Abstract
Background
Mitochondria mediated apoptotic signaling contributes to microvascular hyperpermeability. We hypothesized that cyclosporine A (CsA), that protects mitochondrial transition pores would attenuate hyperpermeability independent of its calcineurin inhibitory property.
Methods
Hyperpermeability was induced in microvascular endothelial cell monolayers using pro-apoptotic BAK or active caspase-3 following CsA or a specific calcineurin inhibitor, calcineurin auto-inhibitory peptide (CIP) treatment. Permeability was measured based on FITC-albumin flux across the monolayers. Mitochondrial transmembrane potential (MTP) was determined using JC-1. Mitochondrial release of cytochrome c was measured using ELISA and caspase-3 activity fluorometrically.
Results
CsA (10 nM) but not CIP (100 μM) attenuated BAK-induced hyperpermeability (p < 0.05) CsA but not CIP attenuated BAK-induced decrease in MTP, increase in cytochrome c levels and caspase-3 activity (p < 0.05). CsA and CIP were ineffective against caspase-3-induced hyperpermeability.
Conclusions
CsA attenuated hyperpermeability by protecting MTP thus preventing mitochondria-mediated apoptotic signaling. CsA’s protective effect is independent of calcineurin inhibition.
INTRODUCTION
Vascular hyperpermeability that occurs due to disruption of the microvascular endothelial cell barrier, is one of the primary clinical manifestations of trauma conditions such as hemorrhagic shock (HS) (1, 2). Recent evidences from our laboratory have demonstrated that activation of mitochondria mediated apoptotic signaling cascade is a major inducer of microvascular hyperpermeability (3, 4). Our studies have further shown that pharmacological intervention of apoptotic signaling can attenuate microvascular hyperpermeability in vitro and in vivo and agents with antioxidant and anti-apoptotic properties have regulatory functions against microvascular permeability (4, 5). The present study is the continuation of our efforts to identify the mechanisms of action of various anti-apoptotic agents that inhibit microvascular permeability acting at the level of mitochondria.
Fundamentally, apoptosis has an ‘intrinsic’ mitochondrial pathway, and an extrinsic “death ligand” pathway. The ‘intrinsic’ pathway of apoptosis is mediated through the decrease in mitochondrial transmembrane potential, the release of cytochrome c, smac (second mitochondrial-derived activator of caspases) and AIF (apoptosis inducing factor), all of which are regulated by proapoptotic and antiapoptotic Bcl-2 family proteins, such as Bax/Bak and Bcl-2/xL (6). Translocation of cytochrome c from mitochondria to the cytosol through mitochondrial transition pores is precisely controlled by the change in mitochondrial transmembrane potential. Cytochrome c triggers the release of apoptosome assembly from apoptotic protease-activating factor-1 (Apaf-1), ATP, and procaspase-9, which activates caspase-3 and caspase-7 (6). Caspases cleave the components of cell-cell (beta- and gamma-catenin) and cell-matrix (focal adhesion kinase and p130(Cas)) adherens junctions during apoptosis with dose and time requirements that paralleled those seen in barrier dysfunction and detachment (7,8). Our recent studies show that a decrease in mitochondrial transmembrane potential, a subsequent increase in mitochondrial release of cytochrome c, activation of caspase-3 and adherens junction damage occurs in vascular hyperpermeability (3, 9).
We have recently shown that cyclosporine A (CsA); a known inhibitor of mitochondrial transition pore opening attenuates vascular hyper permeability following hemorrhagic shock (4). Cyclosporine A is a cyclic nonribosomal peptide containing 11 amino acids produced by the fungus Tolypocladium inflatum Gams and it has been previously shown to inhibit disruption of the mitochondrial membrane function, which plays a key role in apoptosis induction (10). CsA is also a known inhibitor of cellular calcineurin (11). Calcineurin is a Ca (2+)-calmodulin-dependent serine/threonine protein phosphatase that has been implicated in various signaling pathways (12). Among its several functions in controlling intracellular Ca2+ signaling, calcineurin participates in gene regulation and external signal-mediated biological responses in many organisms and in many cell types. Calcineurin inhibition was able to increase the resistance of rats to the pathophysiological consequence of splanchnic artery occlusion shock (12) and attenuate injury in rat model of experimental lung ischemia reperfusion (13). Although recent studies from our laboratory have demonstrated the protective effects of CsA against vascular hyperpermeability, it is not known if this effect is due to inhibition of calcineurin activity or due to the effect at the mitochondrial level.
The purpose of this study was to determine if the protective effects of CsA against hyperpermeability is due to its effects on mitochondrial transition pores and apoptotic signaling or on calcineurin activity or both. Based on our recent observations (4, 14), we have hypothesized that CsA, that is known to protect mitochondrial transition pores, would attenuate microvascular hyperpermeability independent of its calcineurin inhibitory property. For this purpose, we have evaluated the effects of CsA and a specific calcineurin inhibitor, calcineurin autoinhibitory peptide (CIP) on activation of apoptotic signaling and microvascualr endothelial cell hyperpermeability. The peptide corresponds to the residues 467–491 within the inhibitory domain of human calcineurin alpha subunit. CIP has no known effect on mitochondrial transmembrane potential.
MATERIALS AND METHODS
Cyclosporine A, and fluorescein isothiocyanate-bovine albumin (FITC-albumin) were obtained from Sigma (St. Louis, MO). Calcineurin autoinhibitory peptide (CIP) was a gift from Dr. Brett Mitchell (Texas A&M HSC, Temple, Texas). The CIP was originally dissolved in DMSO but diluted in distilled water which gave a less than 1% dilution of DMSO and used at a concentration of 100μM (15). JC-1 (5,5′,6,6′ tetrachoro-1,1′,3,3′ tetraethylbenzimidazolyl carbocyanine iodide) was obtained from Cell Technology Inc. (Mountain View, CA). BAK (BH3) peptide was obtained from R &D Systems (Minneapolis, MN). Active caspase-3 was obtained from Millipore and TransIT was obtained from Mirus Bio-Corporation (Madison, WI).
Effect of Cyclosporine A and CIP on monolayer permeability
Rat lung microvascular endothelial cells (RLMEC) were obtained from VEC Tecnologies Inc. (Rensselaer, NY). The cells were grown as monolayers in transwell plates (Corning Life Sciences, Lowell, MA) in complete MCDB-3 media supplemented with 10 % fetal bovine serum. Prior to 1 hour of the start of experiment, the monolayers were exposed to fresh media without phenol-red dye. The monolayers were treated with Cyclosporine A (10 nM) or CIP (100 μM) for 1 hour. Following this, the cells were transfected with active caspase-3 or pro-apoptotic BAK peptide using TransIT-LT1 polyamine. BAK (BH3) (5 μg/ml) or active caspase-3 (5 μg/ml) was exposed to TransIT (10 μl/ml) for 15 minutes before exposure to the cells (6, 9). FITC-albumin (5 mg/ml) was added to the luminal (upper) chamber of the transwell and left for 30 minutes. Untreated (basal) cells served as controls. Our previous studies show that the transfection medium alone does not induce hyperpermeability in the monolayer [6, 9]. The samples (100 μl) collected from the abluminal (lower) chambers were analyzed for FITC fluorescent intensity using a fluorometric plate reader at excitation 494 nM and 520 nM. The data were calculated as percentage of the control (basal) values.
Effect of Cyclosporine A and CIP on mitochondrial transmembrane potential
The change in mitochondrial transmembrane potential (MTP) was determined using a cationic fluorescent indicator JC-1 that accumulates in intact mitochondria and fluoresces red and when the MTP decreases due to the initiation of apoptosis, the JC-1 cannot accumulate within the mitochondria and remains in the cytoplasm in a green fluorescent monomeric form. To determine MTP, RLMEC were grown on fibronectin coated chamber slides for 24 hours, exposed to media without phenol red for 1 hour followed by CsA or CIP (100 μM) for 1 hour. BAK (BH3) peptide (5 μg/ml) was prepared as described above and transfections were performed for 1 hour as described above. Untreated cells served as controls. The cells were incubated with JC-1 for 15 minutes at 37 °C, washed in a JC-1 wash buffer and observed immediately under a fluorescent microscope for green and red fluorescence.
Effect of Cyclosporine A and CIP on cytochrome c release
RLMEC were treated with CsA (10nM) or CIP (100 μM) for 1 hour followed by BAK (BH3) peptide (5 μg/ml) transfection for 1 hour, as described above. Untreated (basal), CsA alone treated or CIP alone treated cells served as controls. The cytosolic cytochrome c levels were estimated using an enzyme linked immunosorbent assay kit (R&D Systems, Minneapolis, MN). Briefly, the cells were lysed in a cold preparation buffer provided in the kit, centrifuged at 10,000 X g for 60 min at 4°C. The supernatant was subjected to protein assay using Bradford method. The cell lysates were treated with a conjugate reagent, transferred to a microwell plate coated with cytochrome c antibody and incubated at room temperature for 60 minutes. The wells were treated with a substrate reagent and incubated for 30 minutes followed by addition of a stop solution provided in the assay kit. The optical density was read at 450 nm in a calorimetric plate reader.
Effect of Cyclosporine A and CIP on caspase-3 activity
The RLMEC were treated with CsA (10nM) or CIP (100 μM) for 1 hour. BAK (BH3) peptide (5 μg/ml) transfection was performed for 1 hour as described above. Untreated (basal), CsA alone treated or CIP alone treated cells served as controls Caspase-3 activity was determined using a caspase-3 activity assay kit (Calbiochem, La Jolla, CA). The cells were lysed in caspase-3 sample lysis buffer provided in the kit. The homogenates were centrifuged at 13000 X g and the resulting supernatant was used for protein estimation and caspase-3 assays. The cell lysates were exposed to the DEVD substrate conjugate (labeled with a fluorescent molecule, 7-amino-4-trifluoromethyl coumarin) provided in the kit. The resulting fluorescence was measured in a fluorescent plate reader (excitation 400 nm/emission 505 nm).
Statistical analysis
All data are expressed as mean ± SE. The comparisons between groups were made utilizing analysis of variance (ANOVA) followed by the Bonferroni’s post-test for multiple comparisons. The students’ t-test was also employed wherever required. Each experimental value was compared with initial baseline value and expressed as percentage change. A p value of < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Cyclosporine A but not CIP inhibits monolayer hyperpermeability
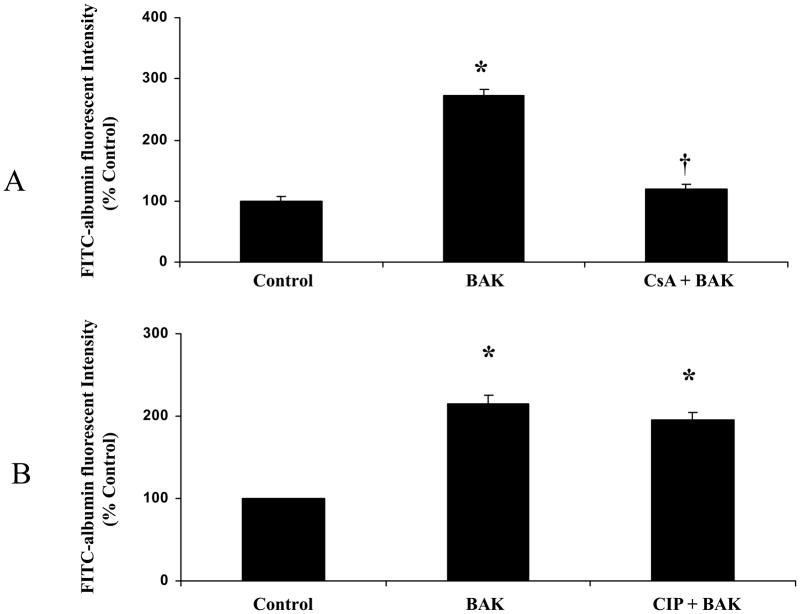
In RLMEC monolayers, transfection of BAK (BH3) peptide (5 μg/ml) induced hyperpermeability (Figure-1A). The FITC-albumin fluorescent intensity was significantly high in the BAK (BH3) peptide transfected group compared with the control group (p < 0.05; Figure-1A) suggesting an increase in leakage of FITC-albumin across the endothelial monolayer. The cells treated with CsA (10 nM) attenuated the BAK-induced hyperpermeability (p < 0.05; Figure-1A). The cells treated with CIP (100 μM) before BAK transfection showed no significant change (Figure 1B).
Figure 1.
Cyclosporine A (CsA) attenuates BAK-induced hyperpermeability in rat lung microvascular endothelial cell monolayers whereas calcineurine specific inhibitor CIP shows no significant effect. Change in permeability is expressed as percentage of the basal fluorescence. (A). BAK transfection induced hyperpermeability in the monolayer compared to control (*p < 0.05; n = 5). CsA (10 nM) pre-treatment in BAK-transfected cells showed decrease in FITC-albumin fluorescence compared with untreated cells (†p < 0.05; n = 5). (B). BAK transfection induced hyperpermeability in the monolayer compared to control (*p < 0.05; n = 5). CIP (100μM) treatment showed no significant effect on BAK-induced hyperpermeability.
Cyclosporine A acts upstream of caspase-3
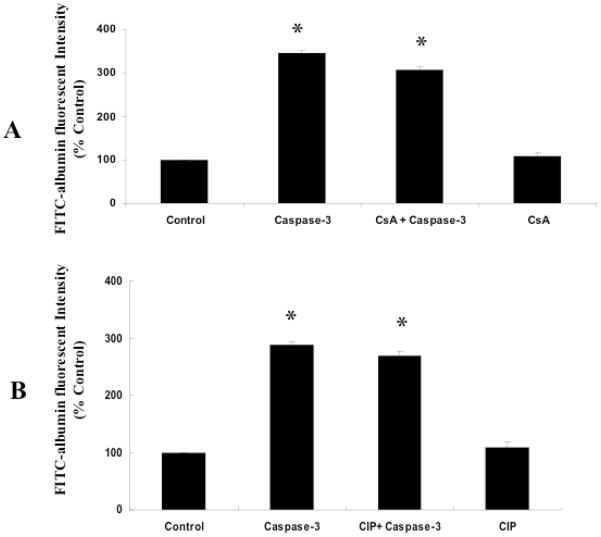
The active caspase-3 transfected (5μg/ml) monolayers showed significant hyperpermeability compared with the control monolayers (p < 0.05; Figure-2A, B). The monolayers pretreated with CsA (10 nM) followed by transfection of caspase-3 did not show significant difference in permeability compared with active caspase-3 transfected monoalyers without CsA treatment (Figure-2A). This shows that CsA is ineffective in directly preventing active caspase-3-mediated hyperpermeability and suggests that CsA mediated protection observed in the previous experiment (BAK-induced permeability) was by acting upstream of caspase-3. The monolayers pretreated with CIP (100 μM) followed by transfection of caspase-3 did not show significant difference in permeability compared with active caspase-3 transfected monoalyers without CIP treatment (Figure-2B).
Figure 2.
CsA or CIP showed no significant effect on active caspase-3 induced-hyperpermeability in rat lung microvascular endothelial cell monolayers. Active caspase-3 transfection induced hyperpermeability in the monolayer compared to control (*p < 0.05; n = 5; A, B). CsA (10 nM) or CIP (100μM) pre-treatment in active acspase-3 transfected cells showed no significant change in FITC-albumin fluorescence compared with untreated cells (A, B).
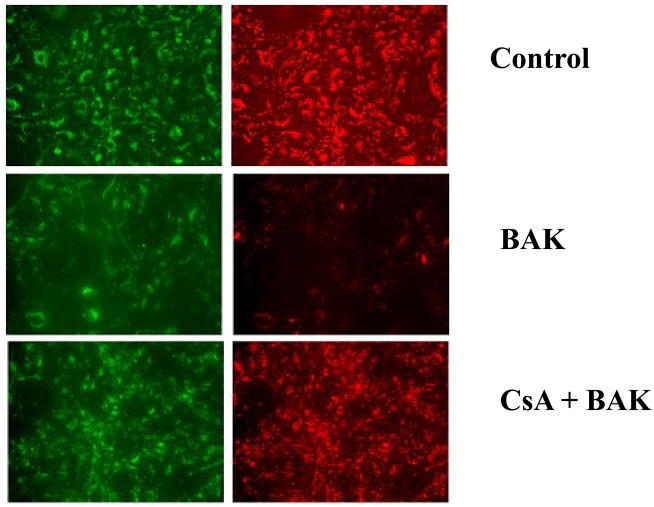
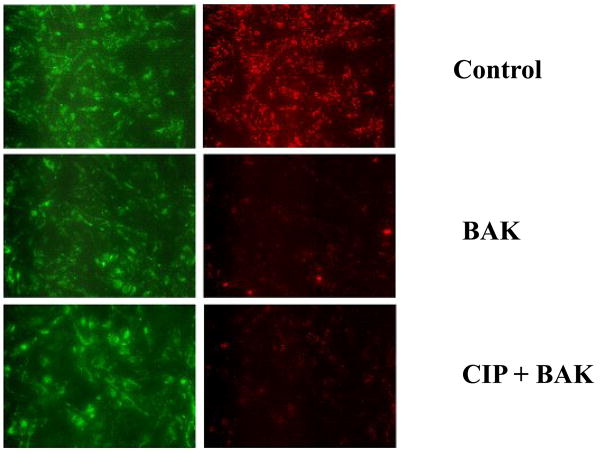
Cyclosporine A but not CIP protects mitochondrial transmembrane potential
The cells from untreated control group showed red fluorescence indicating J-aggregate formation and intact mitochondria. Following BAK (BH3) transfection, there was a decrease in red (mitochondrial) fluorescence indicating the loss of mitochondrial transmembrane potential. BAK (BH3) transfected cells pre-treated with CsA showed an increase in red fluorescence indicating protection of MTP. CIP treatment showed no protective effect against BAK (BH3)-induced decrease in MTP.
Cyclosporine A but not CIP inhibited mitochondrial release of cytochrome c to the cytosol
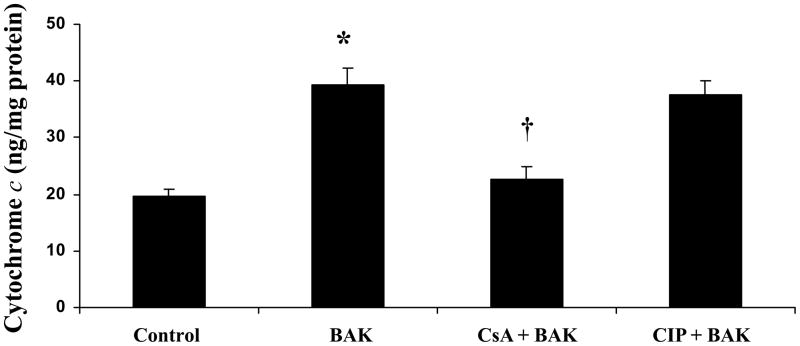
BAK transfected group showed significantly higher levels of cytochrome c versus the control group (p < 0.05; Figure-5). CsA treated-BAK transfected cells showed a significant decrease in cytoplasmic cytochrome c levels compared with BAK (BH3)-transfected cells without CsA pre-treatment whereas CIP treatment showed no significant decrease in BAK-induced cytochrome c levels (p < 0.05; Figure-5). Cytochrome c levels in CsA or CIP alone treated cells were not significantly different from the control group (data not shown).
Figure 5.
CsA treatment prevents cytochrome c release to the cytoplasm in rat lung microvascular endothelial cells (RLMEC) whereas CIP treatment shows no effect. Cytosolic cytochrome c levels are increased following BAK (BH3) transfection compared with control (*p < 0.05; n = 5). CsA pre-treatment in BAK (BH3) transfected cells shows significant decrease in cytochrome c levels compared with BAK (BH3) transfected cells without CsA pre-treatment or with CIP treated-BAK transfected cells (†p < 0.05; n = 5).
Cyclosporine A but not CIP inhibits caspase-3 activity
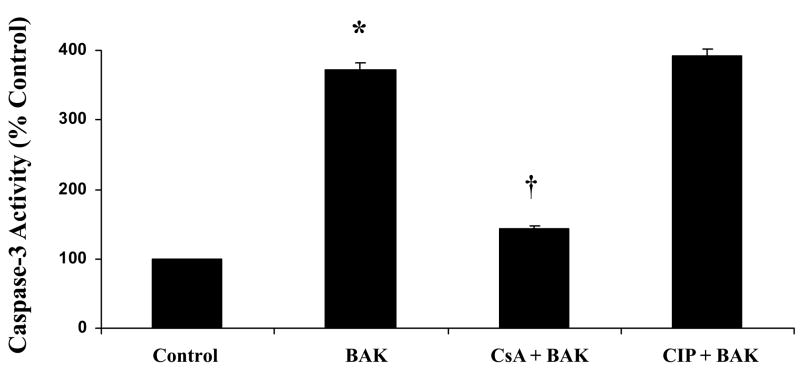
Caspase-3 activity in BAK transfected cells showed significant increase compared with the control group (p < 0.05; Figure-6). CsA treatment significantly decreased BAK-induced increase in caspase-3 activity (p < 0.05; Figure-6). Unlike CsA treatment, the CIP treatment showed no significant decrease in BAK-induced caspase-3 activity. Caspase-3 activity of CsA or CIP alone treated cells were not significantly different from the control group (data not shown). Mitochondrial release of cytochrome c leads to the activation of caspase-3. These results support the above finding hat CsA prevents BAK-induced cytochrome c release whereas CIP has no effect.
Figure 6.
CsA inhibits pro-apoptotic BAK (BH3) peptide induced caspase-3 activity, in rat lung microvascular endothelial cells (RLMEC) whereas CIP treatment shows no significant effect. BAK (BH3) transfection significantly increased caspase-3 activity compared with control cells (*p < 0.05; n = 5). CsA pre-treatment in BAK (BH3) transfected cells shows significant decrease in caspase-3 activity compared with BAK (BH3) transfected cells without CsA pre-treatment or with CIP treated-BAK transfected cells (†p < 0.05; n = 5).
COMMENTS
In this study, we show that cyclosporine A (CsA), an inhibitor of both calcineurin and mitochondrial transmembrane potential attenuates microvascular hyperpermeability by decreasing mitochondrial membrane depolarization, mitochondrial release of cytochrome c and activation of caspase-3. Calcineurin autoinhibitory peptide (CIP) that specifically inhibits calcineurin and shows no effect on mitochondrial transition pores did not show any protective effect. Our findings suggest that CsA mediated attenuation of microvascular hyperpermeability is due to its protective effects on mitochondria mediated apoptotic signalling which is independent of its calcineurin inhibitory property.
Studies from our laboratory and by others have shown that CsA has protective effects on mitochondria at the level of mitochondrial transition pores (4, 14, 16). Various mitochondrial functions, including ion transport and ATP formation require an intact mitochondrial transition pore. A crucial event that occurs in response to apoptotic signaling is loss of this electrochemical proton gradient followed by collapse of mitochondrial transmembrane potential (17). This collapse occurs due to opening of mitochondrial transition pores in the inner mitochondrial membrane that allows the nonselective diffusion of solutes across the membrane. The release of mitochondrial cytochrome c into the cytoplasm takes place through this regulatory mechanism. A decrease in mitochondrial transmembrane potential and an increased mitochondrial release of cytochrome c following pro-apoptotic BAK treatment was observed in our study. Our results show restoration of mitochondrial transmembrane potential and decrease in cytosolic cytochrome c levels in BAK treated cells pre-treated with CsA. This suggests that CsA protects the mitochondrial transition pores and prevents cytochrome c release into the cytoplasm. The calcineurin specific inhibitor CIP showed no protective effect on mitochondrial transition pores and did not prevent cytochrome c release. This was evident from its failure to restore mitochondrial transmembrane potential and prevent BAK-induced rise in cytosolic cytochrome c levels. Our results thus show differential behavior of these calcineurin inhibitors in protecting mitochondrial transition pores and thereby preventing the collapse of the transmembrane potential. In addition to this, our results show that CsA had no effect on caspase-3 mediated hyperpermeability. This indicates that CsA act upstream of caspase-3 to regulate hyperpermeability.
The cellular mechanism by which CsA protects the mitochondrial transition pores has been attributed to its inhibitory effect on the peptidyl-prolyl isomerase activity of cyclophilin D (CyD) (18, 19). In the mitochondria, cytochrome c is originally located in the intermembrane/intercristae spaces where it functions as an electron shuttle in the mitochondrial respiratory chain and interacts with cardiolipin (20). The mitochondrial outer membrane permeabilization occurs in response to several pro-apoptotic stimuli. This facilitates the communication between the intermembrane and the intercristae space which results in mobilization of cytochrome c from cardiolipin leading to it’s releasing into the cytosol (20). Following the release into the cytosol, cytochrome c mediates the allosteric activation of apoptosis-protease activating factor 1, which is required for the proteolytic maturation of caspase-9 and caspase-3 (20, 21). The release of cytochrome c from mitochondria to the cytosol activates caspase-3 leading to cleavage of a variety of cell adhesion proteins (21). The initiation of endothelial apoptosis correlates with cleavage and disassembly of intracellular and extracellular components of cell adherens junctions (22). The major components of endothelial cell adherens junctions are the cadherin family of proteins, alpha-, beta- and gamma-catenins. A stable cell-cell adherens junction requires the close interaction of the cytoplasmic domain of the cadherins with a group of intracellular proteins, the catenins (22). Beta-catenin is a member of the Armadillo repeat protein family with a dual cellular function as a component of both the adherens junction complex and the Wnt signaling pathway. Its absence significantly reduces the capacity of the cells to maintain intercellular contacts that may lead to fluid leakage (23). Proteolytic cleavage of beta catenin occurs following the activation of procaspase-3, -6 or -8 (21, 24, 25, 26). Cleavage of beta-catenin was found to be caspase-dependent and five cleavage products of beta-catenin were identified in vivo and after in vitro cleavage by caspase-3 (27). Thus, CsA mediated inhibition of caspase-3 and subsequent prevention of microvascular endothelial cell-cell detachment is a possible mechanism by which it protected barrier integrity and prevented microvascular hyperpermeability. It is possible that unlike CsA, the calcineurin specific inhibitor CIP failed to protect mitochondrial transition pores and subsequent caspase-3 activation leading to its failure to prevent cell-cell detachment resulting in monolayer hyperpermeability.
Calcineurin is a serine/threonine phosphatase and is controlled by cellular calcium (28). CIP is a specific inhibitor for calcineurin which corresponds to the residues 467–491 within the inhibitory domain of human calcineurin alpha subunit. Once inside the cell, CIP inhibits calcineurin by mimicking the endogenous autoinhibitory domain of the calcineurin A subunit and blocks its catalytic activity. Our results that CIP did not prevent apoptotic signaling or protect endothelial barrier function suggest that the protective effect shown by CsA is not due to its calcineurin inhibitory property. Cyclosporine has been used as an immunosuppressant in organ transplantation. Upon administration CsA binds to and forms a complex with cyclophilin D. This drug-immunophilin complex in turn binds to the calcium-dependent T cell protein phosphatase calcineurin which appears to be critical in its immunosuppressive properties. Calcineurin inhibition results in reduced interleukin-2 synthesis, inhibition of T cell activation, and immunosuppression. Our study showed that calcineurin plays no significant role in regulating mitochondria mediated apoptotic signaling or microvascular permeability.
Our findings show that CsA mediated the protection of mitochondrial transition pores and prevented the mitochondrial release of cytochrome c and the subsequent activation of caspase-3. By inhibiting caspase-3 activation, CsA may be preventing caspase-3 mediated cleavage of cell adherens proteins such as beta catenin and provide protection against cell-cell detachment and hyperpermeability. This study supports our hypothesis that preservation of the mitochondrial transmembrane potential is the mechanism by which CsA protects against microvascular endothelial cell hyperpermeability and shows that its protective effects are independent of its calcineurin inhibitory properties.
SUMMARY
Mitochondria mediated apoptotic signaling contributes to microvascular hyperpermeability following conditions such as hemorrhagic shock. Cyclosporine A (CsA) that protects mitochondrial transition pores and inhibits calcineurin, attenuated apoptotic signaling and microvascular endothelial cell hyperpermeabiltiy. A calcineurin specific inhibitor, calcinueirn auto-inhibitory peptide (CIP) showed no protective effect against apoptotic signaling or hyperpermeability. CsA mediated protection against hyperpermeability is mediated through the inhibition of apoptotic signaling and is independent of its calcineurin inhibitory property.
Figure 3.
CsA protects mitochondrial membrane integrity in rat lung microvascular endothelial cells (RLMEC). Fluorescence microscopy images of the mitochondrial membrane potential indicator JC-1 in its monomeric (green) and dimeric (red) forms are shown. BAK (BH3) transfection leads to the collapse of mitochondrial membrane potential showing predominantly monomeric forms. CsA (10nM) treatment prevents the collapse of mitochondrial membrane potential evidenced by the restoration of dimeric form-red fluorescence.
Figure 4.
CIP shows no protective effect on mitochondrial membrane integrity in rat lung microvascular endothelial cells (RLMEC). Fluorescence microscopy images of the mitochondrial membrane potential indicator JC-1 in its monomeric (green) and dimeric (red) forms are shown. BAK (BH3) transfection leads to the collapse of mitochondrial membrane potential showing predominantly monomeric forms. CIP (100μM) treatment did not prevent the collapse of mitochondrial membrane potential evidenced by the absence of dimeric form-red fluorescence.
Acknowledgments
This work was supported by grant 1K01-HL-07815-01A1 from the National Heart, Lung, and Blood Institute, National Institutes of Health. We thank Dr. Brett Mitchell for the gift of Calcineurin auto-inhibitory peptide.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baluk P, Hirata A, Thurston G, et al. Endothelial gaps: time course of formation and closure in inflamed venules of rats. Am J Physiol. 1997;272:155–170. doi: 10.1152/ajplung.1997.272.1.L155. [DOI] [PubMed] [Google Scholar]
- 2.Childs EW, Udobi KF, Hunter FA, et al. Evidence of transcellular albumin transport after hemorrhagic shock. Shock. 2005;23:565–570. [PubMed] [Google Scholar]
- 3.Childs EW, Tharakan B, Hunter FA, et al. Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2007;292:H3179–189. doi: 10.1152/ajpheart.01337.2006. [DOI] [PubMed] [Google Scholar]
- 4.Tharakan B, Holder-Haynes JG, Hunter FA, Smythe WR, Childs EW. Cyclosporine A prevents vascular hyperpermeability after hemorrhagic shock by inhibiting apoptotic signaling. J Trauma. 2009;66:1033–9. doi: 10.1097/TA.0b013e31816c905f. [DOI] [PubMed] [Google Scholar]
- 5.Tharakan B, Corprew R, Hunter FA, Whaley JG, Smythe WR, Childs EW. 17beta-estradiol mediates protection against microvascular endothelial cell hyperpermeability. Am J Surg. 2009;197:147–54. doi: 10.1016/j.amjsurg.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage. Oncol Rep. 2005;14:595–599. [PubMed] [Google Scholar]
- 7.Herren B, Levkau B, Raines EW, et al. Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol Biol Cell. 1998;9:1589–1601. doi: 10.1091/mbc.9.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998;273:35371–35380. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- 9.Childs EW, Tharakan B, Byrge N, Tinsley JH, Hunter FA, Smythe WR. Angiopoietin-1 inhibits intrinsic apoptotic signaling and vascular hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2008;294:H2285–95. doi: 10.1152/ajpheart.01361.2007. [DOI] [PubMed] [Google Scholar]
- 10.Walter DH, Haendeler J, Galle J, et al. Cyclosporin A inhibits apoptosis of human endothelial cells by preventing release of cytochrome C from mitochondria. Circulation. 1998;98:1153–1157. doi: 10.1161/01.cir.98.12.1153. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree GR. Calcium, Calcineurin, and the Control of Transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- 12.Squadrito F, Altavilla D, Squadrito G, Ferlito M, Deodato B, Arlotta M, Minutoli L, Campo GM, Bova A, Quartarone C, Urna G, Sardella A, Saitta A, Caputi AP. Protective effects of Cyclosporin-A in splanchnic artery occlusion shock. Br J Pharmacol. 2000;130:339–344. doi: 10.1038/sj.bjp.0703310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolley SM, Farivar AS, Naidu BV, Rosengart M, Thomas R, Fraga C, Mulligan MS. Endotracheal calcineurin inhibition ameliorates injury in an experimental model of lung ischemia-reperfusion. J Thorac Cardiovasc Surg. 2004;127:376–84. doi: 10.1016/j.jtcvs.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong JS, Yang H, Duan W, et al. Cytochrome bc(1) regulates the mitochondrial permeability transition by two distinct pathways. J Biol Chem. 2004;279:50420–50428. doi: 10.1074/jbc.M408882200. [DOI] [PubMed] [Google Scholar]
- 15.Santana LF, Chase EG, Votaw VS, et al. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol. 2002;544:57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halestrap AP, Connern CP, Griffiths EJ, et al. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem. 1997;174:167–172. [PubMed] [Google Scholar]
- 17.Kroemer G. Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun. 2003;304:433–435. doi: 10.1016/s0006-291x(03)00614-4. [DOI] [PubMed] [Google Scholar]
- 18.McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J. 2002;367:541–548. doi: 10.1042/BJ20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halestrap AP, Brennerb C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–25. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 20.Garrido C, Galluzi L, Brunet M, et al. Mechanism of cytochrome c release from mitochondria. Cell Death Diff. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 21.Gregorc U, Ivanova S, Thomas M, et al. hDLG/SAP97, a member of the MAGUK protein family, is a novel caspase target during cell-cell detachment in apoptosis. Biol Chem. 2005;386:705–710. doi: 10.1515/BC.2005.082. [DOI] [PubMed] [Google Scholar]
- 22.Hunter I, McGregor D, Robins SP. Caspase-dependent cleavage of cadherins and catenins during osteoblast apoptosis. J Bone Miner Res. 2001;16:466–477. doi: 10.1359/jbmr.2001.16.3.466. [DOI] [PubMed] [Google Scholar]
- 23.Cattelino A, Liebner S, Gallini R, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervello M, Giannitrapani L, La Rosa M, et al. Induction of apoptosis by the proteasome inhibitor MG132 in human HCC cells: Possible correlation with specific caspase-dependent cleavage of beta-catenin and inhibition of beta-catenin-mediated transactivation. Int J Mol Med. 2004;13:741–748. [PubMed] [Google Scholar]
- 25.Van de Craen M, Berx G, Van den Brande I, et al. Proteolytic cleavage of beta-catenin by caspases: an in vitro analysis. FEBS Lett. 1999;458:167–170. doi: 10.1016/s0014-5793(99)01153-9. [DOI] [PubMed] [Google Scholar]
- 26.Nakamoto K, Kuratsu J, Ozawa M. Beta-catenin cleavage in non-apoptotic cells with reduced cell adhesion activity. Int J Mol Med. 2005;15:973–979. [PubMed] [Google Scholar]
- 27.Steinhusen U, Badock V, Bauer A, et al. Apoptosis-induced cleavage of beta-catenin by caspase-3 results in proteolytic fragments with reduced transactivation potential. J Biol Chem. 2000;275:16345–16353. doi: 10.1074/jbc.M001458200. [DOI] [PubMed] [Google Scholar]
- 28.Crabtree GR. Calcium, Calcineurin, and the Control of Transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]