Abstract
Introduction
Nanoparticles represent a potential novel mechanism for transdermal delivery of erectogenic agents directly to the penis.
Aim
To determine if nanoparticles encapsulating known erectogenic agents (tadalafil, sialorphin, and nitric oxide [NO]) can improve erectile function in a rat model of erectile dysfunction (ED) as a result of aging (the Sprague-Dawley retired breeder rat).
Methods
Nanoparticles encapsulating the erectogenic agents were applied as a gel to the glans and penile shaft of anesthetized Sprague-Dawley rats and the intracorporal pressure/blood pressure (ICP/BP) monitored for up to 2 hours with or without stimulation of the cavernous nerve. Control nanoparticles were made without encapsulating erectogenic agents and applied in a similar manner in separate experiments.
Results
Nanoparticles encapsulating NO caused spontaneous visible erections in the rat, with an average time of onset of 4.5 minutes, duration of 1.42 minutes, and ICP/BP of 0.67 ± 0.14. The sialorphin nanoparticles also caused visible spontaneous erections after an average of 4.5 minutes, with a duration of 8 minutes and ICP/BP ratio of 0.72 ± 0.13. The difference in the erectile response between groups of animals treated with NO or sialorphin nanoparticles was significantly different from the control group treated with empty nanoparticles (P < 0.05) Tadalafil nanoparticles showed a significant increase in the mean ICP/BP (0.737 ± 0.029) following stimulation of the cavernous nerve (4 mA) 1 hour after application of the nanoparticles with a visibly improved erectile response.
Conclusions
Nanoparticles encapsulating three different erectogenic agents resulted in increased erectile function when applied to the penis of a rat model of ED. Nanoparticles represent a potential novel route for topical delivery of erectogenic agents which could improve the safety profile for existing orally administered drugs by avoiding effects of absorption and first-pass metabolism, and would be less hazardous than injection.
Keywords: Erectile Dysfunction, Nanoparticles, Transdermal, Small Particle Therapy for ED, Nitric Oxide, Sialorphin
Introduction
The most commonly prescribed class of drugs for treating erectile dysfunction (ED) are phosphodiesterase type 5 (PDE5) inhibitors. However, despite their widespread use and success in treating ED, there are several well-documented side effects associated with their use, such as headache, facial flushing, nasal congestion, and dyspepsia [1]. These side effects are a result of their systemic dispersal following ingestion, and the target enzyme (PDE5) being expressed in a variety of other tissues besides the corpora. In addition, in the presence of high-fat food, absorption of sildenafil and vardenafil may be delayed [1], and certain foods such as grapefruit may also alter the pharmacokinetics [2] potentially leading to variability in dose vs. systemic concentrations. A local topical application could potentially avoid variations in absorption profiles, first-pass metabolism, and systemic effects.
Recently, hybrid hydrogel/glass nanoparticles were developed that could potentially facilitate transcutaneous transport of biologically active substances ranging from nitric oxide (NO) to small peptides and possibly to larger macromolecular entities and assemblies [3]. It is well established that these types of nanoparticles provide excellent matrices for encapsulating a wide variety of organic and inorganic compounds, including many biologically relevant materials, such as proteins [4–6], NO [3], and pharmaceuticals [7]. The resulting material can be converted into powders comprised of nanoparticles with average diameters in the range of tens of nanometers (for comparison, viruses are 10–300 nm or eukaryotic cells 2–100 μm in diameter). We have adapted the procedure for the synthesis of these nanoparticles such that they could encapsulate a commercially available PDE5 inhibitor, tadalafil, or a peptide (sialorphin) which, as we have previously shown, can improve erectile function in animal models [8]. We hypothesized that the sustained release and local delivery of these drugs would be able to effectively treat ED while simplifying and improving the route of administration.
Nanoparticles could potentially deliver both established and novel erectogenic agents to the penis, such as NO, for which our platform has already demonstrated sustained release properties [3]. Although NO is known to reduce corporal smooth muscle tissue tone, a clinically useful method of delivery has not been described. The platform, as described above, has the ability to contain NO in a stable state within dry particles until adequate hydration initiates the release of trapped NO from within the material. Nanoparticles could also be used to deliver erectogenic agents that would otherwise have to be injected into the corpora. One of these reagents is the peptide sialorphin, which, as we have demonstrated, can improve erectile function in the aging rat when intracorporally injected [8].
Given the potential advantages of transdermal delivery of erectogenic agents by nanoparticles, we investigated if nanoparticles encapsulating tadalafil, sialorphin, and NO could improve erectile physiology in an aging model of ED (retired male Sprague-Dawley Rats).
Materials and Methods
Animals and Treatment
Retired breeder male Sprague-Dawley rats weighing >650 g (a commonly employed animal model for the effects of aging on erectile function [9]) were used in these studies and obtained from Charles River Breeding Laboratories (Wilmington, MA, USA). All animal protocols are approved by the Animal Use Committee at the Albert Einstein College of Medicine.
Preparation of Nanoparticles
Nanoparticles with NO were synthesized as we have previously described [3]. Briefly, tetramethylorthosilicate (TMOS) was hydrolyzed and added to a solution of sodium nitrite, glucose, polyethylene glycol (PEG), and chitosan (all materials obtained from Sigma-Aldrich, St. Louis, MO, USA) in 0.05 M phosphate buffer (pH 7). This mixture was allowed to gel into a solid block monolith and was subsequently dried in a lyophilizer to form a fine powder comprised of 10 nm nanoparticles. Control nanoparticles were made by the same procedure; however, sodium nitrite was omitted from the synthesis. A variation of the protocol was implemented to encapsulate functional tadalafil and sialorphin into the platform.
For the tadalafil nanoparticles, four 10-mg tadalafil capsules (Eli Lilly and Company, Indianapolis, IN, USA) were crushed in a mortar and pestle and were subsequently dissolved in 40 mL of 0.05 M phosphate buffer (pH 7). Afterward, 2 mL of PEG, 2 mL of chitosan, and 4 mL of hydrolyzed TMOS were added and the mixture formed a solid gel. The gel was then lyophilized into a dry powder and stored away from moisture. For the sialorphin nanoparticles, 1 mg of sialorphin (Sigma-Aldrich) was dissolved into 40 mL of 0.05M phosphate buffer (pH 7). PEG, chitosan, and TMOS were added as above and the resultant solid gel was lyophilized into powder. This powder was stored away from light and moisture. In addition, five animals were treated with 2.5 mg/kg of tadalafil orally 1 hour prior to the measurement of erectile function.
Measurement of Intracorporal Pressure (ICP)/Blood Pressure (BP)
Rats were first anesthetized via intraperitoneal injection of sodium pentobarbital (35 mg/kg; Abbott Laboratory, Chicago, IL, USA). A cannula was inserted into the carotid artery for systemic pressure (BP). Next, an incision was made in the perineum, the ischiocavernosus muscle removed to expose the corpus cavernosum crus, and a 23-gauge needle was inserted to measure ICP. The changes in ICP and systemic BP were monitored continuously throughout the experiments.
Nanoparticles were applied as a gel to the glans and shaft of the penis and left in place for the duration of the experiment. The nanoparticles were administered as a 50- to 200-μL suspension in 1.5% carboxymethylcellulose (used as a bulking agent). These suspensions represented approximately 10 nMoles NO (steady-state delivery), 50 ng sialorphin, and 1 mg tadalafil.
With the tadalafil nanoparticles, we determined the ICP/BP response following cavernous nerve stimulation before and after administration of the nanoparticles. In order to do this, the cavernous nerves were identified ventrolateral to the prostate gland and carefully isolated. Direct electrostimulation of the cavernous nerve was performed using a delicate stainless steel bipolar hook electrode attached to a multijointed clamp. Each probe was 0.2 mm in diameter with a 1 mm separation between the two poles. Monophasic rectangular pulses were delivered by a signal generator (custom made and with built-in constant current amplifier). Stimulation was performed at 0.75 mA and 4 mA. Following the untreated ICP/BP recording, animals were administered with a 200-μL suspension of the tadalafil nanoparticles. Animals were then stimulated at 0.75 mA and 4 mA at 30-minute intervals after application and the ICP/BP determined.
Results
Effects of NO Nanoparticles on Erectile Activity
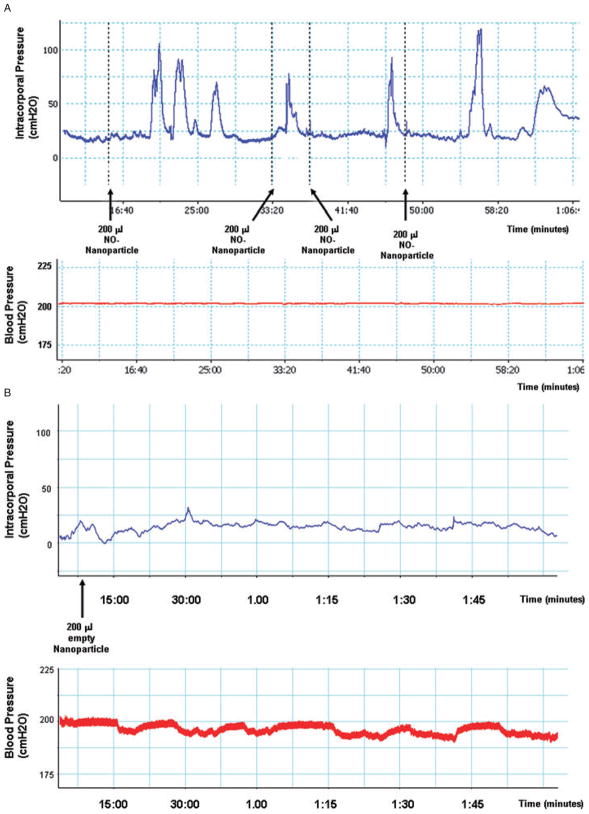
NO is a neurotransmitter that can initiate the development of an erection. Therefore, we hypothesized that if the nanoparticles facilitated transdermal delivery of NO, no electrical stimulation would be necessary to elicit an erection. Following the administration of the nanoparticles in suspension to the penis of the rats, we followed both ICP and BP for approximately 1 hour (Figure 1). None of the control animals (seven retired breeder rats treated with empty nanoparticles) demonstrated any visible erectile response and the ICP/BP remained less than 0.1 for the duration of the experiment (Figure 1B). In contrast, a typical ICP/BP trace for an animal treated with the NO nanoparticles is shown in Figure 1A. Five out of seven animals tested showed an erectile response when treated with the NO nanoparticles. Visible erections would typically begin in the animals approximately 5 minutes (average 4.5 minutes) after the administration of nanoparticles and would be followed by several other erections of diminishing intensity. The average peak ICP/BP ratio for the first erection was 0.67 ± 0.14. The erections were typically of less than 2 minutes duration (average 1.42 minutes). Comparing the observed difference (i.e., an erectile response) between NO nanoparticle treated and empty nanoparticle (control) groups using Fisher’s exact test, the P value is P = 0.02.
Figure 1.
(A) Example of a continuous trace of intracorporal pressure (ICP, upper panel) and systemic blood pressure (BP, lower panel) over the course of an experiment following administration of 200 μL nitric oxide (NO) nanoparticles performed topically on the rat penis. The time points of application of the NO nanoparticles are shown by the arrows. (B) Example of a continuous trace of ICP and BP for animals treated with empty nanoparticles.
The Effect of Sialorphin Nanoparticles on Erectile Activity
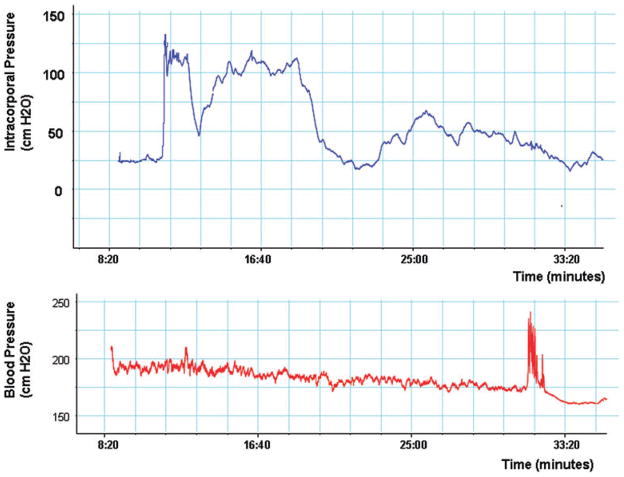
Sialorphin is a neutral endopeptidase inhibitor which can potentially prolong the activity of signal peptides at their receptors [10,11]. We have shown that overexpression of the gene encoding sialorphin (Vcsa1) can result in a priapic-like condition in retired breeder rats [12,13]. The priapic-like condition would suggest that sialorphin can result in an unstimulated erection in animals, and therefore, we reasoned that we would not need cavernous nerve stimulation to elicit an erectile response. We monitored ICP/BP for up to 2 hours following administration of sialorphin. A typical result is shown in Figure 2. Approximately 9 minutes after administration of the sialorphin nanoparticles, a visible erection was observed, which persisted for several minutes. The average peak ICP/BP ratio was 0.72 ± 0.13 (an ICP/BP ratio of 0.6 usually results in a visible erection) and a visible erection lasted an average of 8 minutes. The range of time of onset of an erection for the five animals investigated was between 4 and 12 minutes (average 4.5 minutes). As described above, none of the animals treated with empty nanoparticles exhibited erectile tendencies. Comparing the observed difference (i.e., an erectile response) between sialorphin nanoparticle treated and empty nanoparticle (control) groups using Fisher’s exact test, the P value is P = 0.04.
Figure 2.
A continuous trace of intracorporal pressure (upper panel) and blood pressure (lower panel) over the course of an experiment following administration of 200 μL sialorphin nanoparticles performed topically on the rat penis. The nanoparticles were applied at time = 0 on the trace shown in the figure.
The Effect of Tadalafil Nanoparticles on Erectile Activity
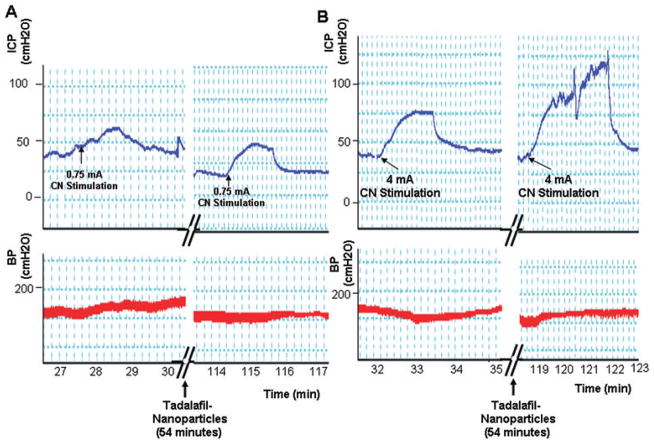
Tadalafil is a PDE5 inhibitor which maintains intracellular levels of cyclic guanosine monophosphate after neuronal stimulation to induce an erection [14]. Therefore, we reasoned that after application of the tadalafil nanoparticles, stimulation of the cavernous nerve would be necessary to obtain an erectile response. Retired breeders, which have impaired erectile function, had their response to stimulation at 0.75 mA and 4 mA measured prior to the addition of the tadalafil nanoparticles. After administration of the tadalafil particles, erectile response to cavernous nerve stimulation was monitored at approximately 30-minute intervals. A typical result is shown in Figure 3 and the average from five experiments is shown in Figure 4. Prior to treatment with the tadalafil nanoparticles, cavernous nerve stimulation at either 0.75 or 4 mA did not result in visible erections, and the ICP/BP at both stimulations were below 0.6 (a ratio which usually indicates a visible erection). However, after 60 minutes, there was a significant improvement (Student’s t-test <0.05) in the erectile response at the 4-mA level of stimulation compared with animals treated with the empty nanoparticles (negative control). The average ICP/BP after 1 hour and 4mA stimulation was 0.737 ± 0.029, and visibly improved erectile responses were observed. We compared the efficacy of transdermal delivery of tadalafil by nanoparticles with orally administered tadalafil. The effect on erectile response, as determined by ICP/BP following cavernous nerve stimulation, was not significantly different between orally and topically administered tadalafil. However, there was significantly improved erectile response in both treated groups after 60 minutes at the 4 mA level of stimulation compared with untreated controls.
Figure 3.
The figure shows a continuous trace of intracorporal pressure (ICP) and systemic blood pressure (BP) over the course of 123 minutes. Administration of the tadalafil nanoparticles (at the 54 minutes time point) was performed topically on the rat penis. The cavernous nerve (CN) was stimulated at 0.75 mA (A) and 4 mA (B), both before (left panel) and at approximately 30-minute intervals after administration of the tadalafil nanoparticles. The effects of CN stimulation after approximately 60 minutes are shown in the right panels.
Figure 4.
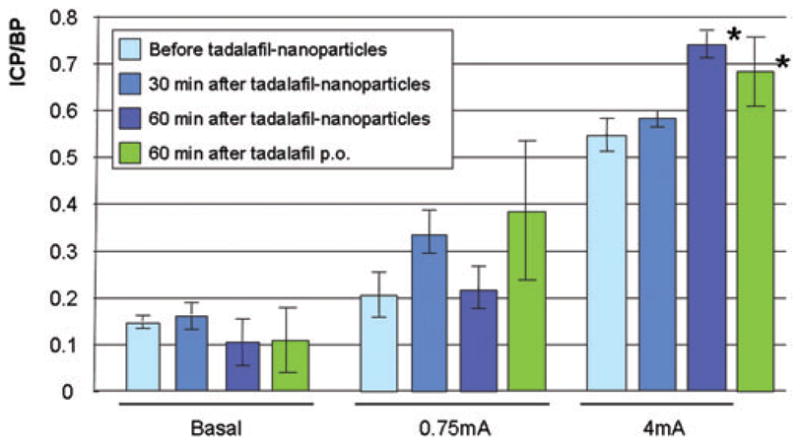
The mean of intracorporal pressure (ICP)/blood pressure (BP) measurements from six rats before treatment with tadalafil nanoparticles, and 30 and 60 minutes following treatment. The average basal ICP/BP is shown and the ICP/BP following stimulation of the cavernous nerve at 0.75 and 4 mA. There is an apparent time-dependent increase in the effect of the tadalafil nanoparticle on erection increasing with time. There was a significant effect 60 minutes after application of 200 μL of tadalafil following 4 mA stimulation. *Significant effect on erectile function compared to pretreatment (P < 0.05, Student’s t-test).
Discussion
In this article, we demonstrate for the first time the feasibility of using nanoparticles to deliver erectogenic agents transdermally to the penis. The glans of the penis may have unique anatomic and physiologic characteristics allowing for efficient transdermal passage of the nanoparticles. Direct delivery to the shaft (or the skin covering other parts of the body) may be inhibited because of the interposing tunica albuginea, which is composed of thickened collagen bundles. However, the glans lacks a thick lamellated stratum corneum, the dry and tightly intercellularly bonded uppermost level of the epidermis, which generally prevents passage of topical agents. The dermis of the glans is, therefore, particularly permeable and directly communicates with the copora cavernosa via a rich venous network [15]. However, the precise route of entry into the cavernous bodies remains to be clarified.
The observed affects on erectile physiology are characteristic of the erectogenic agent. For example, administration of the NO nanoparticles results in spontaneous erections of short duration after a few minutes, whereas sialorphin nanoparticles result in spontaneous erections of extended periods. The average duration of a reflexive erection, or an apomorphine-induced erection in the rat, is less than 1 minute [16]. The longer duration of the sialorphin nanoparticle-induced erection may reflect the reported descriptions where overexpression of the gene encoding sialorphin (Vcsa1) can cause priapic-like conditions when intracorporally injected into retired breeder rats [12,13]. Tadalafil nanoparticles also increased erectile function, but the nature of this erectogenic reagent is such that stimulation of the cavernous nerve was necessary to elicit a response. However, the erectile response, as determined by the ICP/BP following cavernous nerve stimulation, was significantly greater approximately 1 hour after treating animals with the nanoparticles than before treatment.
Sialorphin and the NO nanoparticles both resulted in a demonstrable erectile response within approximately 5 minutes, whereas tadalafil nanoparticles do not cause significantly improved erectile function following cavernous nerve stimulation until approximately 1 hour after treatment. The difference in time for the erectile response may be due to several criteria. NO, being a small molecule, may have faster release and diffusion time from the nanoparticles than the relatively larger molecule tadalafil. The hydrophobicity of the different molecules may also play a role. The action of tadalafil, as a PDE5 inhibitor, is less direct and there may be a delay in the time to reach intracellular levels that effectively block the enzyme. Therefore, the biochemical mechanism of action may also contribute to a delay in the effect of sialorphin compared with the sialorphin and NO nanoparticles. The differences in the time of onset of erectile response of the different nanoparticles may be resolved by future studies on the efficiency of transdermal transport, release, and pharmacokinetics of the erectogenic agents in corporal tissue.
Although this study was conducted primarily as a feasibility study for physiologically relevant changes in erectile function, following ICP/BP determinations animals were euthanized and corporal tissue sections were investigated for histopathology. There was no evidence of inflammation or congestion, and overall, the tissue appeared normal (Figure 5). In a hamster model study, it has been observed that the nanoparticles have a life span in the circulation (after IV infusion) of several hours without any indication of thrombosis of the microcirculation. Following application of the nanoparticles containing the erectogenic agents to the penis, there was no significant change in systemic BP as measured by the carotid artery BP. In future experiments, potential toxicity will be determined for repeated application of the nanoparticles and labeling of the nanoparticle and the encapsulated erectogenic agents could allow the determination of the kinetics and more specific biodistribution of both components of the nanoparticle following topical administration. Application of the NO nanoparticle to the penis did show a tendency to reduce systemic BP over time. However, the protocol in these experiments allowed the nanoparticle gel to remain in place for the duration of the experiments, whereas in clinical applications, we envisage the gel being removed once an erection was attained.
Figure 5.
Representative histological section of penile tissue from animals (A) untreated and (B) following experiments where animals were treated with nitric oxide nanoparticles (10× magnification).
Another organ that may be amenable to treatment by transdermal application of the nanoparticles is the bladder. In female patients, it should be possible to encapsulate treatments for bladder pathologies and instill the nanoparticles into the bladder lumen. The nanoparticles would then cross the bladder wall and act directly, thereby avoiding systemic effects.
The nanotechnology platform used in the present article is based on established silane-based sol-gels, made from tetramethoxysilane (TMOS) or tetraethoxysilane. The sol-gel process involves the transition of a system from a liquid “sol” (generally colloidal) into a solid gel phase. These materials have the unique property of limiting conformational dynamics of the encapsulated compounds while still allowing for the free exchange/access of solvent and small solute molecules due to the complex porous network within the sol-gel matrix [17–19]. However, these attractive aspects of sol-gel technology can limit potential drug delivery applications due to their high porosity, thereby allowing the particle contents to dissipate too rapidly. To address this, shortcoming glass-forming materials, such as sugars and polysaccharides (e.g., chitosan), have been incorporated into the sol-gel procedure in order to modulate the structure of the gel and obstruct the pore network with a relatively stable (when dry) hydrogen-bonded network of molecules. Glass in this case refers to an amorphous solid held together by a strong hydrogen-bonding network—the glass loosens when exposed to water. We have previously demonstrated that the inclusion of both PEG and chitosan as additives to a basic tetramethoxysilane (TMOS) protocol for sol-gel preparation is able to lend such “glassy properties” to our preparation and, in addition, drive the efficient reduction of nitrite to NO resulting in remarkably effective NO formation, NO retention, and slow sustained release of NO [3]. Most significantly, it was also demonstrated that the release profile for the NO is easily tuned through manipulation of the components (e.g., average molecular weight of the added PEG) comprising the particles. In the past, direct application of NO has not been feasible and locally sustained release of NO has only become possible with the development of novel techniques such as these NO-releasing nanoparticles. Nanoparticles may potentially provide a means of delivery that will allow the use of novel erectogenic agents such as NO or sialorphin. Sialorphin has been demonstrated to relax corporal smooth muscle and improve erectile function in rats [8]. If the use of this peptide were to be translated to a clinical treatment, it would, at present, likely involve intra-corporal injection. However, topical application could avoid the potential hazards and anxiety of injection.
Despite the widespread use and success of oral PDE5 inhibitors as treatments for ED, they have several drawbacks, some of which could potentially be improved through topical application. Many patients, particularly diabetic patients, are refractory to treatment by the orally administered PDE5 inhibitors [20–22]. There are several well-documented side effects associated with their use (headache, facial flushing, nasal congestion, and dyspepsia), due to a systemic wide action. In addition, in the presence of high-fat food, absorption of sildenafil and vardenafil may be delayed [1] and certain foods, such as grapefruit, can alter the pharmacokinetics [2]. A topical application of nanoparticles containing erectogenic agents could avoid variation in absorption profiles, first-pass metabolism, and systemic effects. Several reviews of the use of local penile therapy have been published and highlight that results of the use of local penile therapy have been disappointing in clinical trials, namely because of the barrier caused by the penile skin and tunica [15,23]. However, the nanoparticles used in this study represent a novel delivery platform. We have yet to demonstrate whether the nanoparticles penetrate the fibrous, longitudinal fibers of the tunica albuginea or whether they remain lodged in some level of the epidermis or in the dermal collagen. However, the utility of nanoparticles here is primarily to overcome epidermal penetration. The horny layer of intact, healthy skin can prevent substances as small as 100 nm from passage. Since the nanoparticles used in this study are approximately 10 nm in diameter, we believe that they are likely to overcome this barrier due to their size. Once in the epidermis or even dermis, they can release their therapeutic payload, which in our case, consists of small molecules such as NO and sialorphin, which likely can then penetrate the tunica and exert their respective physiological impact on corporal tissue.
The nanoparticle delivery system we have developed has proven to be very flexible with respect to the nature of the agent being carried and is easily administered. With such a wide range of possible therapeutic options for ED via local delivery, a topical formulation of an established pharmaceutical agent or novel treatment agent would be an excellent alternative or even adjunct to existing oral medication. While much work remains to fully develop these systems, especially with respect to dosing and toxicity, these early results indicate that this topical application of nanoparticle platform may be a suitable and effective method for treating ED.
Acknowledgments
This work was partly supported by grant R01DK077665 awarded by the NIH/NIDDK to Kelvin P. Davies and partly through a grant to J. M. Friedman from FJC, A Foundation of Philanthropic Funds. We would like to thank Dr. Rani R. Sellers for performing histopathology.
Footnotes
Conflict of Interest: None.
Statement of Authorship
-
Conception and DesignKelvin P. Davies; Adam Friedman; Joel Friedman; Moses Tar; George Han
-
Acquisition of DataMoses Tar; George Han; Dwaraka S. R. Kuppam
-
Analysis and Interpretation of DataKelvin P. Davies; Adam Friedman; Joel Friedman; Moses Tar; George Han
-
Drafting the ArticleKelvin P. Davies
-
Revising It for Intellectual ContentKelvin P. Davies; Adam Friedman; Joel Friedman; Moses Tar; George Han; Arnold Melman
-
Final Approval of the Completed ArticleKelvin P. Davies
References
- 1.Seftel AD. Phosphodiesterase type 5 inhibitor differentiation based on selectivity, pharmacokinetic, and efficacy profiles. Clin Cardiol. 2004;27(4 suppl 1):114–9. doi: 10.1002/clc.4960271305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jetter A, Kinzig-Schippers M, Walchner-Bonjean M, Hering U, Bulitta J, Schreiner P, Sorgel F, Fuhr U. Effects of grapefruit juice on the pharmacokinetics of sildenafil. Clin Pharmacol Ther. 2002;71:21–9. doi: 10.1067/mcp.2002.121236. [DOI] [PubMed] [Google Scholar]
- 3.Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, Friedman JM. Sustained release nitric oxide releasing nanoparticles: Characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide. 2008;19:12–20. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Kumar A. Bioactive materials for biomedical applications using sol-gel technology. Biomed Mater. 2008;3:034005. doi: 10.1088/1748-6041/3/3/034005. [DOI] [PubMed] [Google Scholar]
- 5.Khan I, Dantsker D, Samuni U, Friedman AJ, Bonaventura C, Manjula B, Acharya SA, Friedman JM. Beta 93 modified hemoglobin: Kinetic and conformational consequences. Biochemistry. 2001;40:7581–92. doi: 10.1021/bi010051o. [DOI] [PubMed] [Google Scholar]
- 6.Khan I, Shannon CF, Dantsker D, Friedman AJ, Perez-Gonzalez-de-Apodaca J, Friedman JM. Sol-gel trapping of functional intermediates of hemoglobin: Geminate and bimolecular recombination studies. Biochemistry. 2000;39:16099–109. doi: 10.1021/bi000536x. [DOI] [PubMed] [Google Scholar]
- 7.Viitala R, Jokinen M, Rosenholm JB. Mechanistic studies on release of large and small molecules from biodegradable SiO2. Int J Pharm. 2007;336:382–90. doi: 10.1016/j.ijpharm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Davies KP, Tar M, Rougeot C, Melman A. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99:431–5. doi: 10.1111/j.1464-410X.2006.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol. 2003;170:285–90. doi: 10.1097/01.ju.0000063375.12512.6e. [DOI] [PubMed] [Google Scholar]
- 10.Tong Y, Tiplitsky SI, Tar M, Melma A, Davies KP. Transcription of G-protein coupled receptors in corporeal smooth muscle is regulated by the endogenous neutral endopeptidase inhibitor sialorphin. J Urol. 2008;180:760–6. doi: 10.1016/j.juro.2008.03.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rougeot C, Messaoudi M, Hermitte V, Rigault AG, Blisnick T, Dugave C, Desor D, Rougeon F. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci USA. 2003;100:8549–54. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98:396–401. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong Y, Tar M, Melman A, Davies KP. The opiorphin gene (ProL1) and its homologues function in erectile physiology. BJU Int. 2008;102:736–40. doi: 10.1111/j.1464-410X.2008.07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turko IV, Ballard SA, Francis SH, Corbin JD. Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (Type 5) by sildenafil and related compounds. Mol Pharmacol. 1999;56:124–30. doi: 10.1124/mol.56.1.124. [DOI] [PubMed] [Google Scholar]
- 15.Yap RL, Mcvary KT. Topical agents and erectile dysfunction: Is there a place? Curr Urol Rep. 2002;3:471–6. doi: 10.1007/s11934-002-0100-x. [DOI] [PubMed] [Google Scholar]
- 16.Bernabe J, Rampin O, Sachs BD, Giuliano F. Intra-cavernous pressure during erection in rats: An integrative approach based on telemetric recording. Am J Physiol. 1999;276(2 Pt 2):pR441–9. doi: 10.1152/ajpregu.1999.276.2.R441. [DOI] [PubMed] [Google Scholar]
- 17.Dunn B, Zink JI. Sol-gel chemistry and materials. Acc Chem Res. 2007;40:729. doi: 10.1021/ar700178b. [DOI] [PubMed] [Google Scholar]
- 18.Ellerby LM, Nishida CR, Nishida F, Yamanaka SA, Dunn B, Valentine JS, Zink JI. Encapsulation of proteins in transparent porous silicate glasses prepared by the sol-gel method. Science. 1992;255:1113–5. doi: 10.1126/science.1312257. [DOI] [PubMed] [Google Scholar]
- 19.Lan EH, Dunn B, Zink JI. Nanostructured systems for biological materials. Methods Mol Biol. 2005;300:53–79. doi: 10.1385/1-59259-858-7:053. [DOI] [PubMed] [Google Scholar]
- 20.Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: A randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421–6. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 21.Vickers MA, Satyanarayana R. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction in patients with diabetes mellitus. Int J Impot Res. 2002;14:466–71. doi: 10.1038/sj.ijir.3900910. [DOI] [PubMed] [Google Scholar]
- 22.Moore RA, Derry S, McQuay HJ. Indirect comparison of interventions using published randomised trials: Systematic review of PDE-5 inhibitors for erectile dysfunction. BMC Urol. 2005;5:18–34. doi: 10.1186/1471-2490-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montorsi F, Salonia A, Zanoni M, Pompa P, Cestari A, Guazzoni G, Barbieri L, Rigatti P. Current status of local penile therapy. Int J Impot Res. 2002;14(1 suppl):S70–81. doi: 10.1038/sj.ijir.3900808. [DOI] [PubMed] [Google Scholar]