings to suggest that social stressors such as the TSST engage the mPFC as part of a regulatory circuitry that modulates downstream stress reactivity and coping processes. Supporting this interpretation, Kern and colleagues used functional connectivity analyses to link increased activation in the mPFC with decreased activation in the hippocampal-amygdala complex, in addition to other limbic areas. These connectivity findings agreed with the notion developed from translational animal models that the mPFC may inhibit HPA activity via regulatory signaling with brain areas innervating the hypothalamus, as reviewed earlier on animal findings detailing the dual role of the amygdala, hippocampus, and prefrontal cortex in visceral and cognitive functions.69,166 In view of these translational findings, an important direction of future research will be to link stress-related variation in hippocampal morphology and functionality to markers of SES, as SES may impact health in part via dysregulated HPA functioning. For example, open questions are whether dimensions of lower SES at the individual, family, or community levels are associated with hippocampal structural or functional plasticity over the lifespan, possibly in association with dysregulated allostatic control over the HPA axis and associated cognitive sequelae.
Summary
Studies on the human hippocampus with structural and functional imaging have produced provocative results that are consistent with the animal models showing a capacity for plasticity that should be followed up by longitudinal studies to demonstrate stress-related changes that are independent of pre-existing individual differences in hippocampal volume and function.
Amygdala and stress processes
Functional neuroanatomy of the amygdala
The amygdala is comprised of distinct cell groups in the medial anterior temporal lobes, adjacent to the hippocampus (see Fig. 1). A critical function of the amygdala in stressor-related processing involves the rapid assignment of emotional and behavioral salience to environmental events167–171 The amygdala supports such processing by integrating multimodal sensory inputs from distributed cortical, thalamic, and brainstem afferent relays. More precisely, sensory input is relayed through thalamic and cortical-thalamic pathways to the basolateral area via the lateral nucleus, basolateral nucleus, and accessory basal nucleus. From the basolateral nucleus, motivationally relevant sensory signals are relayed to the central nucleus. As a primary output nucleus, the central nucleus signals commands for adaptive changes in behavior and supporting physiological adjustments via the stria terminalis to lateral and paraventricular hypothalamic nuclei and to periaqueductal, medullary, and pre-autonomic nuclei. Importantly, the central nucleus is also networked with cortical areas involved in stressor-related processing—principally, areas of the prefrontal cortex, including the anterior cingulate cortex (ACC), ventromedial prefrontal cortex, and orbital prefrontal cortex.172–174 Hence, the amygdala is broadly viewed to interrelate cortical processes supporting the coordination of stressor-evoked changes in behavior and peripheral physiological reactivity, particularly within the context of adverse social environments affecting health.5,24,175
Animal studies of the amygdala
Chronic immobilization stress of the type that causes retraction of dendrites in CA3 region of the hippocampus produces dendritic growth in neurons in basolateral amygdala.176 Moreover, chronic stress of this type not only impairs hippocampal-dependent cognitive function, but also enhances amygdala-dependent unlearned fear and fear conditioning processes177,178 that are consistent with the opposite effects of stress on hippocampal and amygdala structure. Chronic stress also increases aggression between animals living in the same cage, and this is likely to reflect another aspect of hyperactivity of the amygdala.178,179 Moreover, chronic corticosterone treatment in drinking water produces an anxiogenic effect in mice,180 an effect that could be due to the glucocorticoid enhancement of CRF activity in the amygdala.181,182
As for mechanism(s) mediating forms of amygdala neuroplasticity, besides the possible role of glucocorticoids and excitatory amino acids, tPA is required for acute stress to activate not only indices of structural plasticity, but also to enhance anxiety.183 These effects occur in the medial and central amygdala, but not in basolateral amygdala—with the release of CRF acting via CRF-1 receptors appearing to be responsible.109 Furthermore, tPA plays a role in stress-induced decreases in spine density in medial amygdala neurons, but not in the stress-induced increase in spine density in basolateral amygdala neurons.184 However, nothing is yet known about the role of tPA, if any, in the prefrontal cortex. Although, it is noteworthy that tPA does appear to play a role in stress-induced reductions of spine synapse number in the CA1 region of the mouse hippocampus.100
BDNF may also play a role in amygdala, because over-expression of BDNF, without any applied stressor, enhances anxiety in an elevated plus maze and increases spine density on basolateral amygdala neurons and this occludes the effect of immobilization stress on both anxiety and spine density.185 As noted earlier for hippocampus, BDNF over-expressing mice also show reduced behavioral depression in the Porsolt forced-swim task and show protection against stress-induced shortening of dendrites in the CA3 region.185
Summary
Animal studies on the amygdala reveal stress-induced structural plasticity within major subdivisions of this brain region that relate to stress effects on aggression and anxiety.
Human neuroimaging studies of the amygdala
Complimenting the above animal work, the amygdala has been shown to be central to emotion and stress-related processes humans.186–189 Specifically, there is human functional neuroimaging evidence that the amygdala is involved in mediating forms of peripheral stress reactivity that have been linked to physical health outcomes. For example, individual differences in amygdala reactivity to emotionally salient stimuli have been shown to covary with physiological parameters associated with cardiovascular disease risk, including basal levels of autonomic-cardiac control,190 stressor-evoked changes in blood pressure,191 and diurnal variations in the secretion of the stress hormone, cortisol.165 Most recently, it has been demonstrated that individuals who express greater amygdala reactivity to threatening social cues (angry and fearful facial expressions) also exhibit higher levels of preclinical atherosclerosis, as determined noninvasively by a thickening of the intima-media layers of carotid artery vessel wall complex.192 Moreover, in that study, individuals who showed lower levels of preclinical atherosclerosis exhibited a pattern of functional connectivity (correlated activity) between the amygdala and ACC that suggested a potentially greater down-regulation of the amygdala by this area of the prefrontal cortex during the processing of threatening social cues.
These findings are noteworthy from a clinical-translational perspective because the amygdala and its functional interactions with the ACC and other areas of the prefrontal cortex have long been implicated in conferring risk for psychopathologies of mood and anxiety,193–195 which are highly co-morbid with atherosclerotic cardiovascular disease.196–199 Further, functional aspects of the ACC in particular have been recently implicated in atherogenesis in a primate model of comorbid depression and cardiovascular disease.200 In synthesis, the ACC and other areas of the prefrontal cortex may not only plausibly protect against some forms of psychiatric syndromes, but also physical diseases (e.g., atherosclerotic cardiovascular disease) by effectively regulating the amygdala and the peripheral expression of biomediators involved in allostatic load.
In addition to studies of stress reactivity and cardiovascular risk, there is emerging evidence suggesting that amygdala may be involved in linking stress-related processes to health within the context of childhood SES. In particular, social information processing models emphasizing a life course perspective have postulated that lower SES individuals may develop an early sensitivity to social threats in the environment, leading to dysregulated forms of emotional control and recurrent biobehavioral stress responses that increase risk for ill health in later life.175,201,202 This postulate parallels the notion that risk trajectories for ill health may be developmentally “embedded” in the brain and in biobehavioral stress-response systems by early and unfavorable socioeconomic circumstances.203,204
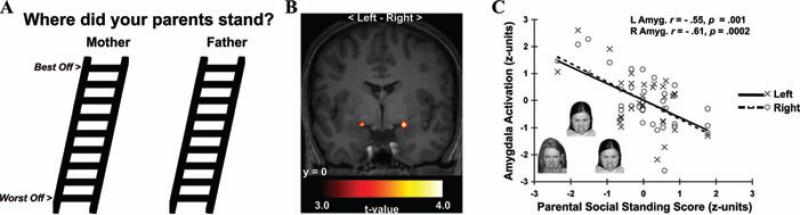
Consistent with this notion, recent neuroimaging evidence has shown that a retrospective measure of lower perceived parental social standing, a putative indicator of socioeconomic disadvantage during childhood and adolescence, is uniquely associated with greater amygdala reactivity to threatening (angry) facial expressions (see Fig. 3).205 Notably, this association was observed among healthy individuals who had not yet reached their adult SES, and it was not explained by several potential confounding factors, including sex, ethnicity, dispositional emotionality, recent symptoms of depression and anxiety, parental education, and participants’ perceptions of their own social standing. Given that the amygdala is (i) instrumental for gauging the emotional salience of social and environmental information, (ii) critical for regulating the neuroendocrine and autonomic stress-response axes, and (iii) sensitive to early life stress, then increased amygdala reactivity to angry or otherwise threat-related facial expressions could represent a neural correlate of a so-called developmental “embedding” of early SES-related experiences that influence sensitivity to perceived social threats—possibly affecting stress regulatory peripheral allostatic systems influencing health or disease vulnerability.
Figure 3.
Lower perceived parental social standing predicted greater amygdala reactivity to angry faces in a functional neuroimaging study of young adults. (A) Social ladders used to assess perceived parental social standing. (B) Statistical parametric maps projected onto an anatomical template. The maps profile amygdala areas where lower perceived parental social standing predicted greater reactivity to angry faces. (C) Plots depicting standardized perceived parental social standing scores (x-axis) and mean-centered, standardized reactivity values derived from left (L, open circles, dashed line) and right (R, closed circles, solid line) amygdala areas in B. Inset in C illustrates exemplar trial of angry faces used to elicit amygdala reactivity. From Gianaros et al. (2008), reprinted with permission.
Most recently, amygdala reactivity has been linked to concurrent changes in the neural representation of social hierarchies in humans.206 In this study, functional magnetic resonance imaging (fMRI) was used to identify neural responses correlated with perceived social rank within the context of an interactive, simulated social context involving exposure to both stable and unstable social hierarchies. Interestingly, in the context of an unstable social hierarchy, viewing a superior ranking individual engaged the amygdala and areas of the mPFC involved in social cognition. These findings are important in that they are among the first to begin to translate animal studies on the role of the amygdala and networked corticolimbic areas in potentially linking stress processes to candidate neurobiological mechanisms mediating the impact of socioeconomic gradients on mental and physical health. Moreover, it is noteworthy that experimentally manipulating social standing—following an interpersonal paradigm similar to that employed by Zink and colleagues206—has recently been shown to increase the subjective experience of negative affect concurrent with elevations in systolic blood pressure.207 In light of translational evidence on the role of the amygdala in mediating negative affect and blood pressure control, it is plausible that the amygdala supports key functions in stress and emotion processes related to SES and health, as speculated previously.208
Summary
Studies on the human amygdala reinforce a large body of animal studies demonstrating the importance of this region for emotion- and stress-related behavioral and physiological processes. Moreover, there is emerging neuroimaging evidence indicating that the functionality of the amygdala may be linked to socioeconomic factors, including childhood SES and the dynamic representation of relative social standing.
Prefrontal cortex and stress processes
Functional neuroanatomy of the prefrontal cortex
As shown in Figure 1, the prefrontal cortex occupies the anterior portion of the frontal lobes and is broadly involved in higher cognitive functions (e.g., working memory and executive control). One such function is the top-down regulation of stress and threat-related responding and coping processes mediated by subcortical limbic areas, including the hippocampus, amygdala, and hypothalamus.209 Importantly, several prefrontal areas send direct projections to the hypothalamus and other areas involved in regulating the peripheral stress-response axes important for health. These prefrontal areas primarily include the orbital and dorsal medial prefrontal cortex and the ACC.
Animal studies of the prefrontal cortex
Chronic stress also causes functional and structural changes in the medial prefrontal cortex, particularly in areas of anterior cingulate, prelimbic, infralimbic, and orbitofrontal regions—corresponding to conventional animal anatomical labeling. For example, CRS and chronic immobilization cause dendritic shortening in medial prefrontal cortex,89,176,210–214 but also produce dendritic growth in orbitofrontal cortex.215 Taken together with the differential effects of the same stressors on the hippocampus and amygdala, these actions of stress are reminiscent of recent work on experimenter versus self-administered morphine and amphetamine, in which different, and sometimes opposite, effects were seen on dendritic spine density in orbitofrontal cortex, medial prefrontal cortex, and hippocampus CA1.216 For example, amphetamine self-administration increased spine density on pyramidal neurons in the medial prefrontal cortex and decreases spine density on orbitofrontal pyramidal neurons.217
Along with many other brain regions, the prefrontal cortex, as well as the amygdala discussed earlier, contain adrenal steroid receptors;218,219 however, the role of adrenal steroids, excitatory amino acids and other mediators has not yet been studied in detail in these brain regions, in contrast to the hippocampus. Nevertheless, glucocorticoids do appear to play a role, since 3 weeks of chronic corticosterone treatment was shown to produce retraction of dendrites in medial prefrontal cortex,210 although with subtle differences in the qualitative nature of the effect from what has been described after chronic restraint stress.212 Another study determined the effect of adrenalectomy or chronic treatment for 4 weeks with corticosterone or dexamethasone on volume and neuron number in the prefrontal cortex.220 Dexamethasone treatment at a dose that may have been high enough to enter the brain (although this was not directly measured) caused a loss of neurons in Layer II of the infralimbic, prelimbic, and cingulate cortex, whereas corticosterone treatment reduced the volume but not the neuron number of these cortical regions.220 The dexamethasone treatment was particularly effective in impairing working memory and cognitive flexibility using working memory task in a Morris water maze.220 Effects of chronic stress were not investigated in this study. These data notwithstanding, the cautions expressed above concerning differences between chronic stress and chronic glucocorticoid treatment must be kept in mind for the prefrontal cortex, as well as the amygdala, which has not been studied yet in this regard.
Behavioral correlates of CRS-induced remodeling in the prefrontal cortex include impairment in attention set shifting, possibly reflecting structural remodeling in the medial prefrontal cortex.215 Attention set shifting is a task in which a rat first learns that either odor or the digging medium in a pair of bowls predicts where food reward is to be found; then new cues are introduced and the rat needs to learn which ones predict the location of food.221 There is also a report that chronic restraint stress impairs extinction of a fear conditioning task.222 This is an important lead since the prefrontal cortex is involved in extinction, a type of learning,223 but much more research is needed to explore the complex relationship between stress, fear conditioning, extinction, and possible morphological remodeling that may well accompany each of these experiences.
Summary
Animal studies on the prefrontal cortex reveal stress-induced changes in neuronal structure and connectivity. On the one hand, the medial prefrontal cortex shows reduced neuronal complexity and loss of synaptic connections as a result of repeated stress, whereas the orbitofrontal cortex shows greater neuronal complexity as a result of chronic stress.
Human studies of the prefrontal cortex
Most of the work on the prefrontal cortex and stress-related processes in humans, particularly within the context of SES research, has focused on areas of the ACC. The ACC is an evolutionally old cortical system common to mammals,224 and it occupies much of the medial wall of the prefrontal cortex surrounding the corpus callosum. Within the ACC, there are regional differences in cellular architecture and efferent and afferent projections to other brain areas that largely correspond to putatively distinct subregions, which have been described in terms of a dorsal cognitive-motor division, a ventral visceral-motor division, and an intermediate affective division anterior to the genu of the corpus callosum, a major white matter tract connecting the two hemispheres of the brain.225–227 Of these cingulate regions, the perigenual anterior cingulate cortex (pACC) has been specifically linked to several emotion and stress-related processes in neuroimaging studies and patient lesion studies. These processes include the appraisal of salient environmental and personal events, the experience of emotional states, and the regulation of behavioral and autonomic responses to emotional and stressful stimuli.225,227–231 Further, there is growing evidence that the pACC is involved in mediating individual differences in stressor-evoked cardiovascular reactivity, which have long been associated with risk for cardiovascular disease.232–234 For example, greater stressor-evoked pACC activity across individuals has been associated with larger magnitude blood pressure reactions to a variant of a Stroop color-word interference stressor,235 particularly in interaction with the amygdala.191 Such a role for the pACC in mediating stressor-evoked cardiovascular reactivity is instantiated through its reciprocal circuitry with adjacent areas of the orbital and medial prefrontal cortex, anterior insula, amygdala, and areas in the hypothalamus, periaqueductal gray (PAG), pons, medulla, and the pre-sympathetic intermediolateral (IML) cell column of the spinal cord.227,236 As such, the pACC—along with other networked cingulate and prefrontal areas—may provide for an interface between stressor appraisal processes and concurrent allodynamic control.237
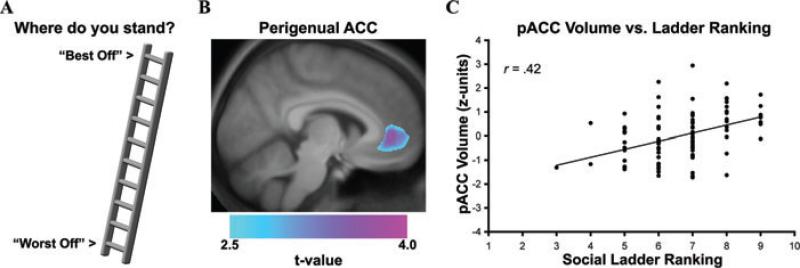
Furthermore and as detailed earlier, translational evidence from animal models has demonstrated that prelimbic and infralimbic areas of the rodent ACC, anatomically homologous areas of the human pACC, show pronounced changes in structural plasticity under conditions of chronic stress. Thus, from a translational perspective developed within the context of these animal findings, stress-related dimensions of low socioeconomic position could plausibly covary with changes in the morphology of the ACC in humans. In support of this speculation, there is structural neuroimaging evidence in humans that individuals who report holding a low social standing in the United States—as reflected by low subjective social status ladder rankings on the MacArthur scale of perceived social standing18—show a reduced gray matter volume in the pACC238 (see Fig. 4). Notably, the relationship between low subjective social status and reduced pACC gray matter volume persisted in this study after accounting for several demographic and psychological factors (e.g., subclinical depressive symptoms, dispositional forms of negative emotionality) and conventionally defined levels of personal and community SES. However, while these cross-sectional findings did not establish a causal direction of association, they do implicate reduced pACC gray matter volume as a structural neural correlate of low subjective social status, a presumptive stress-related dimension of socioeconomic position that has been linked to dysregulated neuroendocrine activity, adverse mental and physical health outcomes, and impaired immune functioning in prior epidemiological studies.18,19,21–23,239
Figure 4.
Lower subjective social status, as reflected by a lower self-reported ranking on a “social ladder”, was associated with reduced gray matter volume in the perigenual area of the anterior cingulate cortex (pACC). (A) Illustration of 10-point social ladder scale used to assess subjective social status. (B) Overlaid on an anatomical template is a statistical parametric map of color-scaled t-values, which illustrate the pACC area where lower subjective social status was associated with reduced gray matter volume across individuals. (C) Plotted along the y-axis is the standardized (z-score) gray matter volume values for pACC area profiled in B. Plotted along the x-axis are social ladder rankings from the scale illustrated in A (1 = “Worst Off,” 10 = “Best Off”). *P < 0.001. From Gianaros et al. (2007), reprinted with permission.
Further, increasing evidence indicates that a compromised structural or functional coupling between the pACC and networked corticolimbic areas such as the amygdala—particularly in the context of environmental adversity and genetic risk—may increase vulnerability to psychiatric and medical syndromes characterized by dysregulated emotion-related behaviors and physiology.240 Finally, there is recent in vivo imaging evidence in humans that reduced ACC volume is associated with HPA axis dysregulation, as indicated by a nonsuppressed cortisol response to a dexamethasone challenge.241 Thus, it is plausible that volumetric or other morphological changes in the pACC could account in part for the dysregulated forms of emotional control and neuroendocrine functioning that have been found among individuals reporting a low subjective social status.
In addition to the pACC, human evidence also implicates dorsal anterior cingulate cortex (dACC) areas in emotion-related processes, particularly those associated with emotion regulation, stressor-evoked physiological reactivity, and subjective distress. Within the cognitive neuroscience literature, areas in the dACC are broadly viewed to support processes related to attention, effortful executive control, and conflict and error monitoring. These processes are instantiated by reciprocal circuitry with the lateral prefrontal cortex, motor and supplementary motor cortex, and posterior parietal cortex.242 A conventional view is that dACC areas monitor for conflicts between competing streams of incompatible information, which foster the potential for behavioral error.243–245 After conflict detection, dACC areas engage prefrontal, motor, and parietal cortices to resolve conflicts and minimize behavioral error by modulating attention, working memory, and motor control processes. In addition to these cognitive processes, dACC areas are also engaged by states of pain-related anxiety,164,246 intentional regulation of autonomic activity,247 and awareness of subjective emotional experiences.248 Based on an integrative translational account of both cognitive and affective neuroscience findings regarding dACC functionality, Critchley228 posits that the dACC may be particularly important for generating autonomic and cardiovascular responses via projections to subcortical areas to support volitional, cognitive, and emotional behaviors. Consistent with this view, several forms of stress-related patterns of cardiovascular and neuroendocrine control have been linked to dCC activity in human neuroimaging studies. For example, stressor-evoked blood pressure reactivity has been shown to covary with heightened dACC activation to demanding cognitive challenges.249,250
There is also evidence that individual differences in dACC and prefrontal functionality are associated with the regulation of the HPA axis. For example, Eisenberger, et al251 demonstrated that cortisol changes elicited by the Trier Social Stress Task (TSST) administered outside of an MRI scanner were correlated with dACC activation during a social rejection task performed inside of the scanner. Specifically, activation of the dACC, in addition to networked areas of the dorsal medial prefrontal cortex, were correlated with larger cortisol responses to TSST. Moreover, activity in these cortical areas statistically mediated the association between individual differences in perceived social support and cortisol responses. An intriguing conclusion drawn by the authors was that a person's level of social support may modulate how specific brain areas, including the dACC, regulate social stress-related cortisol reactivity.
In extension of this work, Taylor and colleagues252 provided recent evidence that individuals who express lesser TSST-evoked cortisol reactivity also express lesser threat-related amygdala reactivity and greater regulatory activity in the ventral portion of the orbitofrontal prefrontal cortex; moreover, these neural activity patterns were observed specifically in association with higher levels of social resources—operationally defined as “personal dispositions that may help people to perceive potentially threatening events as less threatening and/or help them to manage their responses to events perceived to be threatening.”253 In aggregate, there is sufficient human evidence that the availability of social resources, which are often taxed and chronically depleted in the context of lower socioeconomic position, impact neural dynamics important for allostatic control and possibly disease risk.
Summary
Studies on the human prefrontal cortex have revealed an important role for this region and its functional subdivisions, particularly within the anterior cingulate cortex, in mediating stress-related behavioral and biological reactivity and regulation. Translational neuroimaging findings also reveal an association between low subjective social standing, a purported stress-related dimension of low SES, and reduced gray matter volume in the perigenual area of the ACC, an area important for regulating the autonomic and HPA stress-response axes.
Interventions for allostatic load and brain–body interactions
The notion that the brain is the central organ of stress may be used to argue for interventions that are top down and “holistic,” insofar as such interventions stimulate the entire body to help itself and function normally by affecting the neurobiological circuitries detailed above. Importantly, such interventions can be aimed at the individual, in terms of targeting a person's behavioral habits and lifestyle. Moreover, they can be aimed at the level of social organization, in terms of addressing policies of the government and private sector that provide groups of individuals with access to and control over environmental, social, and material resources important for health and well-being.
Interventions for the individual
For the individual, two of the most important interventions are physical activity and arguably social integration. It is well established that a sedentary lifestyle is a major risk factor for many of the diseases of modern life including obesity, diabetes, cardiovascular disease, depression, and dementia. Moreover, recent studies have shown that moderate physical activity can be beneficial for the brain and cardiovascular and metabolic systems.254–258 Voluntary physical activity has been shown to increase neurotrophin expression in cortex and hippocampal regions of the brain,259 as well as to increase neurogenesis in the dentate gyrus of young and even aging animals.260 One mechanism for these effects involves the actions of circulating IGF-1, which is taken up by the brain and acts via receptors found in the hippocampus, as summarized early in this article. Moreover, increased neurogenesis in dentate gyrus has been linked to the actions of antidepressant drugs, providing a potential parallel with the antidepressant actions of physical activity.261 Increased neurogenesis improves memory,262 and new neurons are believed to participate in learning of hippocampal dependent tasks.263 Although the precise role of neurogenesis in dentate gyrus is still controversial, new neurons appear to be more excitable and may contribute to greater cognitive flexibility.262,264 Related to effects of exercise on neurogenesis is the effect of dietary restriction, that also increases neurogenesis and elevates BDNF levels in hippocampus.265 BDNF is an important factor in current thinking about the actions of antidepressant treatments,128 including the consequences for hippocampal volume, memory and mood disorders apparently related to having the Val66Met allele of the BDNF gene.266–269
Physical activity and the human brain
Extending prior work on exercise, physical activity, and neuroplasticity in animal models, an increasing number of neuroimaging studies are beginning to examine these processes in relation to human cognition and brain structure and function.270 Colcombe et al.,271 for example, investigated changes in brain activity using fMRI over the course of a 6-month aerobic exercise program. In this study, older adults performed a cognitive task that places demands on executive control and attention before and after exercise training interventions. Interestingly, adults assigned to an aerobic training group involving brisk, regular walking showed improved cognitive performance and postintervention patterns of fMRI activation in the prefrontal and parietal cortices that were comparable to those displayed by a much younger control group. In contrast, participants assigned to a control group involving toning and stretching showed no such changes in performance or fMRI activity. In a more recent structural neuroimaging study, Colcombe et al.272 demonstrated that older adults who engaged in a 6 month aerobic walking program displayed an increase in the volume gray matter in the prefrontal and temporal cortices. Further, in this study, no such volumetric changes were observed in a nonaerobic control group or in a younger group of control participants. These particular structural neuroimaging findings were extended by Pereira et al.,273 who reported increases in cerebral blood volume—an imaging correlate of neurogenesis—in the dentate gyrus of the hippocampus among middle-aged individuals who completed a 3 month aerobic exercise program. Interestingly, CBV changes in the hippocampus covaried with improved cardiorespiratory fitness and verbal learning and memory. Moreover, as noted earlier, fit individuals are reported to have larger left and right hippocampal volumes than unfit individuals.160 Collectively, these human studies suggest that providing opportunities for voluntary aerobic exercise—which are generally limited in lower SES environments274—are likely to have beneficial effects on the neuroplasticity of prefrontal and hippocampal brain systems important for cognition, stress regulation, and resiliency against ill health.
Social integration
Dimensions of social relationships have long been linked to longevity and aspects of physical and mental health.275–278 These dimensions include social network composition,279 social support,280 social interaction frequency and quality281,282 and the experience of isolation and loneliness accompanying deficient or broken social relationships.283 Importantly, cumulative evidence from social epidemiological studies has repeatedly demonstrated that different dimensions of social relationships can affect longevity and health via unique neurobehavioral pathways.276,277,284 One dimension of social relationships that has been most consistently linked to longevity and health is social integration, a multidimensional construct referring to an individual's (1) effortful behavioral engagement in wide ranging social activities and relationships and (2) cognitive construal of her or his communality and identification with diverse social roles.279 Epidemiological studies have specifically linked measures of social integration (e.g., measures of the number of self-reported social positions or roles, as well as the frequency of participating in social activities) with lifespan,275,278,285,286 trajectories of cognitive aging and risk for dementia,287 severity of subclincial cardiovascular disease,288 risk for stroke,289 survival times in patients with cardiovascular disease,290 and the recurrence of cancer.291
At present, social integration is understood to affect health and longevity through two dissociable pathways.276 One pathway operates by affecting behavioral and biological factors associated with being socially isolated, as opposed to holding a minimum quantity of beneficial social contacts. The other pathways presumably operates by factors associated with the beneficial effects of incremental increases in social network diversity. Critically, the health beneficial effects of being more socially integrated may differ between men and women, depending on the health outcomes of interest.292 Further, social integration may impact longevity and aspects of health through pathways and processes that are fundamentally different from those ascribed to other stress- and health-protective dimensions of social relationships that have also received much attention in epidemiological and laboratory studies of health and aging. For example, social support, which broadly refers to psychological and material resources provided by one's social ties, may enable individuals to cope more adaptively with acute and chronic stressors, specifically when they are encountered. Hence, the protective effects of social support may be context specific rather than health protective in general, as is thought to be the case for social integration.276,293 Thus, social support provided by family or health professionals, who offer emotional support and provide useful information, has been shown to reduce the allostatic load score, which measures key physiological markers related to chronic stress and a potentially health damaging lifestyle.294 Social support also ameliorates reported levels of chronic stress in caregivers, who show a reduced length of telomeres in white blood cells.295
So far, however, little to nothing is known about how social integration, social support, or other social factors may benefit human brain circuits that are affected by chronic stress and allostatic load, although it is clear that these factors are linked to mood, overall mental health, and related brain-based processes.276,296–299 In this regard, there is intriguing translational evidence from animal models that social interactions and social isolation (a possible analog of deficient social integration in humans) have differential effects on neurogenesis and neuroplasticity in the amygdala and hypothalamus, brain systems important for allodynamic regulation.300,301 Further, there is recent neuroimaging evidence that human individual differences in perceived social isolation are associated with regional brain activation to social stimuli, particularly in circuitries involved in the processing of reward (ventral striatum) and in visual attention (occipital cortex and temporo-parietal junction).302 In view of the earlier discussion, an important direction for future research will be to delineate the unique pathways by which dimensions of social relationships and networks affect brain and bodily aging and health, and to design novel interventions impacting health-related aspects of social ties.
Pharmaceutical interventions
For the individual, life-long habits may be hard to change, and it is often necessary to turn to pharmacological interventions: sleeping pills, anxiolytics, β-blockers, and antidepressants are all drugs that are used to counteract some of the problems associated with the accumulation of allostatic overload. Likewise, drugs that reduce oxidative stress or inflammation, block cholesterol synthesis or absorption and treating insulin resistance or chronic pain can help deal with the metabolic and neurological consequences of chronically stressful experiences. All of these agents have value, and yet each one has side effects and limitations that are based in part on the fact that all of the systems that are dysregulated in allostatic overload are also systems that interact with each other and perform normal functions when properly regulated. Because of the nonlinearity of the systems of allostasis, the consequences of any pharmaceutical treatment may be either to inhibit the beneficial effects of the systems in question or to perturb other systems in a direction that promotes an unwanted side effect. Examples of the former include the problems with Cox 2 inhibitors, for example, Vioxx,303 and examples of the latter include the obesity inducing effects of some of the atypical antipsychotics that are widely used to treat schizophrenia and bipolar disorder.304
Can the brain change with therapy?
In light of the potential adverse side effects and limitations of many pharmaceutical treatments, it is important to note that the human brain does appear to change functionally and structurally as a result of experience. Animal models teach us that experiences, including stress-induced changes in brain structure are largely reversible and that resilience in both brain structure and behavior is the name of the game in adapting to changing environments.76 A corollary of this is that failure to show resilience is a feature of maladaptation and pathophysiology, including anxiety and depressive disorders and the downstream effects that these have on the rest of the body via the autonomic, neuroendocrine, and immune systems. But how plastic is the human brain in response to interventions that effectively treat disorders that effect the brain as well as the rest of the body? Cognitive behavioral therapy has been demonstrated to be as efficacious as several medication regimens aimed at treating disorders of mood, particularly depression; moreover, cognitive therapy and medication appear to affect many of the same or overlapping neural mechanisms.305 Moreover, there is recent evidence that successful cognitive therapy can even result in changes in brain morphology that parallel those of physical activity, particularly within the context of chronic fatigue syndrome—a brain–body disorder characterized by unabating or recurrent fatigue adversely affecting allostatic control systems.306 Further, a recent cross-sectional study reports thicker cortical volume in right anterior insula and prefrontal cortex of subjects who had meditated for many years compared to matched controls.307 Therefore, further studies of how the brain is changed by behavioral, as well as by pharmaceutical therapies, are important future directions.
Top-down effects of policies
At the level of social organization, the private sector has a powerful role. Businesses that encourage healthy lifestyle practices among their employees are likely to gain reduced health insurance costs and possibly a more loyal workforce.308,309 Moreover, governmental policies are important, and the Acheson Report310 from the United Kingdom in 1998 recognized that no public policy should be enacted without considering the implications for health of all citizens. Thus basic education, housing, taxation, setting of a minimum wage, and addressing occupational health and safety and environmental pollution regulations are all likely to affect the brain and health via a myriad of mechanisms. In young children, for example, the effects of developing in a low SES environment have been shown to modulate functional neural activity in brain areas important for reading—possibly impacting cognitive development, future academic achievement, and other contributors to adult SES.311–314 At the same time, providing higher quality food and making it affordable and accessible in poor, as well as affluent neighborhoods, is necessary for people to eat better, providing they also learn what types of food to eat and can afford them. Likewise, making neighborhoods safer and more congenial and supportive315 can improve opportunities for positive social interactions and increased recreational physical activity. For the elderly population, community centers and activities that promote social interactions and physical activity have been demonstrated to be beneficial.258,316
Finally, there are programs that combine some of the key elements just described; namely, education, physical activity, and social integration, along with one other ingredient that is hard to quantify: finding meaning and purpose in life. One example is the Experience Corps which takes elderly volunteers and trains them as teachers’ assistants for younger children in the neighborhood schools.317 Not only does this program improve the education of the children, it also benefits the elderly volunteers and improves their physical and mental health.318
Conclusions
To conclude, we reiterate the following key points embodied within this translational review of animal and human neurobiological evidence bearing on the health-related impact of stress processes—particularly those that may be important for understanding SES gradients in well-being and longevity:
The brain is the most important organ mediating stress processes: it determines what is “stressful” to the individual by supporting conscious and unconscious appraisal processes; it determines the health-damaging or health promoting behaviors that result from this appraisal; and it regulates peripheral allodynamic control systems that feed back to the brain to affect functional and structural neuroplasticity.
The brain is arguably the least studied and most important organ in human stress and socioeconomic research on health. Brain imaging measures can be easily incorporated into large scale, longitudinal and cross-sectional studies. Importantly, these measures can help to define the neurobiological and mechanistic pathways by which stress and socioeconomic factors affect health and longevity.
Animal models offer an opportunity to study causal relationships and lifespan processes that can inform translational research in human health neuroscience.
Interventions should focus on top-down strategies intended to alter brain function in ways that will improve allostasis and minimize allostatic load. Instilling optimism, a sense of control and self-esteem, and finding a meaning and purpose in life should be among the chief goals of such interventions. Indeed, virtually all policies of the public and private sector are, in fact, health policies.
Future work in this promising area will require interdisciplinary collaborations between neuroscientists, behavioral geneticists, social and biological psychologists, epidemiologists, policy and intervention researchers focusing on stress and health processes at multiple levels of analysis.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 MH41256; 5PO1 MH58911 (BSM) and K01 MH070616 and R01 HL089850 (PJG). We thank Drs. Karen A Matthews, William H. Dow, Shelley E. Taylor, and members of the John D. and Catherine T. MacArthur Foundation Research Network on Socioeconomic Status and Health for their constructive comments on a draft of this manuscript.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.Holroyd KA, Lazarus RS. Stress, coping, and somatic adaptation. In: Goldberger L, Breznitz S, editors. Handbook of Stress: Theoretical and Clinical Aspects. The Free Press; New York: 1982. [Google Scholar]
- 3.Lazarus RS, Folkman S. Stress, Appraisal and Coping. Guilford; New York: 1984. [Google Scholar]
- 4.Lovallo WR. Stress and Health: Biological and Psycholocial Interactions. Sage; Thousand Oaks, CA: 2005. [Google Scholar]
- 5.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen EI, et al. Economic, neurobiological, and behavioral perspectives on building America's future workforce. Proc. Natl. Acad. Sci. USA. 2006;103:10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shonkoff JP. A promising opportunity for developmental and behavioral pediatrics at the interface of neuroscience, psychology, and social policy: remarks on receiving the 2005 C. Anderson Aldrich Award Pediatrics. 2006;118:2187–2191. doi: 10.1542/peds.2006-1728. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- 9.Adler NE, et al. Socioeconomic status and health: the challenge of the gradient. Am. Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Adler NE, et al. Socioeconomic inequalities in health. No easy solution. JAMA. 1993;269:3140–3145. [PubMed] [Google Scholar]
- 11.Adler NE, et al. Annals of the New York Academy of Sciences. Vol. 896. Academy of Sciences; New York: 1999. Socioeconomic status and health in industrialized nations: social, psychological, and biological pathways. [PubMed] [Google Scholar]
- 12.Kessler RC, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 13.Lorant V, et al. Socioeconomic inequalities in depression: a meta-analysis. Am. J. Epidemiol. 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 14.Marmot M. Henry Holt and Company; New York: 2004. The Status Syndrome: How Social Standing Affects our Health And Longevity. [Google Scholar]
- 15.Sapolsky R. Social status and health in humans and other animals. Annu. Rev. Anthropol. 2004;33:393–418. [Google Scholar]
- 16.Dohrenwend BP. The role of adversity and stress in psychopathology: some evidence and its implications for theory and research. J. Health Soc. Behav. 2000;41:1–19. [PubMed] [Google Scholar]
- 17.Kelly S, Hertzman C, Daniels M. Searching for the biological pathways between stress and health. Annu. Rev. Public Health. 1997;18:437–462. doi: 10.1146/annurev.publhealth.18.1.437. [DOI] [PubMed] [Google Scholar]
- 18.Adler NE, et al. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S, et al. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol. 2008;27:268–274. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- 20.Goodman E, et al. Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obesity Res. 2003;11:1018–1026. doi: 10.1038/oby.2003.140. [DOI] [PubMed] [Google Scholar]
- 21.Ostrove JM, et al. Objective and subjective assessments of socioeconomic status and their relationship to self-rated health in an ethnically diverse sample of pregnant women. Health Psychol. 2000;19:613–618. doi: 10.1037//0278-6133.19.6.613. [DOI] [PubMed] [Google Scholar]
- 22.Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Soc. Sci. Med. 2003;56:1321–1333. doi: 10.1016/s0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 23.Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom. Med. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- 24.Repetti R, Taylor S, Seeman T. Risky families: family social environments and the mental and physical health of offspring. Psychol. Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 25.Dohrenwend BS. Social status and stressful life events. J. Personal. Soc. Psychol. 1973;28:225–235. doi: 10.1037/h0035718. [DOI] [PubMed] [Google Scholar]
- 26.McLeod JD, Kessler RC. Socioeconomic status differences in vulnerability to undesirable life events. J. Health Soc. Behav. 1990;31:162–172. [PubMed] [Google Scholar]
- 27.Aneshensel CS, Sucoff CA. The neighborhood context of adolescent mental health. J. Health Soc. Behav. 1996;37:293–310. [PubMed] [Google Scholar]
- 28.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dial. Clin. Neurosci. Stress. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melin B, et al. Psychological and physiological stress reactions of male and female assembly workers: a comparison between two different forms of work organization. J. Organiz. Behav. 1999;20:47–61. [Google Scholar]
- 30.Ferrie JE, et al. Effects of chronic job insecurity and change in job security on self reported health, minor psychiatric morbidity, physiological measures, and health related behaviours in British civil servants: the Whitehall II study. J. Epidemiol. Commun. Health. 2002;56:450–454. doi: 10.1136/jech.56.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobak M, Marmot M. East-West mortality divide and its potential explanations: proposed research agenda. BMJ. 1996;312:421–425. doi: 10.1136/bmj.312.7028.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan J, et al. Role of sympathoadrenal medullary activation in the initiation and progression of atherosclerosis. Circulation. 1991;84:V123–V132. [PubMed] [Google Scholar]
- 33.Muller JE, Tofler G, Stone P. Circadian variation and triggers of onset of acute caridovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 34.Muller J, Tofler G, Edelman E. Probable triggers of onset of acute myocardial infarction. Clin. Cardiol. 1989;12:473–475. doi: 10.1002/clc.4960120814. [DOI] [PubMed] [Google Scholar]
- 35.Larsson B, et al. Obesity, adipose tissue distribution and health in men-The Study of Men Born in 1913. Appetite. 1989;13:37–44. doi: 10.1016/0195-6663(89)90025-1. [DOI] [PubMed] [Google Scholar]
- 36.Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II study. Am. J. Epidemiol. 2007;165:828–837. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- 37.Dowd JB, Goldman N. Do biomarkers of stress mediate the relation between socioeconomic status and health? J. Epidemiol Commun. Health. 2006;60:633–639. doi: 10.1136/jech.2005.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson MD, Chen E. Socioeconomic status, race, and body mass index: the mediating role of physical activity and sedentary behaviors during adolescence. J. Pediatr. Psychol. 2007;32:250–259. doi: 10.1093/jpepsy/jsl024. [DOI] [PubMed] [Google Scholar]
- 39.Chen E, et al. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J. Allergy Clin. Immunol. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 40.Owen N, et al. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav. Immun. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, et al. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom. Med. 2004;66:553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- 42.Cohen S. Keynote Presentation at the Eight International Congress of Behavioral Medicine: the Pittsburgh common cold studies: psychosocial predictors of susceptibility to respiratory infectious illness. Int. J. Behav. Med. 2005;12:123–131. doi: 10.1207/s15327558ijbm1203_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. John Wiley & Sons; New York: 1988. pp. 629–649. [Google Scholar]
- 44.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 45.McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 46.Cannon WB. The Wisdome of the Body. WW Norton & Company; New York: 1932. [Google Scholar]
- 47.Schulkin J, McEwen BS, Gold PW. Allostasis, Amygdala, and Anticipatory Angst. Neurosci. Biobehav. Rev. 1994;18:385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 48.Kirschbaum C, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Personality and Individual Differences. 1999;27:477–489. [Google Scholar]
- 50.Pruessner JC, et al. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. NeuroImage. 2005;28:815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Lundberg U, et al. Psychological and physiological stress responses during repetitive work at an assembly line. Work Stress. 1989;3:143–153. [Google Scholar]
- 52.Sternberg E, et al. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in lewis rats. Proc. Natl. Acad. Sci. 1989;86:4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griep EN, Boersma JW, De Kloet ER. Altered Reactivity of the Hypothalamic-Pituitary-Adrenal Axis in the Primary Fibromyalgia Syndrome. J. Rheumatol. 1993;20:469–474. [PubMed] [Google Scholar]
- 54.Demitrack MA. Neuroendocrine Research Strategies in Chronic Fatigue Syndrome. In: Goodnick PJ, Klimas NG, editors. Chronic Fatigue and Related Immune Deficiency Syndromes. Vol. 40. American Psychiatric Press, Inc.; 1996. pp. 45–66. [Google Scholar]
- 55.Crofford LJ, et al. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheumatism. 1994;37:1583–1592. doi: 10.1002/art.1780371105. [DOI] [PubMed] [Google Scholar]
- 56.Yehuda R, et al. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol. Psychiatry. 1991;30:1031–1048. doi: 10.1016/0006-3223(91)90123-4. [DOI] [PubMed] [Google Scholar]
- 57.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 58.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 59.Eichenbaum H, Otto T. The hippocampus—what does it do? Behav. Neural. Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- 60.Pitkanen A, et al. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Annals N. Y. Acad. Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 61.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res. Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 62.Knigge K. Adrenocortical response to stress in rats with lesions in hippocampus and amygdala. Proc. Soc. Exp. Biol. Med. 1961;180:18–20. doi: 10.3181/00379727-108-26832. [DOI] [PubMed] [Google Scholar]
- 63.Ikegaya Y, Saito H, Abe K. High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neuroscience Research. 1995;22:203–207. doi: 10.1016/0168-0102(95)00894-7. [DOI] [PubMed] [Google Scholar]
- 64.Ikegaya Y, Saito H, Abe K. Attenuated hippocampal long-term potentiation in basolateral amygdala-lesioned rats. Brain Res. 1994;656:157–164. doi: 10.1016/0006-8993(94)91377-3. [DOI] [PubMed] [Google Scholar]
- 65.McEwen BS. Adrenal steroid feedback on neuroendocrine tissues. Ann. N.Y. Acad. Sci. 1977;297:568–579. doi: 10.1111/j.1749-6632.1977.tb41883.x. [DOI] [PubMed] [Google Scholar]
- 66.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 67.Herman JP, Prewitt CMF, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit. Rev. Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 68.Dunn J, Orr S. Differential plasma corticosterone responses to hippocampal stimulation. Exp. Brain Res. 1984;54:1–6. doi: 10.1007/BF00235813. [DOI] [PubMed] [Google Scholar]
- 69.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J. Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akana SF, et al. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J. Neuroendocrin. 2001;13:625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 72.Jacobson L, et al. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- 73.Akana S, et al. Constant corticosterone replacement normalizes basal adrenocorticotropin (ACTH) but permits sustained ACTH hypersecretion after stress in adrenalectomized rats. Endocrinology. 1988;122:1337–1342. doi: 10.1210/endo-122-4-1337. [DOI] [PubMed] [Google Scholar]
- 74.Spano MS, et al. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiat. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 75.Laugero KD, et al. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology. 2001;142:2796–2804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- 76.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 77.McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 78.Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- 79.Popov VI, Bocharova LS, Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48:45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- 80.Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- 81.Magarinos AM, et al. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc. Natl. Acad. Sci. USA. 2006;49:18775–18780. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seri B, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kempermann G, Gage FH. New nerve cells for the adult brain. Sci. Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- 84.Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 85.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Czeh B, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aberg MA, et al. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gould E, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sousa N, et al. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 90.McKittrick CR, et al. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 91.Arendt T, et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16:1091–1101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- 93.Maguire EA, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur. Neuropsychopharm. 2004;14:S497–S502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 95.Wood GE, et al. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. PNAS. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ejchel-Cohen TF, et al. Chronic restraint stress decreases the expression of glutathione S-transferase pi1 in the mouse hippocampus. Brain Res. 2006;1090:156–162. doi: 10.1016/j.brainres.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 97.Grillo CA, et al. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136:477–486. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 98.Magarinos AM, Verdugo Garcia JM, McEwen BS. Chronic restraint stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- 100.Pawlak R, et al. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. PNAS. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bianchi M, Heidbreder C, Crespi F. Cytoskeletal changes in the hippocampus following restraint stress: role of serotonin and microtubules. Synapse. 2003;49:188–194. doi: 10.1002/syn.10230. [DOI] [PubMed] [Google Scholar]
- 102.Smith MA, Cizza G. Stress-induced changes in brain-dervied neurotrophic factor expression are attenuated in aged Fischer 344/N rats. Neurobiol. Aging. 1996;17:859–864. doi: 10.1016/s0197-4580(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 103.Isgor C, et al. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 104.Kuroda Y, McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res. Mol. Brain Res. 1998;59:35–39. doi: 10.1016/s0169-328x(98)00130-2. [DOI] [PubMed] [Google Scholar]
- 105.Kuroda Y, McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, GAP-43 and MAP2 mRNA expression in the rat hippocampus. Mol. Brain Res. 1998;59:35–39. doi: 10.1016/s0169-328x(98)00130-2. [DOI] [PubMed] [Google Scholar]
- 106.Marmigere F, et al. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- 107.Hansson AC, et al. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by Exon IV promoter. J. Neuroendocrin. 2006;18:104–114. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- 108.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 109.Matys T, et al. Tissue plasminogen activator promotes the effects of corticotropin releasing factor on the amygdala and anxiety-like behavior. Proc. Natl. Acad. Sci. USA. 2004;101:16345–16350. doi: 10.1073/pnas.0407355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brunson KL, et al. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci. USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brunson KL, et al. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 114.Bremner JD, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gurvits TV, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol. Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bremner JD, et al. Magnetic resonance imaging-based measurement of hippocampal volume in post-traumatic stress disorder related to childhood physical and sexual abuse–a preliminary report. Biol. Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sheline YI, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol. Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 119.Villarreal G, King CY. Brain imaging in post-traumatic stress disorder. Semin. Clin. Neuropsychiatry. 2001;6:131–145. doi: 10.1053/scnp.2001.21840. [DOI] [PubMed] [Google Scholar]
- 120.Vermetten E, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fuchs E, Czeh B, Flugge G. Examining novel concepts of the pathophysiology of depression in the chronic psychosocial stress paradigm in tree shrews. Behav. Pharmacol. 2004;15:315–325. doi: 10.1097/00008877-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 122.Fuchs E, et al. Alterations of neuroplasticity in depression: the hippocampus and beyond. European Journal of Neuropsychopharmacology. 2004;14:481–490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 123.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol. Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- 124.Lupien SJ, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 125.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 126.Karl A, et al. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioal Reviews. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Nemeroff CB, et al. Posttraumatic stress disorder: a state-of-the-science review. J. Psychiatr. Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 128.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 129.Starkman MN, et al. Hippocampal formation volume, memory dysfunction, and cortisol levels in partients with Cushing's syndrome. Biol. Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 130.Starkman MN, et al. Improvement in learning associated with increase in hippocampal formation volume. Biol. Psychiatry. 2003;53:233–238. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- 131.Starkman MN, et al. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol. Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 132.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 133.Gianaros PJ, et al. Prospective reports of chronic life stress predict decreased gray matter volume in the hippocampus. NeuroImage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ganzel BL, et al. Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. NeuroImage. 2008;40:788–795. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 136.Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- 137.Sapolsky RM. Chickens, eggs and hippocampal atrophy. Nat. Neurosci. 2002;5:1111–1113. doi: 10.1038/nn1102-1111. [DOI] [PubMed] [Google Scholar]
- 138.Lupien SJ, et al. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. NeuroImage. 2007;34:479–485. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 139.Buss C, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J. Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pezawas L, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol. Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- 141.Bueller JA, et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol. Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 142.Szeszko PR, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 143.Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- 144.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci. STKE. 20042004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 145.Duman RS. Neurotrophic factors and regula tion of mood: role of exercise, diet and metabolism. Neurobiol. Aging. 2005;26(Suppl 1):88–93. doi: 10.1016/j.neurobiolaging.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 146.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Banks WA, Kastin AJ. Leucine modulates peptide transport system-1 across the blood-brain barrier at the stereospecific site within the central nervous system. J. Pharm. Pharmacol. 1991;43:252–254. doi: 10.1111/j.2042-7158.1991.tb06678.x. [DOI] [PubMed] [Google Scholar]
- 148.Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48:PL117–121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- 149.Maier SF, et al. The role of the vagus nerve in cytokine-to-brain communication. Ann. N. Y. Acad. Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 150.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 151.Rosell DR, et al. Spatiotemporal distribution of gp130 cytokines and their receptors after status epilepticus: comparison with neuronal degeneration and microglial activation. Neuroscience. 2003;122:329–348. doi: 10.1016/s0306-4522(03)00593-1. [DOI] [PubMed] [Google Scholar]
- 152.Rosell DR, et al. Differential expression of suppressors of cytokine signaling-1, -2, and -3 in the rat hippocampus after seizure: implications for neuromodulation by gp130 cytokines. Neuroscience. 2003;122:349–358. doi: 10.1016/s0306-4522(03)00594-3. [DOI] [PubMed] [Google Scholar]
- 153.Marsland AL, et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom. Med. 2006;68:895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- 154.Marsland AL, et al. Interleukin-6 Covaries Inversely with Hippocampal gray Matter Volume in Middle-Aged Adults. Biol. Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Friedman EM, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc. Natl. Acad. Sci. USA. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cho K. Chronic “jet lag” produces temporal lobe atrophy and spatial cognitive deficits. Nature Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 157.Bierhaus A, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 158.Gold SM, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 159.Convit A, et al. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc. Natl. Acad. Sci. USA. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Erickson KI, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 162.Pruessner JC, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. Psychiatry. 2007;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 163.Kern S, et al. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 165.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 167.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 168.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol. Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 170.Sah P, et al. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 171.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 172.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J. Comp. Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 173.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 174.Morecraft RJ, et al. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J. Comp. Neurol. 2007;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- 175.Taylor SE, et al. Early environment, emotions, responses to stress, and health. J. Pers. 2004;72:1376–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 176.Vyas A, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Conrad CD, et al. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 178.Wood GE, et al. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav. Neurosci. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- 179.Wood GE, et al. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm & Behav. 2003;43:205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 180.Ardayfio P, Kim K-S. Anxiogenic-like effect of chronic corticosterone in the light-dark emergency task in mice. Behav. Neurosci. 2006;120:249–256. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
- 181.Corodimas KP, et al. Corticosterone potentiation of learned fear. Annals of the New York Acad. Sci. 1994;746:392. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- 182.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 183.Pawlak R, et al. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nature Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- 184.Bennur S, et al. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 185.Govindarajan A, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. USA. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect Behav. Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 187.Phan KL, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 188.Phillips ML, et al. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 189.Phillips ML, et al. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 190.Neumann SA, et al. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biol. Psychiatry. 2006;60:1155–1162. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 191.Gianaros PJ, et al. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J. Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gianaros PJ, et al. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol. Psychiatry. 65:943–950. doi: 10.1016/j.biopsych.2008.10.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 194.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: toward development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 195.Phillips ML, et al. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 196.Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav. Brain Res. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 197.Carney R, Freedland K. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol. Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 198.Grippo A, Johnson K. Biological mechanisms in the relationship between depression and heart disease. Neurosci. Behav. Rev. 2002;26:941–962. doi: 10.1016/s0149-7634(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 199.Rudisch B, Nemeroff C. Epidemiology of co-morbid coronary artery disease and depression. Biol. Psychiatry. 2003;54:227–240. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 200.Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci. Biobehav. Rev. 2009;33:133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen E, Matthews KA. Cognitive appraisal biases: an approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann. Behav. Med. 2001;23:101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- 202.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: how and why do these relationships change with age? Psychol. Bull. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 203.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann. N.Y. Acad. Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 204.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom. Med. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 205.Gianaros PJ, et al. Potential neural embedding of parental social standing. Soc. Cog. Affective Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zink CF, et al. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mendelson T, Thurston RC, Kubzansky LD. Affective and cardiovascular effects of experimentally-induced social status. Health Psychol. 2008;27:482–489. doi: 10.1037/0278-6133.27.4.482. [DOI] [PubMed] [Google Scholar]
- 208.Roy JP. Socioeconomic status and health: a neurobiological perspective. Med. Hypotheses. 2004;62:222–227. doi: 10.1016/S0306-9877(03)00315-3. [DOI] [PubMed] [Google Scholar]
- 209.Compas BE. Psychobiological processes of stress and coping: implications for resilience in children and adolescents–comments on the papers of Romeo & McEwen and Fisher et al. Ann. N.Y. Acad. Sci. 2006;1094:226–234. doi: 10.1196/annals.1376.024. [DOI] [PubMed] [Google Scholar]
- 210.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 211.Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J. Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 213.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 214.Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex. 2005;30:1–9. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- 215.Liston C, et al. Stress-induced alterations in pre-frontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Crombag HS, et al. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cerebral Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 218.Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- 219.Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J. Comp. Neurol. 1991;313:522–538. doi: 10.1002/cne.903130312. [DOI] [PubMed] [Google Scholar]
- 220.Cerqueira JJ, et al. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Miracle AD, et al. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol. Learning Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 223.Santini E, et al. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Allman JM, et al. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann. New York Academy Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- 225.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cog. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 226.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 227.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 229.Paus T. Primate anterior cingulate cortex: where motor control, drive, and cognition interface. Nat. Neurosci. Rev. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 230.Lane RD, et al. The rebirth of neuroscience in psychosomatic medicine, Part II: clinical applications and implications for research. Psychosom. Med. 2009;71:135–151. doi: 10.1097/PSY.0b013e318198a11f. [DOI] [PubMed] [Google Scholar]
- 231.Lane RD, et al. The rebirth of neuroscience in psychosomatic medicine, part I: historical context, methods and relevant basic science. Psychosom. Med. 2009;71:117–134. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]
- 232.Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychol. Bull. 1984;96:435–464. [PubMed] [Google Scholar]
- 233.Manuck SB. Cardiovascular reactivity in cardiovascular disease: “Once more unto the breach.”. Int. J. Behav. Med. 1994;1:4–31. doi: 10.1207/s15327558ijbm0101_2. [DOI] [PubMed] [Google Scholar]
- 234.Treiber FA, et al. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom. Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 235.Gianaros PJ, et al. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Buchanan SL, Powell DA. Cingulothalamic and prefrontal control of autonomic function. In: Vogt BA, Gabriel M, editors. Neurobiology of the Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Birkhauser; Boston: 1993. pp. 381–414. [Google Scholar]
- 237.Berntson GG, Cacioppo JT. Integrative physiology: homeostasis, allostasis and the orchestration of systemic physiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. Cambridge University Press; Cambridge, UK: 2007. [Google Scholar]
- 238.Gianaros PJ, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc. Cog. Affect Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–590. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 240.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cog. Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 241.MacLullich AM, et al. Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic-pituitary-adrenal axis regulation in healthy elderly men. J. Clin. Endocrinol. Metabol. 2006;91:1591–1594. doi: 10.1210/jc.2005-2610. [DOI] [PubMed] [Google Scholar]
- 242.Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 243.Botvinick MM, et al. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 244.Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 245.Ridderinkhof KR, et al. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 246.Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. Eur. J. Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Critchley HD, et al. Volitional control of autonomic arousal: a functional magnetic resonance study. NeuroImage. 2002;16:909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- 248.Lane RD, et al. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J. Cogn. Neurosci. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- 249.Critchley HD, et al. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J. Physiol. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gianaros PJ, et al. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–635. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Eisenberger NI, et al. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Taylor SE, et al. Neural bases of moderation of cortisol stress responses by psychosocial resources. J. Pers. Soc. Psychol. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- 253.Taylor SE, Stanton AL. Coping resources, coping processes, and mental health. Ann. Rev. Clin. Psychol. 2007;3:377–401. doi: 10.1146/annurev.clinpsy.3.022806.091520. [DOI] [PubMed] [Google Scholar]
- 254.Bernadet P. Benefits of physical activity in the prevention of cardiovascular disease. J. Cardiovasc. Pharmacol. 1995;25:S3–S8. doi: 10.1097/00005344-199525001-00003. [DOI] [PubMed] [Google Scholar]
- 255.Perseghin G, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. The New England J. Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 256.Kramer AF, et al. Enhancing brain and cognitive function of older adults through fitness training. J. Mol. Neurosci. 2003;20:213–221. doi: 10.1385/JMN:20:3:213. [DOI] [PubMed] [Google Scholar]
- 257.Barbour KA, Blumenthal JA. Exercise training and depression in older adults. Neurobiol. Aging. 2005;26S:S119–S123. doi: 10.1016/j.neurobiolaging.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 258.Rovio S, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. The Lancet. Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 259.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 260.van Praag H, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Olson AK, et al. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 262.Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- 263.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;26:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 264.Karten YJG, et al. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus. 2006;16:312–320. doi: 10.1002/hipo.20165. [DOI] [PubMed] [Google Scholar]
- 265.Lee J, Seroogy KB, Mattson MP. Dietary re striction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 266.Hariri AR, et al. Brain-derived neurotrophic factor val66 met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pezawas L, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jiang X, et al. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- 269.Szeszko PR, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiat. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 270.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 271.Colcombe SJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Colcombe SJ, et al. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 273.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Moore LV, et al. Availability of recreational resources in minority and low socioeconomic status areas. Am. J. Prev. Med. 2008;34:16–22. doi: 10.1016/j.amepre.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Berkman LF, Glass T. Social integration, social networks, social support, and health. In: Berkman LF, Kawachi I, editors. Social Epidemiology. Oxford Press; New York: 2000. [Google Scholar]
- 276.Cohen S. Social relationships and health. Am. Psychol. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- 277.Cohen S, Janicki-Deverts D. Can we improve our physical health by altering our social networks? Perspect. Psychol. Sci. doi: 10.1111/j.1745-6924.2009.01141.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Uchino BN. Social support and physical health. Yale University Press; New Haven: 2004. [Google Scholar]
- 279.Brissette I, Cohen S, Seeman TE. Measuring social integration and social networks. In: Cohen S, Underwood L, Gottlieb B, editors. Measuring and intervening in social support. Oxford Press; New York: 2000. [Google Scholar]
- 280.Helgeson VS. Social support and quality of life. Qual. Life Res. 2003;12(Suppl 1):25–31. doi: 10.1023/a:1023509117524. [DOI] [PubMed] [Google Scholar]
- 281.Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol. Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 282.Reis HT, Collins WA, Berscheid E. The relationship context of human behavior and development. Psychol. Bull. 2000;126:844–872. doi: 10.1037/0033-2909.126.6.844. [DOI] [PubMed] [Google Scholar]
- 283.Cacioppo JT, et al. Loneliness and health: potential mechanisms. Psychosom. Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 284.Cohen S. Psychosocial models of the role of social support in the etiology of physical disease. Health Psychol. 1988;7:269–297. doi: 10.1037//0278-6133.7.3.269. [DOI] [PubMed] [Google Scholar]
- 285.Berkman LF. The role of social relations in health promotion. Psychosom. Med. 1995;57:245–254. doi: 10.1097/00006842-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 286.Seeman TE. Social ties and health: the benefits of social integration. Ann. Epidemiol. 1996;6:442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- 287.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 288.Kop WJ, et al. Social network and coronary artery calcification in asymptomatic individuals. Psychosom. Med. 2005;67:343–352. doi: 10.1097/01.psy.0000161201.45643.8d. [DOI] [PubMed] [Google Scholar]
- 289.Rutledge T, et al. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosom. Med. 2008;70:282–287. doi: 10.1097/PSY.0b013e3181656e09. [DOI] [PubMed] [Google Scholar]
- 290.Rutledge T, et al. Social networks are associated with lower mortality rates among women with suspected coronary disease: the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation study. Psychosom. Med. 2004;66:882–888. doi: 10.1097/01.psy.0000145819.94041.52. [DOI] [PubMed] [Google Scholar]
- 291.Helgeson VS, Cohen S, Fritz HL. Social ties and cancer. In: Holland JC, Breitbart W, editors. Psychooncology. Oxford Press; New York: 1998. pp. 99–109. [Google Scholar]
- 292.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 293.Finfgeld-Connett D. Clarification of social support. J. Nurs. Scholarsh. 2005;37:4–9. doi: 10.1111/j.1547-5069.2005.00004.x. [DOI] [PubMed] [Google Scholar]
- 294.Seeman TE, et al. Social relationships, gender, and allostatic load across two age cohorts. Psychosom. Med. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 295.Epel ES, et al. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Adams RE, Boscarino JA. Predictors of PTSD and delayed PTSD after disaster: the impact of exposure and psychosocial resources. J. Nerv. Ment. Dis. 2006;194:485–493. doi: 10.1097/01.nmd.0000228503.95503.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Leskela U, et al. The influence of adversity and perceived social support on the outcome of major depressive disorder in subjects with different levels of depressive symptoms. Psychol. Med. 2006;36:779–788. doi: 10.1017/S0033291706007276. [DOI] [PubMed] [Google Scholar]
- 298.Saxena S, Jane-Llopis E, Hosman C. Prevention of mental and behavioural disorders: implications for policy and practice. World Psychiatry. 2006;5:5–14. [PMC free article] [PubMed] [Google Scholar]
- 299.Silver EJ, et al. The relationship of depressive symptoms to parenting competence and social support in inner-city mothers of young children. Matern. Child. Health J. 2006;10:105–112. doi: 10.1007/s10995-005-0024-4. [DOI] [PubMed] [Google Scholar]
- 300.Fowler CD, et al. The effects of social environment on adult neurogenesis in the female prairie vole. J. Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- 301.Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res. Rev. 2008;57:342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cacioppo JT, et al. In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. J. Cogn. Neurosci. 2009;21:83–92. doi: 10.1162/jocn.2009.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sanghi S, et al. Cyclooxygenase-2 inhibitors: a painful lesson. Cardiovasc. Hematol. Disord. Drug Targets. 2006;6:85–100. doi: 10.2174/187152906777441803. [DOI] [PubMed] [Google Scholar]
- 304.Vestri HS, et al. Atypical antipsychotic drugs directly impair insulin action in adipocytes: effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology. 2007;32:765–772. doi: 10.1038/sj.npp.1301142. [DOI] [PubMed] [Google Scholar]
- 305.de Lange FP, et al. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131:2172–2180. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- 306.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat. Rev. Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lazar SW, et al. Meditation experience is associated with increased cortical thickness. NeuroReport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Aldana SG. Financial impact of health promotion programs: a comprehensive review of the literature. Am. J. Health Promotion. 2001;15:296–320. doi: 10.4278/0890-1171-15.5.296. [DOI] [PubMed] [Google Scholar]
- 309.Pelletier KR. A review and analysis of the clinical and cost-effectiveness studies of comprehensive health promotion and disease management programs at the worksite: 1998–2000 update. Am. J. Health Promotion. 2001;16:107–115. doi: 10.4278/0890-1171-16.2.107. [DOI] [PubMed] [Google Scholar]
- 310.Acheson SD. Independent Inquiry into Inequalities in Health Report. The Stationary Office; London: 1998. [Google Scholar]
- 311.Farah MJ, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev. Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 312.Farah MJ, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 313.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 314.Noble KG, et al. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev. Sci. 2006;9:642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 315.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective effects. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 316.Larson EB, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 317.Frick KD, et al. Modeled cost-effectiveness of the experience corps Baltimore based on a pilot randomized trial. J. Urban Health Bull. N.Y. Acad. Med. 2004;81:106–117. doi: 10.1093/jurban/jth097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Fried LP, et al. A social model for health promotion for an aging population: initial evidence on the experience corps model. J. Urban Health: Bull. N.Y. Acad. Med. 2004;81:64–78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]