Abstract
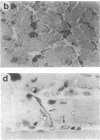
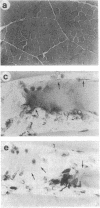
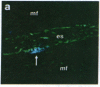
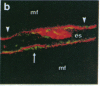
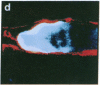
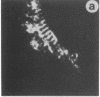
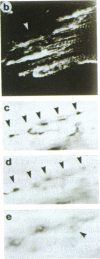
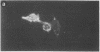
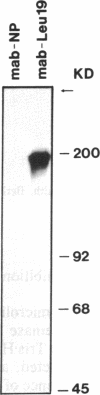
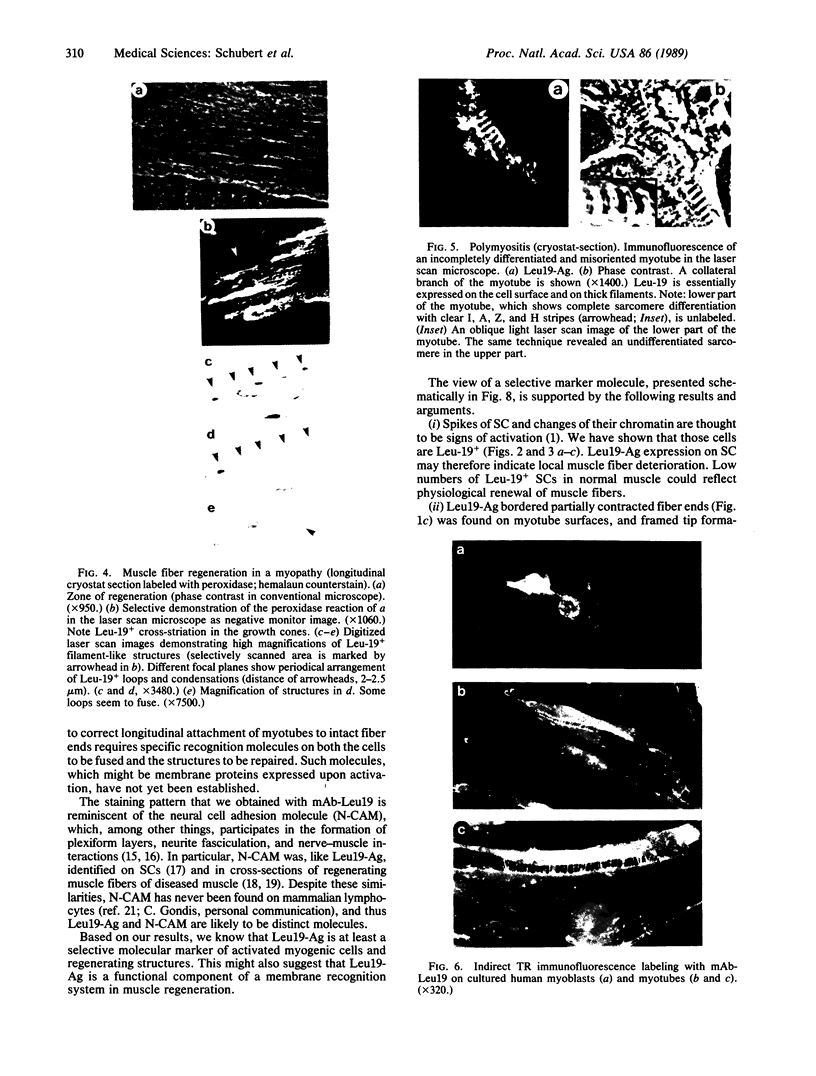
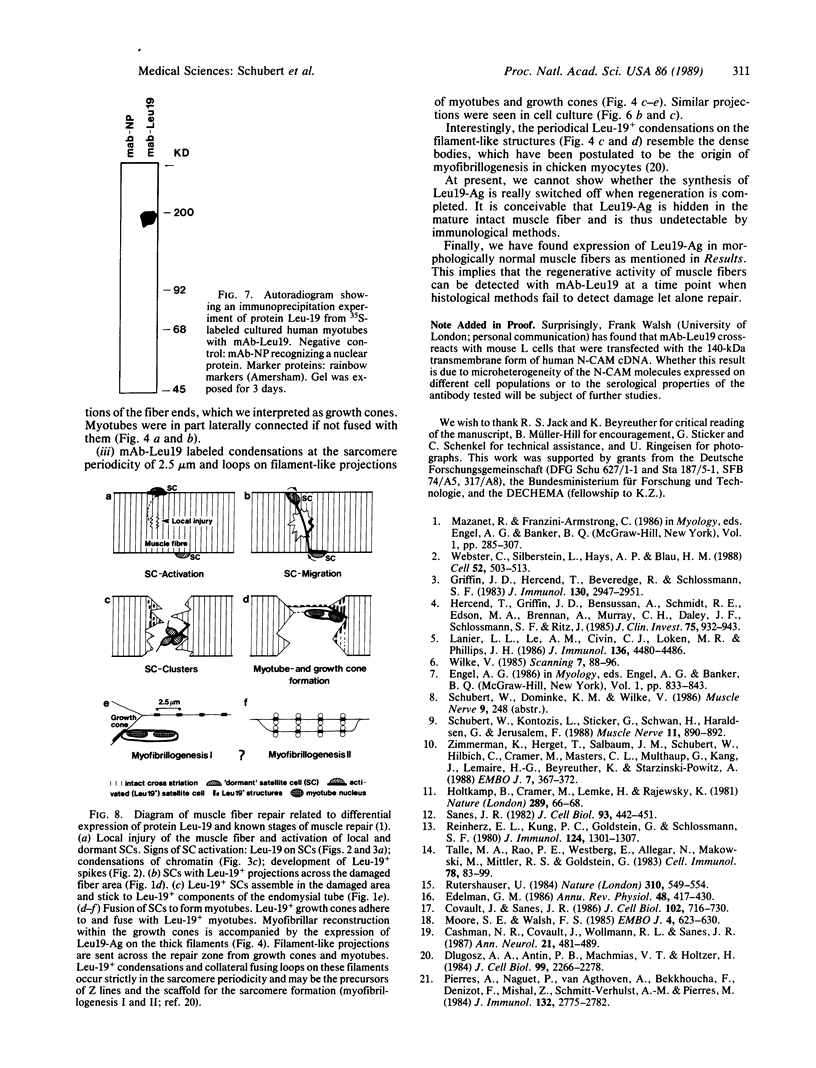
Antigen Leu-19 (Leu19-Ag), a 200- to 220-kDa surface glycoprotein, was originally identified on a subset of human peripheral lymphocytes exhibiting non-major histocompatibility complex-restricted cytotoxicity. Here we report that monoclonal antibody Leu-19 (mAb-Leu19) labels structures in human skeletal muscle: (i) satellite cells, which form the stem cell pool of muscle fiber regeneration, both in normal and diseased muscle; (ii) myotubes and myotube projections in regions of muscle fiber repair; (iii) periodically organized fibrillar structures in areas of regeneration; (iv) the surface of myoblasts and developing myotubes in culture. mAb-Leu19 precipitated a protein of approximately 200 kDa from cultured muscle cells. Our data show that Leu19-Ag is expressed on muscle-specific components of myosegments in repair and thus represents a molecular marker of muscle regeneration. On the basis of this molecular marker and using laser scan microscopy, it is possible to visualize at the light microscopic level hitherto undetectable details of muscle regeneration in routine cryostat sections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashman N. R., Covault J., Wollman R. L., Sanes J. R. Neural cell adhesion molecule in normal, denervated, and myopathic human muscle. Ann Neurol. 1987 May;21(5):481–489. doi: 10.1002/ana.410210512. [DOI] [PubMed] [Google Scholar]
- Covault J., Sanes J. R. Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J Cell Biol. 1986 Mar;102(3):716–730. doi: 10.1083/jcb.102.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz A. A., Antin P. B., Nachmias V. T., Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984 Dec;99(6):2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in neural histogenesis. Annu Rev Physiol. 1986;48:417–430. doi: 10.1146/annurev.ph.48.030186.002221. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Hercend T., Beveridge R., Schlossman S. F. Characterization of an antigen expressed by human natural killer cells. J Immunol. 1983 Jun;130(6):2947–2951. [PubMed] [Google Scholar]
- Hercend T., Griffin J. D., Bensussan A., Schmidt R. E., Edson M. A., Brennan A., Murray C., Daley J. F., Schlossman S. F., Ritz J. Generation of monoclonal antibodies to a human natural killer clone. Characterization of two natural killer-associated antigens, NKH1A and NKH2, expressed on subsets of large granular lymphocytes. J Clin Invest. 1985 Mar;75(3):932–943. doi: 10.1172/JCI111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp B., Cramer M., Lemke H., Rajewsky K. Isolation of a cloned cell line expressing variant H-2Kk using fluorescence-activated cell sorting. Nature. 1981 Jan 1;289(5793):66–68. doi: 10.1038/289066a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Moore S. E., Walsh F. S. Specific regulation of N-CAM/D2-CAM cell adhesion molecule during skeletal muscle development. EMBO J. 1985 Mar;4(3):623–630. doi: 10.1002/j.1460-2075.1985.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Rutishauser U. Developmental biology of a neural cell adhesion molecule. Nature. 1984 Aug 16;310(5978):549–554. doi: 10.1038/310549a0. [DOI] [PubMed] [Google Scholar]
- Sanes J. R. Laminin, fibronectin, and collagen in synaptic and extrasynaptic portions of muscle fiber basement membrane. J Cell Biol. 1982 May;93(2):442–451. doi: 10.1083/jcb.93.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W., Kontozis L., Sticker G., Schwan H., Haraldsen G., Jerusalem F. Immunofluorescent evidence for presence of interleukin-1 in normal and diseased human skeletal muscle. Muscle Nerve. 1988 Aug;11(8):890–892. doi: 10.1002/mus.880110814. [DOI] [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983 May;78(1):83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A. P., Blau H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988 Feb 26;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann K., Herget T., Salbaum J. M., Schubert W., Hilbich C., Cramer M., Masters C. L., Multhaup G., Kang J., Lemaire H. G. Localization of the putative precursor of Alzheimer's disease-specific amyloid at nuclear envelopes of adult human muscle. EMBO J. 1988 Feb;7(2):367–372. doi: 10.1002/j.1460-2075.1988.tb02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]