Abstract
Rationale
Loss of Tbx1 and decrease of retinoic acid (RA) synthesis result in DiGeorge/Velo-Cardio-Facial syndrome (DGS/VCFS)-like phenotypes in mouse models, including defects in septation of the outflow tract (OFT) of the heart and anomalies of pharyngeal arch-derived structures including arteries of the head and neck, laryngeal-tracheal cartilage, and thymus/parathyroid. Wild-type levels of Tbx1 and RA signaling are required for normal pharyngeal arch artery (PAA) development. Recent studies have shown that reduction of RA or loss of Tbx1 alters the contribution of second heart field (SHF) progenitor cells to the elongating heart tube.
Objective
Here we tested whether Tbx1 and the RA signaling pathway interact during the deployment of the SHF and formation of the mature aortic arch.
Methods and Results
Molecular markers of the SHF, neural crest cells (NCC) and smooth muscle cells (SMC) were analyzed in Raldh2;Tbx1 compound heterozygous mutants. Our results revealed that the SHF and OFT develop normally in Raldh2+/−;Tbx1+/− embryos. However, we found that decreased levels of RA accelerate the recovery from arterial growth delay observed in Tbx1+/− mutant embryos. This compensation coincides with the differentiation of SMC in the 4th PAAs, and is associated with severity of NCC migration defects observed in these mutants.
Conclusions
Our data suggest that differences in levels of embryonic RA may contribute to the variability in great artery anomalies observed in DGS/VCFS patients.
Keywords: Tbx1, Retinoic Acid, Pharyngeal arch, DiGeorge syndrome, Congenital heart defects
INTRODUCTION
Cardiovascular development is a complex and ordered process that is regulated by both genetic and epigenetic factors, including dynamic remodeling of the pharyngeal arch arteries (PAAs) and cardiac function.1 Failure of blood flow during heart development and remodeling leads to cardiovascular malformations, including interruption of the aortic arch, double aortic arches, and coarctation of the aorta.2 Despite the significant prevalence of congenital cardiovascular diseases little is known about molecules that control the subsequent remodeling into a functional vasculature. Cardiovascular defects are a major feature of the DiGeorge/velocardiofacial syndrome (DGS/VCFS), the most common genetic deletion syndrome in humans, with an incidence of 1 in 4000 live births.3
Most DGS/VCFS patients share a 3Mb deletion at 22q11.2, while 8% harbor a smaller 1.5Mb deletion in the centromeric half of the 3Mb region.3 Extensive genetic analysis in human and mouse indicates that the T-box family gene, TBX1, is the major gene responsible for DGS/VCFS defects (reviewed in 4). Homozygous Tbx1 mutant mice have craniofacial and cardiovascular defects associated with pharyngeal hypoplasia.4 These mice fail to form PAAs 3–6 and die at term with a single ventricular outlet. In addition, Tbx1 is required for normal addition of OFT progenitor cells of the second heart field (SHF) to the developing heart.5–7 Tbx1 heterozygous mutant mice display a high frequency of absence or hypoplasia of the 4th PAA at E10.5 and associated great artery anomalies at later stages of development, including interrupted aortic arch, aberrant right subclavian artery and high aortic arch, components of DGS/VCFS in man. Intriguingly, the incidence of PAA associated anomalies decreases between E10.5 and fetal stages suggesting the intervention of compensatory mechanisms leading to partial phenotypic recovery.8 Such mechanisms, likely modifying the plasticity of PAA development, remain unidentified, although several major signaling pathways that affect development and morphogenesis of PAA derivatives have been shown to interact with Tbx1, including fibroblast growth factor (FGF),5, 9, 10 hedgehog,11 vascular endothelial growth factor,12 and retinoic acid 13, 14 pathways.
Retinoic acid (RA), the active derivative of vitamin A, by activating its nuclear receptors, critically regulates a variety of steps and stages of cardiovascular and pharyngeal development.15 Knockout mice deficient for retinaldehyde dehydrogenase 2 (RALDH2), which catalyzes the second oxidative step in RA biosynthesis, have been used to examine RA-dependent PAA development (reviewed in 15). Raldh2−/− mutants exhibit a severe deficiency in RA production and defects in early heart morphogenesis,16 while RA-supplemented Raldh2−/− embryos display OFT defects and impaired development of posterior (3rd-6th) PAA.17 Moreover, disruption of RA levels can result in embryonic defects similar to those seen in DGS/VCFS.17–19 Raldh2−/− mutants display a posterior expansion of Fgf8 and Isl1 positive cardiac progenitors into lateral plate mesoderm; subsequently, the addition of SHF cells to the heart tube is highly compromised, especially in the OFT region.20, 21 Recent studies suggest a potential genetic interaction between Tbx1 and RA signaling. Local increases of RA in the pharyngeal region down-regulate Tbx1 expression,14 while expanded Raldh2 expression and ectopic RA signaling is observed in embryos lacking Tbx1.13, 22–24
In this study we have investigated the possibility that reduction of RA synthesis modulates development of the 4th PAA in Tbx1+/− embryos, a feature of DGS/VCFS. During this analysis we found that reduced levels of RA associated with Raldh2 heterozygosity accelerate recovery from arterial growth delay in Tbx1+/− embryos. Unlike the situation in homozygous mutant Raldh2 and Tbx1 embryos, the cardiac contribution of the SHF is unaffected in compound heterozygous mutant embryos. Accelerated phenotypic recovery coincides with the initiation of arch artery vascular smooth muscle (VSM) differentiation. Hence, VSM differentiation is sensitive to the RA-levels in Tbx1+/− embryos, and is associated with more severe NCC migration defects in Tbx1+/− than Raldh2+/−;Tbx1+/− embryos. RA deficiency therefore induces earlier recovery of DiGeorge-related aortic arch defects, suggesting that differences in levels of embryonic RA may contribute to the phenotypic variability observed in DGS/VCFS patients.
METHODS
Mouse mutants
Mice carrying null alleles at Raldh2 and Tbx1 have been previously described.16, 25 Genotyping details are provided in the online data supplement (available in the Online Data Supplement at http://circres.ahajournals.org).
Ink injection in situ hybridization and immunohistochemistry
For India ink injection, embryos were collected at E10.5 or E11.5 and injected intracardially using drawn Pasteur pipettes. Details of procedures are provided in the online data supplement.
Quantitative RT-PCR
Details of procedures and primer sequences are provided in the online data supplement.
Embryonic explant cultures
Detail of explant cultures are provided in the online data supplement
RESULTS
Local antagonism between RA signaling and Tbx1 expression
Retinoic acid (RA) generated by Raldh2 in lateral mesoderm establishes the posterior border of the second heart field (SHF).20 One major SHF regulatory gene is the T-box transcription factor, Tbx1.5–7 To investigate the effect of RA deficiency we examined the distribution of Tbx1 in Raldh2−/− embryos at embryonic day (E) 8.5. In wild-type (WT) embryos, Tbx1 transcripts accumulate in pharyngeal ectoderm, endoderm and cranial and splanchnic mesoderm including the SHF (Figure 1A). In Raldh2+/− and Raldh2−/− mutants, this expression is progressively expanded in a caudal direction (Figure 1B and C; 20). Real-time quantitative PCR confirmed that Tbx1 transcript levels are increased in Raldh2 heterozygous embryos (~1.5-fold; online Figure I). To evaluate whether modulation of Tbx1 expression is a direct consequence of RA signaling alteration, we investigated the effect of RA deprivation on development of the pharyngeal region in cultured explants by inhibiting RA synthesis with disulfiram, an inhibitor of the RALDH2 enzyme.26 Treatment of pharyngeal mesoderm explants isolated from 6–8 somites stage embryos with disulfiram caused an increase of Tbx1 transcripts (Online Figure II). In contrast, trans-RA-treated explants show a reduction of Tbx1 (Online Figure II), consistent with the observations of Roberts et al., (2005) in avian embryos.14 These results suggest that the level of RA can modify Tbx1 expression in the pharyngeal region. However RA impacts differently on Tbx1 expression in different pharyngeal cell types (insets in Figure 1A,B and C). Indeed, Tbx1 expression is expanded in the splanchnic mesoderm/SHF, while its expression in the ectoderm and endoderm is severely reduced in Raldh2−/− embryos.
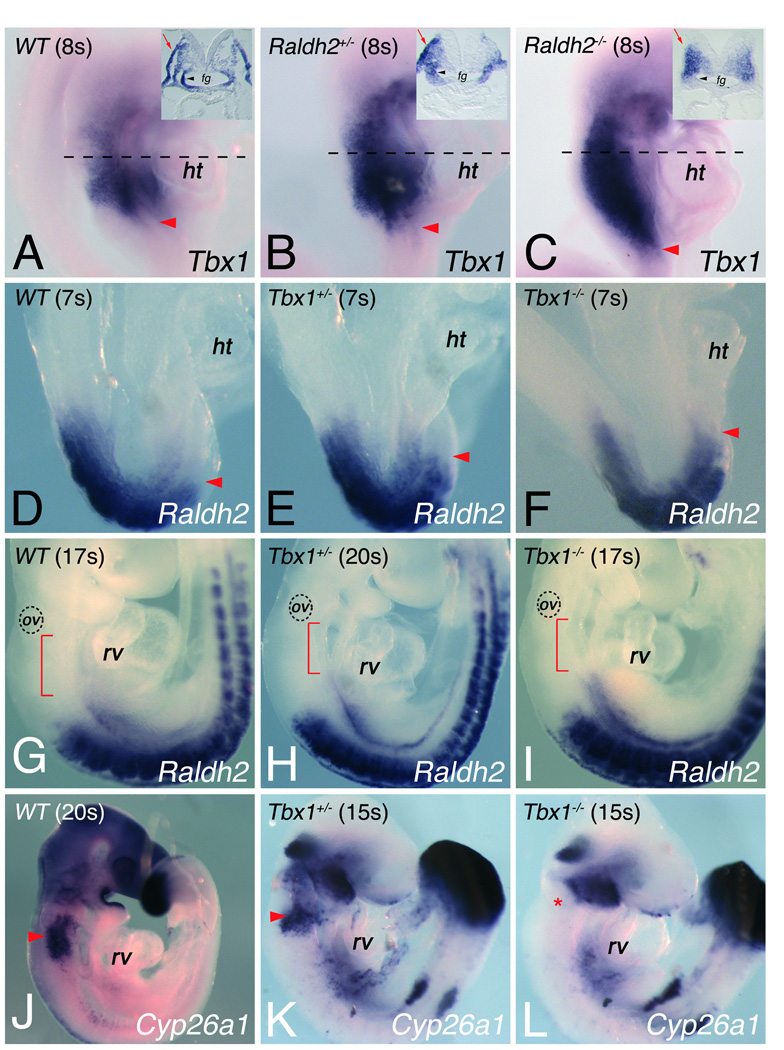
Figure 1.
Alteration of Tbx1 and retinoic acid signaling in Raldh2 and Tbx1 mutant embryos. In situ hybridization on whole mount embryos shows (A-C) Tbx1, (D-I) Raldh2 and (J-L) Cyp26a1 (I,J) in different mutant backgrounds. (A-C) Red arrowheads indicate the most posterior limit of Tbx1 expression in (A) wild-type (WT), (B) Raldh2+/− and (C) Raldh2−/− embryos at the 8-somite stage (8s), dotted lines indicating planes of sections. Transverse sections of embryos displayed in insets in A-C, indicate that RA signaling is required for Tbx1 transcript accumulation in pharyngeal ectoderm (red arrows) and endoderm (black arrowheads). Tbx1 expression is expanded caudally in absence of Raldh2 (compare C with A). (D-I) Raldh2 expression in (D,G) WT, (E,H) Tbx1+/− and (F,I) Tbx1−/− embryos at the 7-somite stage (7s) and at embryonic day (E) 9.5. Arrowheads in D-F show the anterior limit of Raldh2 expression. Red brackets in G-I indicate the interval between the most anterior domain of Raldh2 expression and the otic vesicle (ov). (J-L) In situ hybridization showing Cyp26a1 expression in (J) WT, (K) Tbx1+/− and (L) Tbx1−/− embryos at 15s stage, revealing the absence of Cyp26a1 expression in ectoderm caudal to pharyngeal brachial two (asterisk) in absence of Tbx1−/− embryos (compare L with K). fg, foregut; ht, heart tube; rv, right ventricle.
Raldh2 levels are altered in the pharyngeal mesoderm of Tbx1−/− mice.13, 22–24 We analyzed the expression pattern of Raldh2, in WT and Tbx1 mutant embryos before and during the formation of the pharyngeal region at E8.5 and E9.5. In splanchnic mesoderm of WT embryos at the 7 somite (s) stage, Raldh2 is expressed in the caudal part of the SHF (Figure 1D). In contrast, Raldh2 expression was progressively expanded cranially in Tbx1+/− and Tbx1−/− embryos (Figure 1E,F), and this expansion was maintained in the splanchnic mesoderm at the 17–20s stage consistent with previous reports (Figure 1G-I). Since there was no detectable morphological difference between WT and Tbx1−/− embryos at E8.5 (compare Figure 1D and F) these results are consistent with a direct regulation of Raldh2 by Tbx1.22, 23 Recent data have also shown that disruption of Tbx1 function affects expression of Cyp26a1, encoding the major RA-degrading enzyme in the pharyngeal region at E9.5.13, 22–24 At the 15s stage, we confirm that expression of Cyp26a1 was notably reduced in the pharyngeal region of Tbx1−/− embryos versus comparable WT embryos (Figure 1J-L).
Pharyngeal segmentation in Raldh2 and Tbx1 mutants
Abnormalities of the pharyngeal derivatives constitute the major defects of DGS/VCFS patients. In order to evaluate the pharyngeal morphology of Raldh2+/−, Tbx1+/−, and Raldh2+/−; Tbx1+/− mutants, we crossed Raldh2+/−;Tbx1+/− with WT C57Bl/6 mice (see Online supplements) and performed a histological and gene expression pattern analysis at E9.5 and E10.5. Fgf8 is required for pharyngeal development,27 and its expression in the pharyngeal region is reduced in Tbx1−/− mutant embryos.28 At E9.5, Fgf8 is expressed in the lateral pharyngeal ectoderm and endoderm. Experiments of in situ hybridization and quantitative RT-PCR for Fgf8 revealed no differences between WT, single and compound Raldh2+/−;Tbx1+/− embryos (Figure 2A-D; online Figure III). Normal levels of Raldh2 and Tbx1 are crucial for the development of the fourth pharyngeal pouch.17, 28 Pax1 expression revealed three distinct pharyngeal pouches in compound heterozygous embryos at E10.5 (Figure 2E-H). However, sectional analysis showed that the fourth pouch was unilaterally absent in 37.5% Tbx1+/− embryos (n=8), whereas all Raldh2+/−;Tbx1+/− (n=5) had four distinct pharyngeal pouches at E10.5 (Online Figure IV), suggesting that these two genes interact genetically to play critical, dose-dependent roles during pouch formation.
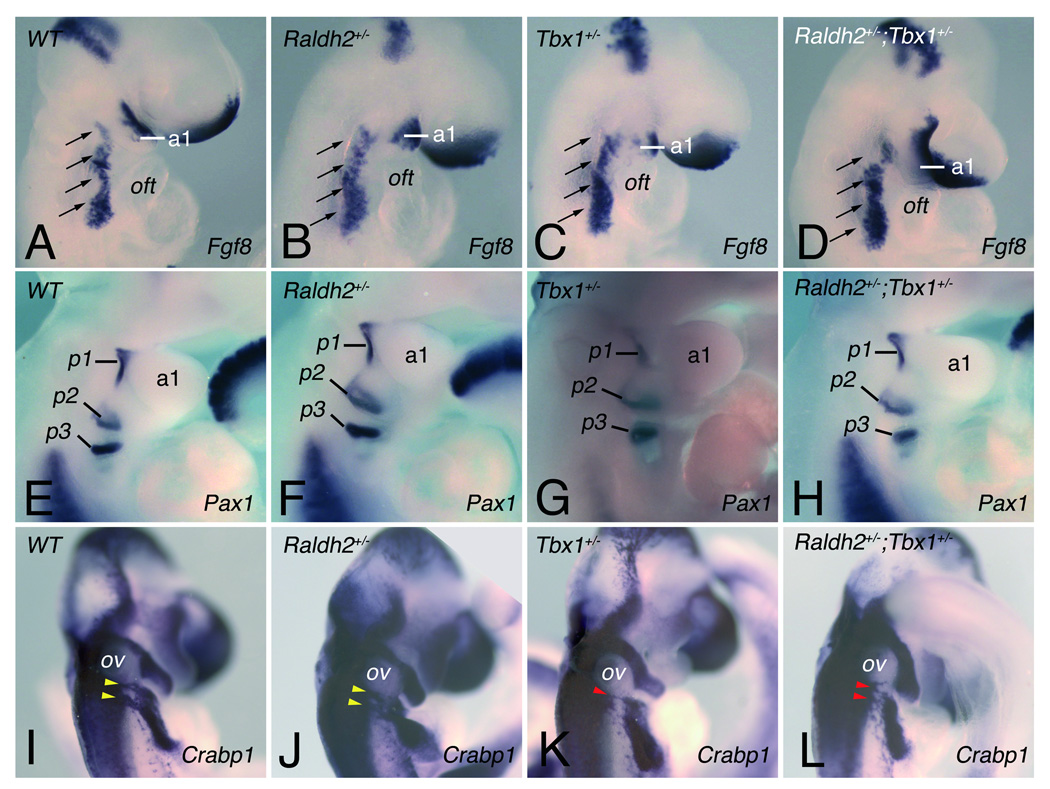
Figure 2.
Pharyngeal and neural crest development in single and compound heterozygous Tbx1 and Raldh2 mutant embryos. (A-D) Fgf8 expression in the pharyngeal region at E9.5 detected by in situ hybridization in (A) WT, (B) Raldh2+/−, (C) Tbx1+/− and (D) Raldh2+/−;Tbx1+/− embryos. Analysis of Fgf8 (A-D) transcripts displays no difference between single and compound heterozygous embryos. Arrows indicate the pharyngeal region where Fgf8 is expressed. (E-F) Endodermal pouch formation was assessed by expression of Pax1 detected at E10.5 (~30–34 somites). Pharyngeal pouches 1–3 were bilaterally present in all embryos (see Online Figure II for the fourth pharyngeal pouch). (I-L) Migratory cardiac neural crest cells (NCC) were detected by ISH for Crabp1 in WT, in single and compound heterozygous embryos at E10.5. Yellow and red arrowheads indicate normal and abnormal post-otic streams of migrating NCCs, respectively. a, arch; oft, outflow tract; ov, otic vesicle; p, pouch.
The development of neural crest cells (NCC) infiltrating the third, fourth and sixth pharyngeal arches, as well as the forming outflow tract (OFT), is crucially involved in cardiovascular patterning and is disrupted in Tbx1 homozygous and heterozygous mutants.29, 30 Analysis of Crabp1 expression at E9.5, which marks migrating NCCs, revealed migration defects in 100% of Tbx1+/− and 66.6% of Raldh2+/−;Tbx1+/− embryos. Whereas two streams of NCCs were observed in WT and Raldh2+/− embryos (Figure 2I,J), these streams were abnormally fused in all Tbx1+/− (n=5) and in four out of six Raldh2+/−;Tbx1+/− embryos (Figure 2K,L; online Figure V). Furthermore, the NCC migration defects was more severe in Tbx1+/− than Raldh2+/−;Tbx1+/− embryos, in agreement with a genetic interaction between Tbx1 and Raldh2 during pharyngeal development. This incorrect migration has been shown to misguide these cells from their intended destination, consistent with a role for Tbx1 in NCC development.8, 29–31 Although our analysis of the number of AP-2α-positive cells revealed no significant difference in Tbx1+/− compared to Raldh2+/−;Tbx1+/− embryos, we cannot exclude a subtle difference in the number of NCCs around the 4th PAA between these mutant embryos (Online Figure V).
Second heart field development and OFT morphology in Raldh2 and Tbx1 mutants
A subset of the SHF (pharyngeal mesoderm cells), has been shown to be important for the development of the cardiac OFT.32 Both Tbx1 and Raldh2 are required for correct deployment of the SHF.5, 6, 20 We therefore asked whether single or compound heterozygous mutant embryos had OFT defects by analyzing the expression profile of the Fgf10-enhancer (Mlc1v-nlacZ-24) transgene expressing β-galactosidase in the anterior part of the SHF and OFT myocardium in single and compound heterozygote embryos.32 While dramatic caudal expansion of the SHF was observed in the absence of RA signal (Online Figure VI),20 we found no disruption of Mlc1v-nlacZ-24 expression in Raldh2+/− embryos at E9.5 (data not shown) and E10.5 stages (Online Figure VII). In contrast to Tbx1−/− embryos that exhibit reduction of Mlc1v-nlacZ-24 transgene expression in the lateral region of the dorsal pericardial wall and future pharyngeal arch region (Online Figure VI),7 no differences were detected in Tbx1+/− or in compound Raldh2+/−; Tbx1+/− mutants compared to WT embryos at E9.5 (data not shown) and E10.5 stages (Online Figure VII).
Pharyngeal arch artery abnormalities in Tbx1+/− and Raldh2+/−;Tbx1+/− mutants
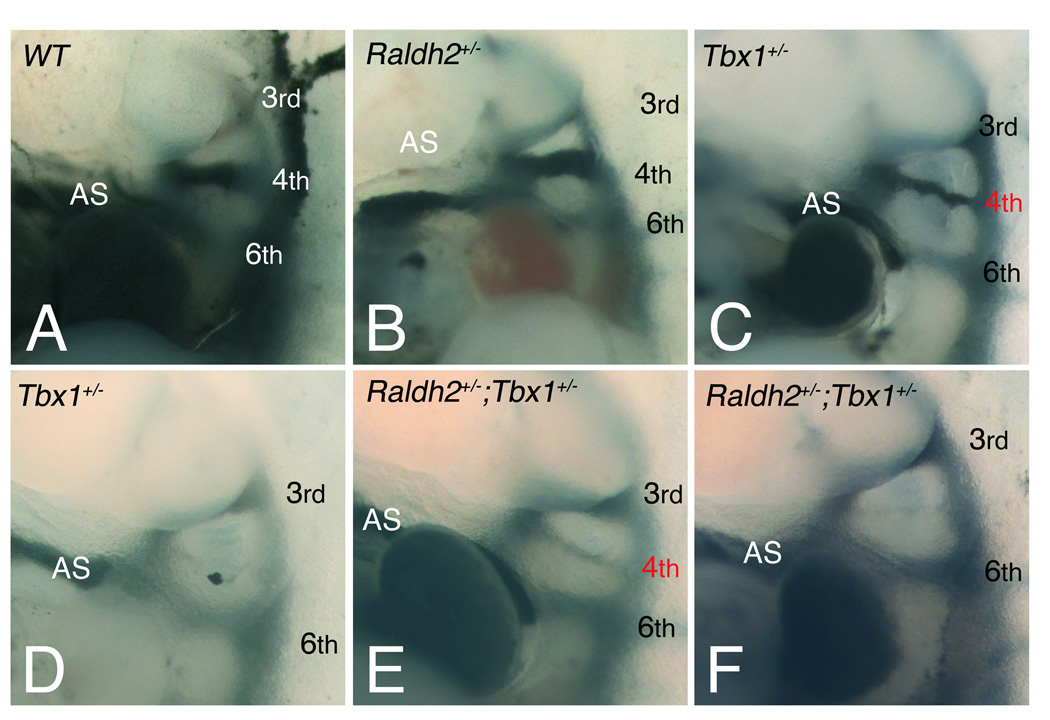
We subsequently investigated the effect of reduction of Raldh2 levels on pharyngeal arch artery (PAA) development in Tbx1+/− embryos at E10.5, a stage when the sixth PAAs form and the third regresses. The most frequent Tbx1+/− anomalies at this stage are unilateral or bilateral loss or hypoplasia of the 4th PAAs.8 Intracardiac injection of India ink revealed normal morphology of WT 4th PAAs at E10.5 (Figure 3A; Table 1). While the majority of Raldh2+/− embryos displayed normal 4th PAA morphology (Figure 3B), 8% of Raldh2+/− embryos (n=25) had hypoplasia of the 4th PAAs (Table 1). Significantly higher numbers of 4th PAA anomalies were observed in Tbx1+/− embryos (Table 1), as previously reported.8, 33 Defects in 4th PAA development were similar in Tbx1+/− and Raldh2+/−;Tbx1+/− embryos at E10.5 (Figure 3C-F; Table 1), indicating no major difference in early pharyngeal artery development when RA-levels are reduced in Tbx1+/− embryos.
Figure 3.
Pharyngeal arch artery (PAA) abnormalities visualized by intracardiac injection of India ink at E10.5. (A-F) Left lateral views of injected embryos displaying normal formation of the left 3rd, 4th and 6th PAAs in (A) WT and (B) Raldh2+/− embryos, whereas a defect of one or both 4th PAAs is observed in (C,D) Tbx1+/− and (E,F) compound heterozygous embryos. Defects include hypoplasia (red numbers) in (C) Tbx1+/− and (E) Raldh2+/−;Tbx1+/− embryos, or absence of the 4th PAAs in (D) Tbx1+/− and (F) Raldh2+/−;Tbx1+/− embryos. See Table 1 for detail. Arabic numerals indicate PAAs. AS, aortic sac.
Table 1.
Fourth pharyngeal arch artery defects in E10.5 embryos scored by ink Injection
| Bilateral defects |
|||||||
|---|---|---|---|---|---|---|---|
| Genotype | n | Abnormal (%) |
Monolateral defect |
Bilateral defect |
Sm/Sm | Sm/N-P | N-P/N-P |
| Wild-type | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| Raldh2+/− | 25 | 2 (8) | 1 | 1 | 1 | 0 | 0 |
| Tbx1+/− | 17 | 14 (82) | 61 | 8 | 5 | 23 | 1 |
| Raldh2+/−;Tbx1+/− | 20 | 17 (85) | 82 | 9 | 4 | 34 | 2 |
n, number of scorable embryos.
Sm/Sm, both fourth PAAs are thin but patent to ink; Sm/N-P, one artery is thin, the other is not patent to ink; N-P/N-P, both arteries are not patent to ink.
One left PAA absent, two right PAA absent, one left and two right PAA hypoplastics.
One left PAA absent, four right PAA absent, one left and two right PAA hypoplastics.
One left PAA hypoplastic with one right PAA absent.
Two left PAA hypoplastic with two right PAA absent and one left PAA absent with one right hypoplastic.
Analysis of aortic arch artery patterning in heterozygous Df1/+ mice, a model of del22q11.2 syndrome, has shown that ~70% of defective arteries at E10.5 recover and develop normally.8 To establish if the formation of the 4th PAA is delayed in compound heterozygous as it is in Tbx1+/− embryos, we performed intracardiac India ink injection at E11.5. While a similar proportion of defects was observed in Tbx1+/− and Raldh2+/−; Tbx1+/− embryos at E10.5, a reduced penetrance of 4th PAA defects occurred when RA-levels are decreased in Tbx1 heterozygous mutants at E11.5 (57% of Tbx1+/− versus 29% of Raldh2+/−;Tbx1+/− [Table 2, χ2=30.47; p<0.01]). This lower penetrance is not a survival effect, because an equal ratio of Tbx1+/−:Raldh2+/−;Tbx1+/− embryos are observed at all timepoints analyzed between E8.5 and E14.5 (Online Table I).
Table 2.
Fourth pharyngeal arch artery defects in E11.5 embryos scored by ink injection
| Bilateral defects |
|||||||
|---|---|---|---|---|---|---|---|
| Genotype | n | Abnormal (%) |
Monolateral defect |
Bilateral defect |
Sm/Sm | Sm/N-P | N-P/N-P |
| Wild-type | 40 | 0 | 0 | 0 | 0 | 0 | 0 |
| Raldh2+/− | 41 | 2 (5) | 21 | 0 | 0 | 0 | 0 |
| Tbx1+/− | 30 | 17 (57) | 92 | 8 | 4 | 24 | 2 |
| Raldh2+/−;Tbx1+/− | 24 | 7 (29)** | 43 | 3 | 3 | 0 | 0 |
p<0.01.
n, number of scorable embryos; Sm/Sm, both fourth PAAs are thin but patent to ink ; Sm/N-P, one fourth PAA is thin and one non patent to ink ; N-P/N-P, both arteries are not patent to ink.
One right PAA hypoplastic and one absent.
Two left PAA hypoplastic and four right PAA hypoplastic.
Two right and one left PAA hypoplastic and one right PAA absent.
Two left PAA hypoplastic and one right PAA absent.
An absence of vascular smooth muscle (VSM) is observed in the malformed and growth impaired 4th PAAs of Tbx1+/− embryos.8 Potentially the growth restoring differences due to combined Raldh2+/−;Tbx1+/− haploinsufficiency (versus single Tbx1 mutation) occurring from E10.5 and E11.5 correlates with normalization of VSM differentiation, indicated by α-smooth muscle actin (αSMA) expression (Figure 4A-H). At E10.5, embryos with 4th PAA monolateral defects were sectioned and stained with anti- αSMA. Histological analysis revealed that all reduced-size 4th PAAs of Tbx1+/− (n=6) and Raldh2+/−;Tbx1+/− (n=8) were devoid or deficient of VSM respectively (Figure 4 C,D), again indicating no striking difference in 4th PAA VSM differentiation between Tbx1+/− and Raldh2+/−;Tbx1+/− embryos at E10.5. In contrast, at E11.5, 83.3% of Tbx1+/− embryos (n=6) had no or few αSMA positive cells in the 4th PAAs (Figure 4G), while 66.6% of Raldh2+/−;Tbx1+/− embryos (n=9) had normal αSMA expression (Figure 4H). The other embryos had 4th PAAs with a thin layer containing few αSMA positive cells. These data indicate that recovery from arterial growth delay in Raldh2+/−;Tbx1+/− embryos is associated with improved differentiation of VSM.
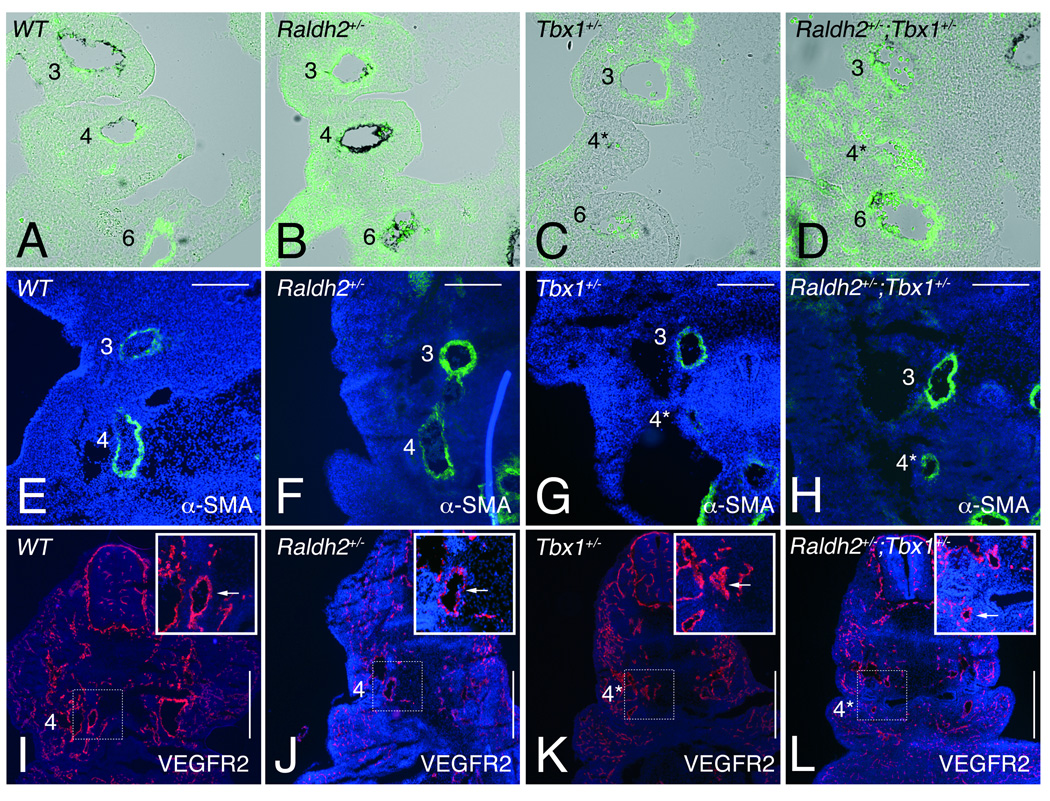
Figure 4.
Vascular smooth muscle differentiation in single and compound heterozygous Tbx1 and Raldh2 mutant embryos. (A-H) Vascular smooth muscle (VSM) differentiation was followed by anti-αSMA on front sections through the pharyngeal region of embryos at (A-D) E10.5, and (E-H) E11.5. (A-D) Abnormally small 4th PAAs (asterisk in C) in Tbx1+/− (n=6) embryos at E10.5 were devoid of VSM staining, while weak staining was detected (asterisk in D) in Raldh2+/−,Tbx1+/− (n=8) embryos at the same stage. At E11.5 4th PAAs of (G) Tbx1+/− embryos had a thin and incomplete layer of VSM in the vessel wall (n=5), while normal VSM differentiation was observed in the 4th PAAs of (H) Raldh2+/−;Tbx1+/− embryos (n=6). (I-L) Immunofluorescent analysis of VEGFR2 of the 4th PAAs in front sections of (I) WT, (J) Raldh2+/−, (K) Tbx1+/− and (L) Raldh2+/−;Tbx1+/− embryos at E11.5. Insets show high magnification views of the 4th PAA (arrow) as indicated by the boxed areas in I-L. VEGFR2 was not affected in hypoplatic 4th PAAs (asterisk) in (K) Tbx1+/− and (L) Raldh2+/−;Tbx1+/− embryos. See Table 1 and Table 2 for details. l, left; r, right. Scale bars: 200µm (E through H); 500µm (I through L)
We next examined whether vascular growth factor signaling associated with endothelial differentiation and PAA remodeling might change in Tbx1+/− and Raldh2+/−;Tbx1+/− embryos.2 Among these molecules we have analyzed platelet-derived growth factor (PDGF) receptor A and vascular endothelial growth factor receptor 2 (VEGFR2) as markers of PAA endothelial cells.34 Immunohistochemistry with VEGFR2 revealed no difference in hypoplastic 4th PAA in Tbx1+/− and Raldh2+/−;Tbx1+/− embryos at E11.5 (Figure 4K,L). Similar results were found with PDGFR-A (data not shown), suggesting that VEGF and PDGF signaling are not involved in the difference of arterial growth observed in Raldh2+/−;Tbx1+/− embryos.
Great artery patterning in Tbx1+/− and Raldh2+/−; Tbx1+/− mutants
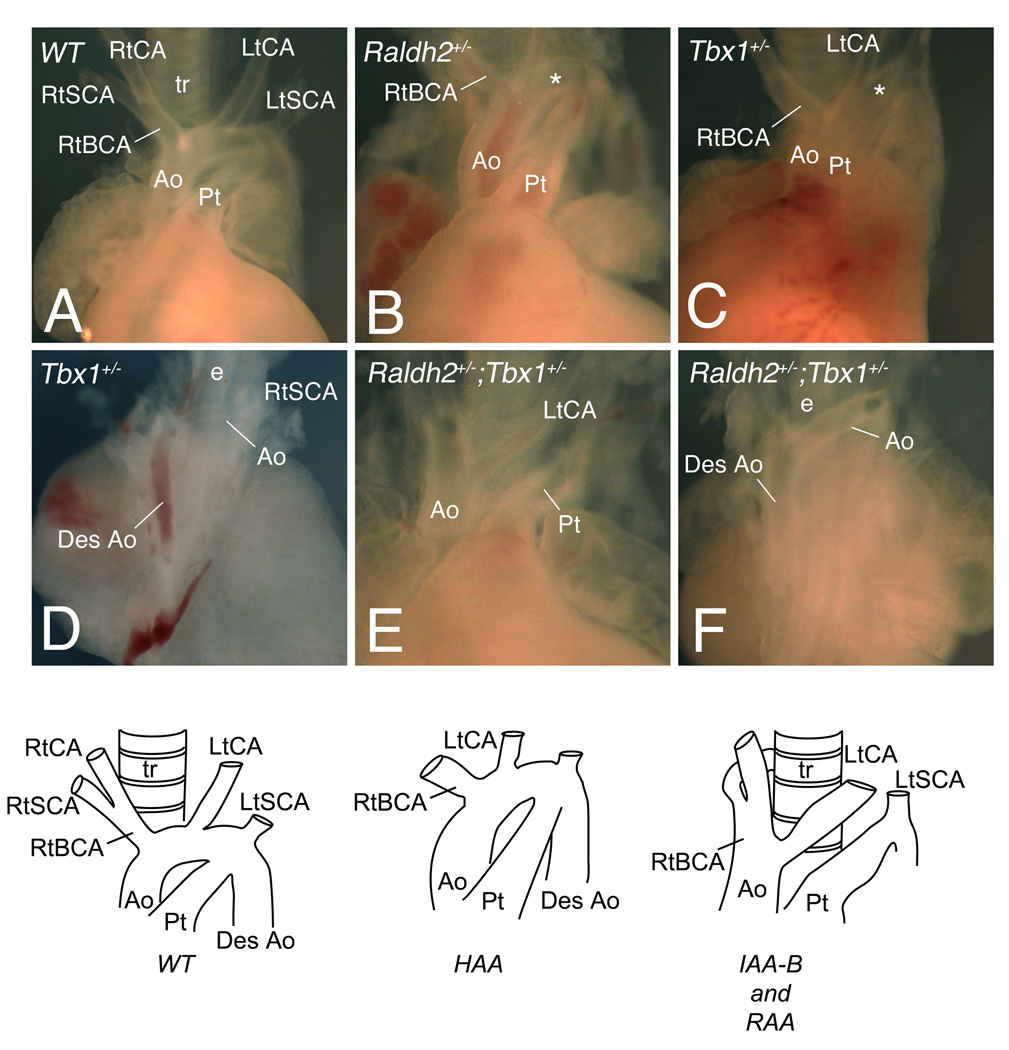
As reduction of RA-levels in Raldh2+/−; Tbx1+/− mutants increases the number of embryos that overcome the primary defect of the 4th PAAs, we examined aortic arch artery patterning of single and compound heterozygous mutant embryos at E14.5 and E18.5 to establish whether this effect is maintained (Figure 5; Table 3). All WT fetuses analyzed (n=43) had normal aortic arch morphology (Figure 5A). Similarly to earlier stages, one Raldh2+/− embryo (3%) exhibited a high aortic arch phenotype (Figure 5B; Table 3). This defect likely reflects genetic background-dependent effects. Moreover 75% of Tbx1+/− fetuses (Online Figure VIII; Table 3) had normal aortic arch anatomy, which confirms a significant decrease in phenotypic penetrance compared to E11.5 embryos. While we observe that Tbx1 haploinsufficient embryos display a spectrum of phenotypes similar to previous reports (Figure 5C,D; Table 3), the high aortic arch defect observed in C57BL/6 genetic background was comparatively more frequent than in the other genetic backgrounds (Table 3).33 However, when compound heterozygote fetuses were examined at later stages by gross dissection (Figure 5E,F; Table 3), the penetrance of aortic arch defects was different from those observed at E11.5 and reduced but not significantly less than that of Tbx1+/− embryos (Online Figure VIII). This indicated that primary normalization of the 4th PAA phenotype observed at E11.5 in Raldh2+/−;Tbx1+/− embryos was not further improved during later arch artery development in contrast to the case of Tbx1+/− embryos. Together these results suggest that reduction of RA-levels (due to Raldh2+/− mutation) accelerates the recovery from arterial growth delay observed in Tbx1+/− embryos (Online Figure VIII). We therefore conclude that the difference in penetrance of aortic arch defects at E11.5 suggests a role of regulated RA synthesis in determining the pathogenesis of DGS/VCFS phenotypes associated with heterozygous loss of Tbx1.
Figure 5.
Aortic arch abnormalities in single and compound heterozygous Tbx1 and Raldh2 mutants at fetal stages. (A,B,C,E) Ventral or (D,F) dorsal views of dissected fetal hearts showing the great arteries of WT and mutant embryos. (A) WT embryo showing a left aortic arch and normal origin of the right subclavian and left carotid arteries at E14.5. Examples of (B) Raldh2+/− and (C) Tbx1+/− embryos with a high aortic arch (asterisk). The dorsal views (D,F) show a retro-esophageal connection between the ascending and descending aorta (desAo) in Tbx1+/− and compound Raldh2+/−;Tbx1+/− embryos respectively. The frontal view (E) reveals lack of segment of the arch, a type B interruption (IAA-B) in Raldh2+/−;Tbx1+/− mutant. Schemas represent a WT, high aortic arch (HAA) and interruption (IAA-B) with retro-esophageal connection respectively. See Table 3 for detail. Ao, aorta; BCA, brachiocephalic artery; CA, carotid artery; e, esophagus; Lt, left; Pt, pulmonary trunk; Rt, right; SCA, subclavian artery; t, trachea.
Table 3.
Aortic arch patterning defects in E14.5-E18.5 embryos
| Genotype | n | Abnormal (%) |
HAA | Ab-RSA | IAA-B and RAA |
|---|---|---|---|---|---|
| Wild-type | 43 | 0 | 0 | 0 | 0 |
| Raldh2+/− | 35 | 1 (3) | 1 | 0 | 0 |
| Tbx1+/− | 36 | 9 (25) | 4 | 4 | 1 |
| Raldh2+/−;Tbx1+/− | 36 | 7 (19) | 2 | 4 | 1 |
Ab-RSA, aberrant origin of the right subclavian artery including retroesophageal right subclavian artery; HAA, high aortic arch; IAA-B, interrupted aortic arch type B; RAA, right aortic arch.
DISCUSSION
DiGeorge/velocardiofacial/del22q11.2 syndrome (DGS/VCFS) is characterized genetically by heterozygous deletions within chromosome 22q11 and clinically by cardiovascular defects, thymic, parathyroid and craniofacial anomalies. An important aspect of the human syndrome is phenotypic variability, which contrasts with remarkable homogeneity of the genetic defect,3 suggesting the presence of genetic modifiers. Hence important genetic interactions exist between Tbx1 and selective genes which give DGS/VCFS-like phenotype in mice.35 In this study we demonstrate that Tbx1 and Raldh2, encoding the enzyme responsible for synthesizing the majority of embryonic retinoic acid (RA), interact genetically to regulate the formation of structures affected in human patients with DGS/VCFS, and that reductions of RA-levels accelerate recovery from arterial growth delay observed in del22q11 mouse models.8
Independent studies of Tbx1 mutants demonstrate disruption in expression of RA metabolism genes including Raldh2 in the pharyngeal mesoderm of Tbx1−/− embryos after E9.5, but it remains unclear whether this mis-regulation is direct or secondarily due to morphological alterations.13, 22, 23 We observed cranial expansion of Raldh2 within the lateral mesoderm at E8.0 in Tbx1−/− embryos (Figure 1). At this stage the caudal pharyngeal region has not yet developed and no morphological differences between WT and Tbx1−/− embryos are apparent, suggesting that Tbx1 may directly regulate Raldh2 expression levels in the pharyngeal region.22, 23 Furthermore, increased Tbx1 expression in the P19CL6 mouse carcinoma cell line induces down-regulation of Raldh2.36 Liao et al.23 reported the identification of a T-box consensus binding site proximal to the first exon of Raldh2, reinforcing the hypothesis that Raldh2 is a direct Tbx1 target. In addition, both Tbx1 and RA regulate the expression of Cyp26 genes, responsible for inactivation of RA.13, 24, 37 These findings raise the possibility of multifaceted regulation of local RA-levels, including its synthesis and its metabolism. Regulation of the expression of Cyp26 enzymes involves multiple inputs including RA,15 suggesting a negative feedback mechanism triggered by aberrant RA signaling in Tbx1 mutant embryos. Ectopic expression of Tbx1 in Raldh2 mutant mice is similar to alterations in Tbx1 levels in vitamin A-deficient quail embryos.14 Our results suggest that the relationship between Tbx1 transcriptional activity and RA signaling may be cell-type specific, as Tbx1 is down-regulated in the pharyngeal ectoderm and endoderm of Raldh2 mutant embryos, but is expanded caudally in lateral mesoderm. Indeed, Tbx1 dosage manipulations have revealed that different tissues have different sensitivity to Tbx1 levels.38, 39
The variable penetrance of the 4th pharyngeal arch artery (PAA) phenotype in del22q11 mouse model is associated with impaired vascular smooth muscle (VSM) differentiation.8 Our results suggest that reduction of RA-levels affects the timing of growth recovery of the 4th PAAs in compound Raldh2+/−;Tbx1+/− embryos, by increasing VSM differentiation. Although the vascular system is not considered a traditional target of RA signaling, VSM cells are sensitive to its action.40 Studies from Hirschi and colleagues have shown that RA is crucial for vascular development and for endothelial cell maturation.41, 42 In addition, studies using all-trans RA have shown that RA can affect the proliferation, migration and differentiation of smooth muscle cells (SMC) in vitro.43 More recently, a study using Cyp26a1−/− embryonic stem cells with high intracellular RA-levels, revealed decreased differentiation, as assessed by SMC gene expression.44 We propose that RA affects a local signaling pathway between pharyngeal endoderm and mesenchyme in the 4th PAA. Examination of the pathways involved in remodeling of PAAs revealed that TGF-β signaling enhances expression of VSM cell-specific proteins that are essential for PAA wall structure.45 Regulation of TGF-β signaling by RA has been extensively reported in different cell types, indicating complex cross-talk between signals that regulate growth and differentiation.15 Thus, it is possible that RA reduction promotes differentiation of VSM of the 4th PAA through up-regulation of TGF-β signaling pathways. Indeed, TGF-β expression has been shown to be negatively and indirectly regulated by RA signaling in other contexts.46, 47 An alternative hypothesis is that modulation of NCCs migration during PAA development in compound heterozygous compared to single Tbx1+/− embryos is sufficient to modulate the penetrance of the 4th PAA defects that we observed. Several studies have revealed a role for Tbx1 in NCC differentiation into VSM as a possible explanation of the phenotype observed in Tbx1+/− mutants.8, 30, 31 We and others30 have provided evidence that migrating streams of NCC are disorganized in Tbx1+/− mutant embryos. This defect has been shown to result in a reduced number of these cells reaching the 4th PAA and is associated with abnormal differentiation of arterial endothelial cells.30 We hypothesize that reduction of RA-levels may affect the haploinsufficiency of Tbx1+/− mice by increasing the number of NCC reaching the 4th PAA, and accelerating subsequent VSM differentiation.
The majority of patients with DGS/VCFS features (~80%) have a similar chromosomal 22q11.2 deletion (del22q11)48 of 3Mb encompassing TBX1 and other genes, but with considerable phenotypic variability. It has been shown that neither the size of the deletion nor the parental origin of the deleted chromosome influenced the phenotype.49 While TBX1 has been shown to be the major gene underlying DGS/VCFS, genetic studies suggest that other loci may contribute to the variability of the cardiac phenotype.49, 50 To date only one modifier of the cardiovascular phenotype, VEGF, has been identified in del22q11 patients.12 Our data, which demonstrate that reduced penetrance of cardiovascular defects in Tbx1+/− mice in mid-gestation is sensitive to RA deficiency, support the hypothesis that aberrant level of embryonic RA could be another such modifier and that genes involved in RA synthesis, metabolism and signaling should be considered candidate modifiers of the DGS/VCFS phenotype associated with 22q11deletion.
Novelty and Significance.
What Is Known?
Abnormal development of the pharyngeal apparatus has been implicated in the pathogenesis of the DGS/VCFS syndrome, the most common genetic deletion syndrome in humans.
DGS/VCFS syndrome is characterized by a high clinical variability.
In the mouse, loss of Tbx1 and aberrant retinoic acid (RA) synthesis results in cardiovascular defects similar to the phenotype found in DGS patients.
What New Information Does This Article Contribute?
It reveals a genetic interaction between Tbx1 and RA signaling.
It reveals that reduction of RA-levels decreases cardiovascular malformations found normally in Tbx1 heterozygous embryos.
The common clinical features associated with DiGeorge/velo-cardio-facial syndrome (DGS/VCFS) are congenital cardiovascular malformations, thymic and parathyroid aplasia or hypoplasia and craniofacial defects. However, despite the vast majority of patients diagnosed with DGS/VCFS having the same region of 22q11.2 deleted, there is wide phenotypic variation. The causes of this phenotypic variability remain unknown. Genetic studies have identified the transcription factor encoding gene Tbx1 as a major candidate gene for DGS/VCFS. Tbx1 heterozygous mutant mice display absence or hypoplasia of the 4th pharyngeal arch artery (PAA) at E10.5 and associated great artery anomalies at later stages of development. Retinoic acid (RA) interacts with Tbx1, as Tbx1 expression was increased in Raldh2−/− deficient embryos and reduction of RA synthesis modulates development of the 4th PAA in Tbx1+/− embryos by accelerating the recovery of the arterial growth delay. RA deficiency therefore induces earlier recovery of DiGeorge-related aortic arch defects, supporting the hypothesis that differences in levels of embryonic RA may contribute to the phenotypic variability observed in DGS/VCFS patients. Genes involved in RA synthesis, metabolism and signaling should be considered candidate modifiers of the DGS/VCFS phenotype associated with 22q11deletion.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Virginia Papaioannou for Tbx1+/− mice and Pascal Dollé for Raldh2+/− mice. We are grateful to Marc Bartoli for help with statistical analysis.
SOURCES OF FUNDING
This work is supported by the “Agence Nationale de la Recherche” (ANR-07-MRAR-003), (to R.K and S.Z), the “Association Français contre les Myopathies” (AFM 13517) (to S.Z.), the EU CardioGeNet program (to R.K) and the National Institutes of Health (R01 HL070733) (to K.N.). L.R. received fellowships from the “Ministère de l'Enseignement Supérieur et de la Recherche” and the “Université de la Méditerranée” (Monitorat).
ABBREVIATIONS
- αSMA:
α-smooth muscle actin
- DGS
DiGeorge syndrome
- E
embryonic day
- FGF
Fibroblast growth factor
- PCR
polymerase chain reaction
- NCC
Neural crest cells
- OFT
Outflow tract
- PAA
Pharyngeal arch artery
- PDGF
platelet-derived growth factor
- RA
retinoic acid
- RALDH2
retinaldehyde dehydrogenase 2
- SHF
Second heart field
- Tbx1
T-box transcription factor
- VCFS
velo-cardio-facial syndrome
- VEGFR2
vascular endothelial growth factor receptor 2
- VSM
vascular smooth muscle
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: [6] Cardiac development; [139] Developmental biology; [142] Gene expression; [89] Genetics of cardiovascular disease
DISCLOSURE
“NONE”
REFERENCES
- 1.Poelmann RE, Gittenberger-de Groot AC, Hierck BP. The development of the heart and microcirculation: role of shear stress. Med Biol Eng Comput. 2008;46:479–484. doi: 10.1007/s11517-008-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450:285–288. doi: 10.1038/nature06254. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay EA. Chromosomal microdeletions: dissecting del22q11 syndrome. Nat Rev Genet. 2001;2:858–868. doi: 10.1038/35098574. [DOI] [PubMed] [Google Scholar]
- 4.Baldini A. Dissecting contiguous gene defects: TBX1. Curr Opin Genet Dev. 2005;15:279–284. doi: 10.1016/j.gde.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Hu T, Yamagishi H, Maeda J, McAnally J, Yamagishi C, Srivastava D. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development. 2004;131:5491–5502. doi: 10.1242/dev.01399. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, Lindsay EA, Baldini A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- 7.Kelly RG, Papaioannou VE. Visualization of outflow tract development in the absence of Tbx1 using an FgF10 enhancer trap transgene. Dev Dyn. 2007;236:821–828. doi: 10.1002/dvdy.21063. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay EA, Baldini A. Recovery from arterial growth delay reduces penetrance of cardiovascular defects in mice deleted for the DiGeorge syndrome region. Hum Mol Genet. 2001;10:997–1002. doi: 10.1093/hmg/10.9.997. [DOI] [PubMed] [Google Scholar]
- 9.Moon AM, Guris DL, Seo JH, Li L, Hammond J, Talbot A, Imamoto A. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev Cell. 2006;10:71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitelli F, Zhang Z, Huynh T, Sobotka A, Mupo A, Baldini A. Fgf8 expression in the Tbx1 domain causes skeletal abnormalities and modifies the aortic arch but not the outflow tract phenotype of Tbx1 mutants. Dev Biol. 2006;295:559–570. doi: 10.1016/j.ydbio.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamagishi H, Maeda J, Hu T, McAnally J, Conway SJ, Kume T, Meyers EN, Yamagishi C, Srivastava D. Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev. 2003;17:269–281. doi: 10.1101/gad.1048903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stalmans I, Lambrechts D, De Smet F, Jansen S, Wang J, Maity S, Kneer P, von der Ohe M, Swillen A, Maes C, Gewillig M, Molin DG, Hellings P, Boetel T, Haardt M, Compernolle V, Dewerchin M, Plaisance S, Vlietinck R, Emanuel B, Gittenberger-de Groot AC, Scambler P, Morrow B, Driscol DA, Moons L, Esguerra CV, Carmeliet G, Behn-Krappa A, Devriendt K, Collen D, Conway SJ, Carmeliet P. VEGF: a modifier of the del22q11 (DiGeorge) syndrome? Nat Med. 2003;9:173–182. doi: 10.1038/nm819. [DOI] [PubMed] [Google Scholar]
- 13.Guris DL, Duester G, Papaioannou VE, Imamoto A. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Dev Cell. 2006;10:81–92. doi: 10.1016/j.devcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Roberts C, Ivins SM, James CT, Scambler PJ. Retinoic acid down-regulates Tbx1 expression in vivo and in vitro. Dev Dyn. 2005;232:928–938. doi: 10.1002/dvdy.20268. [DOI] [PubMed] [Google Scholar]
- 15.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 16.Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 17.Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130:2525–2534. doi: 10.1242/dev.00463. [DOI] [PubMed] [Google Scholar]
- 18.Vermot J, Niederreither K, Garnier JM, Chambon P, Dolle P. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. Proc Natl Acad Sci U S A. 2003;100:1763–1768. doi: 10.1073/pnas.0437920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wendling O, Dennefeld C, Chambon P, Mark M. Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development. 2000;127:1553–1562. doi: 10.1242/dev.127.8.1553. [DOI] [PubMed] [Google Scholar]
- 20.Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci U S A. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivins S, Lammerts van Beuren K, Roberts C, James C, Lindsay E, Baldini A, Ataliotis P, Scambler PJ. Microarray analysis detects differentially expressed genes in the pharyngeal region of mice lacking Tbx1. Dev Biol. 2005;285:554–569. doi: 10.1016/j.ydbio.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Liao J, Aggarwal VS, Nowotschin S, Bondarev A, Lipner S, Morrow BE. Identification of downstream genetic pathways of Tbx1 in the second heart field. Dev Biol. 2008;316:524–537. doi: 10.1016/j.ydbio.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts C, Ivins S, Cook AC, Baldini A, Scambler PJ. Cyp26 genes a1, b1 and c1 are down-regulated in Tbx1 null mice and inhibition of Cyp26 enzyme function produces a phenocopy of DiGeorge Syndrome in the chick. Hum Mol Genet. 2006;15:3394–3410. doi: 10.1093/hmg/ddl416. [DOI] [PubMed] [Google Scholar]
- 25.Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- 26.Vermot J, Pourquie O. Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature. 2005;435:215–220. doi: 10.1038/nature03488. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- 28.Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A. A genetic link between Tbx1 and fibroblast growth factor signaling. Development. 2002;129:4605–4611. doi: 10.1242/dev.129.19.4605. [DOI] [PubMed] [Google Scholar]
- 29.Vitelli F, Morishima M, Taddei I, Lindsay EA, Baldini A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum Mol Genet. 2002;11:915–922. doi: 10.1093/hmg/11.8.915. [DOI] [PubMed] [Google Scholar]
- 30.Calmont A, Ivins S, Van Bueren KL, Papangeli I, Kyriakopoulou V, Andrews WD, Martin JF, Moon AM, Illingworth EA, Basson MA, Scambler PJ. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating Gbx2 expression in the pharyngeal ectoderm. Development. 2009;136:3173–3183. doi: 10.1242/dev.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kochilas L, Merscher-Gomez S, Lu MM, Potluri V, Liao J, Kucherlapati R, Morrow B, Epstein JA. The role of neural crest during cardiac development in a mouse model of DiGeorge syndrome. Dev Biol. 2002;251:157–166. doi: 10.1006/dbio.2002.0819. [DOI] [PubMed] [Google Scholar]
- 32.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 33.Taddei I, Morishima M, Huynh T, Lindsay EA. Genetic factors are major determinants of phenotypic variability in a mouse model of the DiGeorge/del22q11 syndromes. Proc Natl Acad Sci U S A. 2001;98:11428–11431. doi: 10.1073/pnas.201127298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Vitelli F, Baldini A. Generating and modifying DiGeorge syndrome-like phenotypes in model organisms: is there a common genetic pathway? Trends Genet. 2003;19:588–593. doi: 10.1016/j.tig.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Caterino M, Ruoppolo M, Fulcoli G, Huynth T, Orru S, Baldini A, Salvatore F. Transcription factor TBX1 overexpression induces downregulation of proteins involved in retinoic acid metabolism: a comparative proteomic analysis. J Proteome Res. 2009;8:1515–1526. doi: 10.1021/pr800870d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, Dolle P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat Genet. 2002;31:84–88. doi: 10.1038/ng876. [DOI] [PubMed] [Google Scholar]
- 38.Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA, Brown MC, Adams J, Morrow BE. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Baldini A. In vivo response to high-resolution variation of Tbx1 mRNA dosage. Hum Mol Genet. 2008;17:150–157. doi: 10.1093/hmg/ddm291. [DOI] [PubMed] [Google Scholar]
- 40.Miano JM, Topouzis S, Majesky MW, Olson EN. Retinoid receptor expression and all-trans retinoic acid-mediated growth inhibition in vascular smooth muscle cells. Circulation. 1996;93:1886–1895. doi: 10.1161/01.cir.93.10.1886. [DOI] [PubMed] [Google Scholar]
- 41.Bohnsack BL, Lai L, Dolle P, Hirschi KK. Signaling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation. Genes Dev. 2004;18:1345–1358. doi: 10.1101/gad.1184904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai L, Bohnsack BL, Niederreither K, Hirschi KK. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development. 2003;130:6465–6474. doi: 10.1242/dev.00887. [DOI] [PubMed] [Google Scholar]
- 43.Neuville P, Yan Z, Gidlof A, Pepper MS, Hansson GK, Gabbiani G, Sirsjo A. Retinoic acid regulates arterial smooth muscle cell proliferation and phenotypic features in vivo and in vitro through an RARalpha-dependent signaling pathway. Arterioscler Thromb Vasc Biol. 1999;19:1430–1436. doi: 10.1161/01.atv.19.6.1430. [DOI] [PubMed] [Google Scholar]
- 44.Langton S, Gudas LJ. CYP26A1 knockout embryonic stem cells exhibit reduced differentiation and growth arrest in response to retinoic acid. Dev Biol. 2008;315:331–354. doi: 10.1016/j.ydbio.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Gittenberger-de Groot AC, Azhar M, Molin DG. Transforming growth factor beta-SMAD2 signaling and aortic arch development. Trends Cardiovasc Med. 2006;16:1–6. doi: 10.1016/j.tcm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Kubalak SW, Hutson DR, Scott KK, Shannon RA. Elevated transforming growth factor beta2 enhances apoptosis and contributes to abnormal outflow tract and aortic sac development in retinoic X receptor alpha knockout embryos. Development. 2002;129:733–746. doi: 10.1242/dev.129.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- 48.McDermid HE, Morrow BE. Genomic disorders on 22q11. Am J Hum Genet. 2002;70:1077–1088. doi: 10.1086/340363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelmann L, Pandita RK, Spiteri E, Funke B, Goldberg R, Palanisamy N, Chaganti RS, Magenis E, Shprintzen RJ, Morrow BE. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum Mol Genet. 1999;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- 50.Voelckel MA, Girardot L, Giusiano B, Levy N, Philip N. Allelic variations at the haploid TBX1 locus do not influence the cardiac phenotype in cases of 22q11 microdeletion. Ann Genet. 2004;47:235–240. doi: 10.1016/j.anngen.2004.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.