Abstract
Introduction
The opiorphins are a newly characterized class of peptides that act as potent endogenous neutral endopeptidase (NEP) inhibitors. Recent reports have suggested that they play an important role in erectile physiology.
Aim
This article reviews recent developments that increase our understanding of the role of the opiorphin family of peptides in erectile physiology.
Methods
During a microarray screen of gene changes that occur in a rat diabetic model of erectile dysfunction (ED), Vcsa1 was one of the most down-regulated genes in the rat corpora. Quantitative real-time polymerase chain reaction demonstrated that in at least three models of diseases that result in ED (diabetes, aging, and cavernous nerve [CN] transection), Vcsa1 was down-regulated in the rat corpora. The human opiorphin family of genes (hSMR3A/B and ProL1) also acts as markers of erectile function in patients with ED.
Main Outcome Measures
The reader will be informed of the most current research regarding the role of opiorphins in urogenital smooth muscle biology.
Results
These observations led to the suggestion that genes encoding opiorphins (and potentially their peptide products) can act as markers of ED. Gene transfer of plasmids overexpressing Vcsa1 in aging rats, as well as intracorporal injection of sialorphin, led to an improvement in erectile function. In organ bath studies, we demonstrated that sialorphin can cause increased rates of relaxation of corporal smooth muscle (CSM). We have also demonstrated that in vitro, Vcsa1 causes changes in the expression of G-protein-coupled receptors (GPCRs). This has led us to suggest that the action of Vcsa1 on erectile physiology may act through relaxation of CSM by its ability to act as an inhibitor of NEP, therefore prolonging the action of peptide agonists at their GPCRs.
Conclusions
Overall, there is a growing body of evidence that the opiorphins play a role in regulating CSM tone and thereby erectile function.
Keywords: Opiorphins, Smooth Muscle, Erectile Dysfunction, Urogenital Dysfunction, Neutral Endopeptidase, G-Protein-Coupled Receptor
Introduction
There is a growing evidence that a family of peptides, called opiorphins, plays an important role in the regulation of a diverse range of physiologic processes, including erectile function. The opiorphins are pentapeptides derived by post-translational processing from their parent proteins [1,2]. In humans, there are three genes encoding opiorphin homologs: ProL1, hSMR3A, and hSMR3B [3]. The name opiorphin was first applied to the pentapeptide product of ProL1 by Catherine Rougeot of the Pasteur Institute in 2007 [1]. The other human homologs, hSMR3A and hSMR3B, are two very closely related genes, both of which are potentially post-translationally processed to the same opiorphin homolog, which we call hSMR3, to distinguish it from opiorphin (Table 1). In rats, the Vcsa1 gene encodes the opiorphin homolog, called sialorphin [2]. Vcsa1 has a very tissue-specific distribution. In the rat, the main tissues where the transcript is significantly expressed are the corporal smooth muscle (CSM), submandibular gland (SMG), and prostate [2,4].
Table 1.
The opiorphin family of genes and the mature peptides they encode
| Gene sequence | Pentapetide | Species | Amino acid |
|---|---|---|---|
| Vcsa1 | Sialorphin | Rat | QHNPR |
| ProL1 | Opiorphin | Human | QRFSR |
| hSMR3A/B | hSMR3 | Human | QRGPR (hypothetical peptide) |
| Consensus | Q r · p R |
Vcsa1 expression is hormonally regulated by androgens, resulting in a marked gender-specific expression of Vcsa1. There is a 1,000-fold greater expression in the SMG of male rats vs. female rats [5]. The expression of Vcsa1 in the two other major sites of synthesis in the rat (the CSM and prostate) is also potentially regulated in an androgen-sensitive manner, as both organs have been shown to express androgen receptors [6,7]. In addition, the release of the sialorphin pentapeptide from the SMG into the bloodstream or saliva has been shown to be subject to regulation by epinephrine. Circulating levels of sialorphin under resting conditions are approximately 0.4 ng/mL; however, following intraperitoneal injection of epinephrine, serum levels increased to about 1.2 ng/mL [2].
Genes for the Opiorphins Are Down-Regulated in the Corporal Tissue of Animals with Erectile Dysfunction (ED)
Our interest in the role of Vcsa1 in erectile function began in 2005. During that year, we were performing microarray analysis of gene expression changes that occur in the corpora of a Type 1 model for diabetes (streptozotocin-treated rats). One of the results of this analysis was that expression of the Vcsa1 gene is significantly down-regulated in corporal tissue with the onset of diabetes-induced ED. The down-regulation of the gene is related to the duration of diabetes. It is the most down-regulated gene (14.3-fold) by microarray analysis after 4 months of diabetes (Table 2). We focused on the Vcsa1 gene because of a publication in 2003 from Kevin McVary’s group at Northwestern University that had shown that the same gene (called SMR3A at that time) was down-regulated in the corpora of rats in a neurogenic model of ED [8]. In both models of ED at later time points (in the neurogenic model after 28 days [8] and in the diabetic model at 6 months [Table 2]), there was some reversal of the down-regulation of Vcsa1, which was suggested to be a compensatory mechanism in the neurogenic model of ED [8].
Table 2.
Vcsa1 expression in corpora of diabetic compared with normal animals as determined by microarray analysis using the RGU34A chip
| Duration of diabetes | Rank | Fold change (average) | Number of microarrays |
|---|---|---|---|
| 1 week | 480 | −1.5 | 7 |
| 2 months | 5 | −3.6 | 5 |
| 4 months | 1 | −14.3 | 3 |
| 6 months | 65 | −3.2 | 3 |
M = log fold difference; rank = position on a ranked list of all genes (approximately 8,800) going from most fold change to least fold change; N = number of diabetic microarray chip analyzed in this analysis.
Using quantitative real-time polymerase chain reaction, we demonstrated that the Vcsa1 transcript was down-regulated not only in diabetic animals but also in the corpora of old animals with ED. In order to determine erectile function, we measured the intracorporal pressure/blood pressure (area under the curve, ICP/BP) ratio following electrostimulation of the cavernous nerve (Figure 1A). In older rats, there is a decrease in the ICP/BP ratio when the cavernous nerve is electro-stimulated compared with younger animals. In Sprague–Dawley rats, this occurs at around 9–10 months of age. We demonstrated that this decrease of erectile function with age was accompanied by a decrease in the levels of Vcsa1 transcript in the corpora (Figure 1B). This work led us to suggest that Vcsa1 acts as a marker of ED resulting from several etiologies [4].
Figure 1.
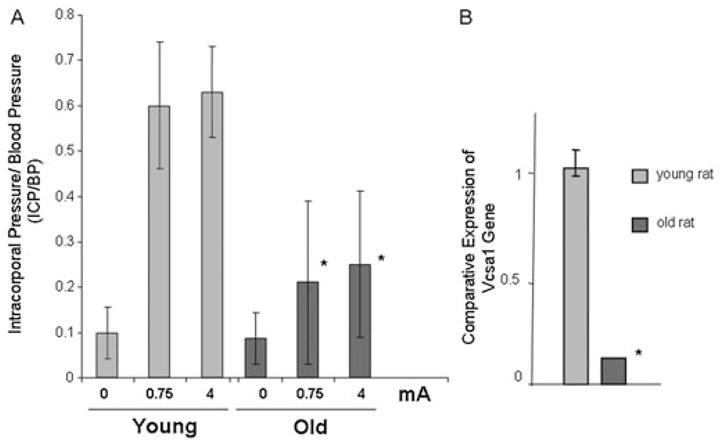
(A) The erectile function was determined in seven young (3–4 months) Sprague–Dawley rats and six old (9–10 months) Sprague–Dawley rats. The average intracorporal pressure/blood pressure (ICP/BP) following stimulation of the cavernous nerve with 0.75 or 4 mA is shown. *Significantly increased ICP/BP response at the level of stimulation in old compared with young animals (P < 0.05). (B) The expression of Vcsa1 in the corpora of young and old (retired breeder) Sprague–Dawley rats. The expression of Vcsa1 transcripts was normalized to the housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase) and was analyzed using the comparative crossing threshold method. The control bladder tissue was used as the calibrator tissue (set as 1). Each quantitative polymerase chain reaction measurement was performed in duplicate on six animals. The bars represent the mean comparative expression of the gene, and the error bars represent the standard deviation. *Significantly different expression of Vcsa1 compared with the young animals (P < 0.05). (Figure is based on data published in Tong et al. [4].)
In two recent articles, we have shown that compared with patients without ED, the human homologs to Vcsa1 (hSMR3A/B and ProL1) are also reduced in expression in corporal tissue from patients with ED as a result of diabetes or with ED not related to diabetes [3,9]. In addition, by determining the effects of gene transfer of plasmids expressing hSMR3A/B or ProL1 on erectile function in the rat, we hypothesized that both ProL1 and hSMR3A/B have similar roles in erectile physiology to Vcsa1 [3].
We have therefore proposed that the opiorphin family of genes may act as a biomarker for organic ED. The importance of identifying markers for ED has developed with an increasing realization within the medical community that ED is strongly correlated with the overall health of men. ED may act as a predictor for the development of cardiovascular disease (CVD) and diabetes. It is now recommended that patients with ED should be put on cardioprotective regimens. Given this scenario, it would be useful to distinguish between disease-and aging-based (organic) dysfunction from potentially reversible anxiety or psychogenic ED using an objective test with a biomarker. Also, it could be potentially useful to inform patients that ED is psychogenic and a reversible condition, as well as medical insurance reimbursement issues. In addition, such biomarkers may act as objective measures of the type and degree of ED and as a measure of the efficacy of treatment.
Sialorphin Can Directly Impact Erectile Physiology
We have demonstrated that the Vcsa1 gene can have a direct effect on erectile physiology. We intracorporally injected plasmids expressing Vcsa1 (pVAX-Vcsa1) into the corpora of old (9–10 months) Sprague–Dawley rats and compared the effect on erectile function with the empty vector (pVAX) and a positive control (pVAX-hSlo) ([4,9]. pVAX-hSlo is a potential gene therapy treatment of ED in clinical trials [10–12]. Erectile function was determined 1 week by observation, histology, and ICP/BP following electrical stimulation of the cavernous nerve with 0.75 or 4 mA. At the higher doses (80 μg or higher) of plasmids expressing Vcsa1 or hSMR3A, there were visible indications of edema, a possible indication of a vasocongested state, whereas in untreated control animals, corporal morphology appeared normal [3,4,9]. Histologic examination and comparison to control animals also suggest that Vcsa1 and SMR3A cause changes in the morphology of the penis, which might be a result of vasocongestion (priapism-like state). The dorsal vein is often enlarged in the treated animals, which would occur if there was increased blood flow or post-penile obstruction (not observed). In addition, there is evidence of sinusoidal congestion of blood in animals treated with Vcsa1 and hSMR3A but not observed in the control animals. We hypothesized that this priapic-like condition resulted in damage to the penile tissue, which reduced the ICP/BP response to electrical stimulation (Figure 2). However, at lower levels of gene transfer of pVAX-Vcsa1 (5 and 25 μg), there was a significant increase in erectile function compared with the control.
Figure 2.
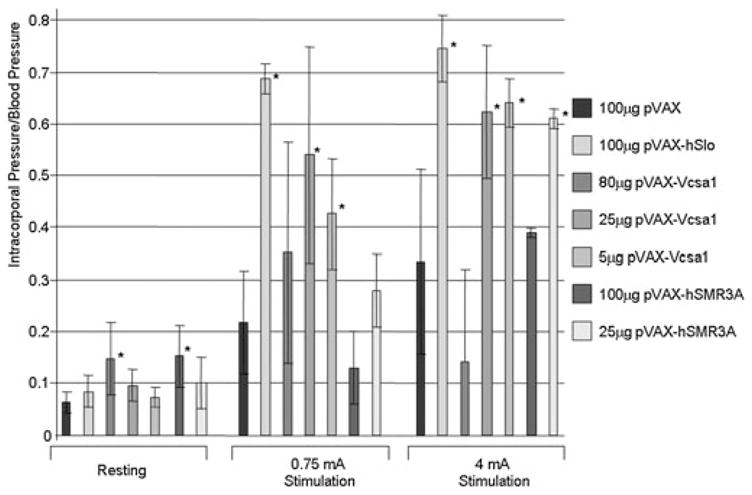
Erectile function was determined by measuring intracorporal pressure/blood pressure (ICP/BP) following electrical stimulation of old rats following various intracorporal gene transfer treatments using 5, 25, or 80 μg pVAX-Vcsa1 or empty vector (100 μg pVAX). A positive control was the plasmid expressing hSlo (100 μg pVAX-hSlo), which is currently undergoing clinical trials for treatment of patients with erectile dysfunction, and a negative control was the empty plasmid. *Significant difference in ICP/BP to the control animals (100 μg pVAX) at the same level of stimulation (P < 0.05). These data are a compilation of data published and described in more detail by our group [4].
In a separate article, we also demonstrated that the gene product of Vcsa1, sialorphin, also has a direct effect on erectile physiology [13]. We injected 100 μg sialorphin directly into the corpora of old (9–10 months) rats. Over the course of 1 hour, we measured the ICP/BP ratio following stimulation at 0.75 and 4 mA. We observed that there was a significant improvement of erectile function by sialorphin after 35–45 minutes, which increased with a longer time point (55–65 minutes) [13]. The long-lasting effect of sialorphin suggests that it binds tightly to its targets in the corpora.
Erectile function is physiologically related to the ability of the CSM tissue to “relax.” Therefore, we determined the effects of sialorphin on the tension of CSM strips under various conditions in an organ bath. Tissue strips were first contracted with phenylepherine. C-type natriuretic peptide was then added to the bath, which induces relaxation through activation of GPCR signaling pathways [14]. Addition of sialorphin to the media caused an increase in the rate of relaxation of CSM tissue. We proposed that the ability of sialorphin to improve erectile function was an increase in the ability of CSM to undergo relaxation.
The Opiorphin Homologs Are Inhibitors of Neutral Endopeptidase (NEP)
Both sialorphin and opiorphin have been shown to be inhibitors of NEP [1,15]. NEP plays an important role in regulating the activity of peptide agonists by catalyzing their proteolysis and removal from membrane receptors to which they are bound. The inhibition of NEP by the opiorphin homologs could therefore influence the physiology of a wide variety of tissues by causing extended binding time of peptide agonists to their receptors. One of the most important groups of peptide receptors are G-protein-coupled receptors (GPCRs), which are a superfamily of proteins with over 800 estimated to be encoded by the human genome [16–18]. The GPCRs function in the regulation of transmission of signals from the cell membrane to the interior of cells. Defects in their regulation can result in uncontrolled stimulation of cellular processes resulting in diseases such as blindness, obesity, inflammation, depression, asthma, and hypertension [19,20]. Given the central importance of GPCR in regulating cellular pathways, it is not surprising that the majority of all prescribed drugs target either activation of GPCR or their downstream pathways [21,22]. Cells have evolved several mechanisms to prevent overstimulation of GPCR signaling pathways; for example, long-term exposure of cells to GPCR agonists results in the down-regulation of GPCR expression through altered rates of receptor degradation and synthesis [23].
The Opiorphins Regulate GPCR Expression
Recent results from our laboratory demonstrate that in CSM in vitro, down-regulation of Vcsa1 through siRNA knockdown causes a compensatory up-regulation in the expression of GPCR [24]. We hypothesized that in CSM cells, there is a compensatory up-regulation of the GPCR as a result of the decreased stimulation of agonists at the GPCR because of the increased activity of NEP in the absence of sialorphin (as shown in Figure 3). The down-regulation of Vcsa1 expression, although effecting up-regulating GPCR receptors as a group, changes the expression of individual receptors to different levels. Although the activation of GPCR could potentially result in either an increase or decrease in smooth muscle tone, a possible explanation for the decrease in smooth muscle tone caused by Vcsa1 may be a result of this differential targeting of specific GPCR.
Figure 3.
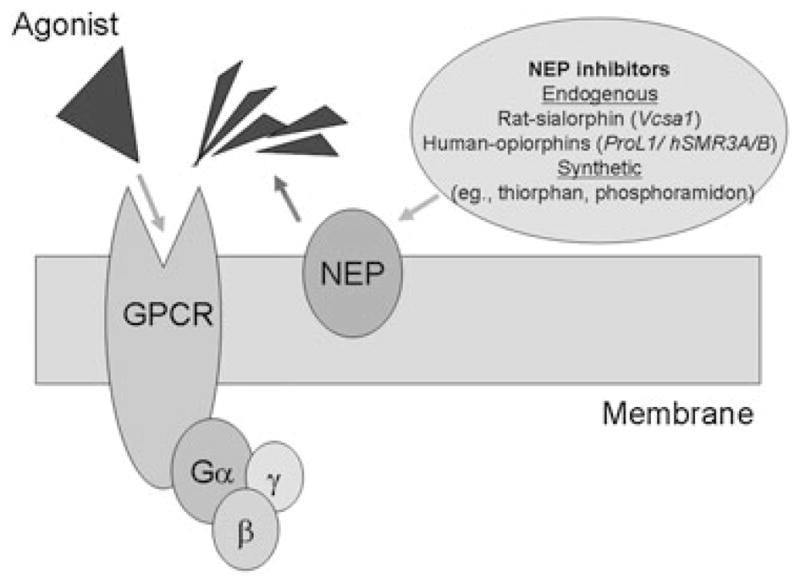
An agonist binds to G-protein-coupled receptors (GPCRs) to activate Ga-subunit. Neutral endopeptidase (NEP) acts on the agonist to remove it. The action of sialorphin is to prolong agonist stimulation of its receptor.
As a wide range of cellular processes are regulated through GPCR signaling, it is not surprising that sialorphin, which can alter the duration of binding of an agonist to its receptor, has been demonstrated to be involved in several physiologic processes, such as pain perception, antidepressant effects, sexual behavior, and erectile function in rats [4,13,15,25]. Other synthetic NEP inhibitors, such as phosphoramidon and thiorphan, have also been shown to have similar physiologic effects on the vascular, cardiovascular, and renal function of rats [14,26].
We hypothesized that the ability of the opiorphins to relax CSM, and thereby cause an erection (or at higher levels, a priapic-like condition), is mediated through their ability to modulate NEP activity and, therefore, the activity of peptide agonists bound to the cell membrane. As the opiorphins are released into the bloodstream, they also have the potential to act on organs distal to their sites of synthesis. This could be a possible explanation for ED being a predictor of CVD [27–29]. It is assumed that this correlation is because of the common mechanisms of generation of vascular disease acting in the endothelial cells of blood vessels in the penis and heart [30–32]. However, our work raises the possibility that the down-regulation of expression of human opiorphin genes in the penis and, thereby, circulating levels of sialorphin, may potentially modulate GPCR activity in other vascular tissues resulting in pathophysiology.
Acknowledgments
Kelvin P. Davies is supported by grants awarded by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R21DK079594 and R01DK077665).
Footnotes
Conflict of Interest: None declared.
References
- 1.Wisner A, Dufour E, Messaoudi M, Nejdi A, Marcel A, Ungeheuer MN, Rougeot C. Human opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc Natl Acad Sci USA. 2006;103:17979–84. doi: 10.1073/pnas.0605865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rougeot C, Rosinski-Chupin I, Njamkepo E, Rougeon F. Selective processing of submandibular rat 1 protein at dibasic cleavage sites. Salivary and bloodstream secretion products. Eur J Biochem. 1994;219:765–73. doi: 10.1111/j.1432-1033.1994.tb18556.x. [DOI] [PubMed] [Google Scholar]
- 3.Tong Y, Tar M, Melman A, Davies K. The opiorphin gene (ProL1) and its homologues function in erectile physiology. BJU Int. 2008;102:736–40. doi: 10.1111/j.1464-410X.2008.07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98:396–401. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosinski-Chupin I, Huaulme JF, Rougeot C, Rougeon F. The transcriptional response to androgens of the rat VCSA1 gene is amplified by both binary and graded mechanisms. Endocrinology. 2001;142:4550–9. doi: 10.1210/endo.142.10.8428. [DOI] [PubMed] [Google Scholar]
- 6.DiSanto ME. Corpus cavernosum smooth muscle physiology: A role for sex hormones? J Androl. 2003;24:S6–16. doi: 10.1002/j.1939-4640.2003.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 7.Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: Role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–63. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 8.User HM, Zelner DJ, McKenna KE, McVary KT. Microarray analysis and description of SMR1 gene in rat penis in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;170:298–301. doi: 10.1097/01.ju.0000060882.75475.5a. [DOI] [PubMed] [Google Scholar]
- 9.Tong Y, Tar M, Monrose V, DiSanto M, Melma A, Davies KP. hSMR3A as a marker for patients with erectile dysfunction. J Urol. 2007;178:338–43. doi: 10.1016/j.juro.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. Plasmid-based gene transfer for treatment of erectile dysfunction and overactive bladder: Results of a phase I trial. Isr Med Assoc J. 2007;9:143–6. [PubMed] [Google Scholar]
- 11.Melman A, Biggs G, Davies K, Zhao W, Tar MT, Christ GJ. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther. 2008;15:364–70. doi: 10.1038/sj.gt.3303093. [DOI] [PubMed] [Google Scholar]
- 12.Melman A, Feder M. Gene therapy for the treatment of erectile dysfunction. Nat Clin Pract Urol. 2008;5:60–1. doi: 10.1038/ncpuro1014. [DOI] [PubMed] [Google Scholar]
- 13.Davies KP, Tar M, Rougeot C, Melman A. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99:431–5. doi: 10.1111/j.1464-410X.2006.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marton Z, Pataricza J, Krassoi I, Varro A, Papp JG. NEP inhibitors enhance C-type natriuretic peptide-induced relaxation in porcine isolated coronary artery. Vascul Pharmacol. 2005;43:207–12. doi: 10.1016/j.vph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Rougeot C, Messaoudi M, Hermitte V, Rigault AG, Blisnick T, Dugave C, Desor D, Rougeon F. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci USA. 2003;100:8549–54. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchese A, George SR, Kolakowski LF, Jr, Lynch KR, O’Dowd BF. Novel GPCRs and their endogenous ligands: Expanding the boundaries of physiology and pharmacology. Trends Pharmacol Sci. 1999;20:370–5. doi: 10.1016/s0165-6147(99)01366-8. [DOI] [PubMed] [Google Scholar]
- 17.Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 18.Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 19.Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, Pardo L, Leurs R. Pharmacogenomic and structural analysis of constitutive G protein-coupled receptor activity. Annu Rev Pharmacol Toxicol. 2007;47:53–87. doi: 10.1146/annurev.pharmtox.47.120505.105126. [DOI] [PubMed] [Google Scholar]
- 20.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- 21.Armbruster BN, Roth BL. Mining the receptorome. J Biol Chem. 2005;280:5129–32. doi: 10.1074/jbc.R400030200. [DOI] [PubMed] [Google Scholar]
- 22.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–50. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 23.Bohm SK, Grady EF, Bunnett NW. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong Y, Tiplitsky SI, Tar M, Melman A, Davies KP. Transcription of G-protein coupled receptors in corporeal smooth muscle is regulated by the endogenous neutral endopeptidase inhibitor sialorphin. J Urol. 2008;180:760–6. doi: 10.1016/j.juro.2008.03.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messaoudi M, Desor D, Nejdi A, Rougeot C. The endogenous androgen-regulated sialorphin modulates male rat sexual behavior. Horm Behav. 2004;46:684–91. doi: 10.1016/j.yhbeh.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Park KS, Li Y, Zhang Y, Liu H, Swain MG, Lee SS. Effects of the neutral endopeptidase inhibitor thiorphan on cardiovascular and renal function in cirrhotic rats. Br J Pharmacol. 2003;139:81–8. doi: 10.1038/sj.bjp.0705219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grover SA, Lowensteyn I, Kaouache M, Marchand S, Coupal L, DeCarolis E, Zoccoli J, Defoy I. The prevalence of erectile dysfunction in the primary care setting: Importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166:213–9. doi: 10.1001/archinte.166.2.213. [DOI] [PubMed] [Google Scholar]
- 28.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 29.Kupelian V, Shabsigh R, Araujo AB, O’Donnell AB, McKinlay JB. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: Results from the Massachusetts Male Aging Study. J Urol. 2006;176:222–6. doi: 10.1016/S0022-5347(06)00503-9. [DOI] [PubMed] [Google Scholar]
- 30.Kirby M, Jackson G, Simonsen U. Endothelial dysfunction links erectile dysfunction to heart disease. Int J Clin Pract. 2005;59:225–9. doi: 10.1111/j.1742-1241.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 31.Muller A, Mulhall JP. Cardiovascular disease, metabolic syndrome and erectile dysfunction. Curr Opin Urol. 2006;16:435–43. doi: 10.1097/01.mou.0000250284.83108.a6. [DOI] [PubMed] [Google Scholar]
- 32.Eaton CB, Liu YL, Mittleman MA, Miner M, Glasser DB, Rimm EB. A retrospective study of the relationship between biomarkers of atherosclerosis and erectile dysfunction in 988 men. Int J Impot Res. 2007;19:218–25. doi: 10.1038/sj.ijir.3901519. [DOI] [PubMed] [Google Scholar]


