Abstract
Objective:
To explore the expression of Notch1 signaling pathway in nasopharyngeal carcinoma (NPC).
Methods:
We performed immunocytochemistry on surgically resected NPC using antibodies against embryonic stem (ES) cell proteins and against Notch1 signaling components.
Results:
We found that ES cell protein markers SOX2 and OCT4 were expressed in a subpopulation of cells for all three subtypes of NPC but barely in the normal control. Double immunostaining shows that SOX2- and OCT4-positive cells coexpressed proliferative markers, suggesting that human NPC may contain cancer stem–like cells. In addition, we found that Notch1 signaling was activated in NPC. Confocal images show that the Notch1 signaling activated form and Hes1, a downstream target of Notch1 signaling, was predominantly found in SOX2- and OCT4-positive cells.
Conclusion:
Our findings suggest that the Notch1 signaling pathway might be a regulator of cancer stem–like cells in NPC.
Keywords: cancer stem cells, nasopharyngeal carcinoma, Notch1 signaling, OCT4, proliferation, SOX2
Nasopharyngeal carcinoma (NPC), which arises from the mucosal epithelium of the nasopharynx, is one of the most poorly understood and commonly misdiagnosed diseases.1 Although rare in most parts of the world, it is one of the most common cancers found in southern China and Southeast Asia, where an annual incidence of more than 20 cases per 100,000 is reported.1-3 Because NPC occurs in an anatomic site that is poorly accessible to surgeons, high-dose radiotherapy with or without concurrent chemotherapy is the primary treatment.4,5 The 5-year survival rate of about 34 to 52% has not changed significantly over the decades, despite intensive efforts to develop effective therapy.4,5 Numerous studies have linked NPC to infection of the Epstein-Barr virus.6 The molecular mechanisms underlying the carcinogenesis of NPC, however, remain unresolved.
Studies have shown that a small subpopulation of cells present in leukemic humans exhibit marked heterogeneity with respect to extensive proliferation and a self-renewal capacity that is not found in the majority of tumour cells.7,8 After transplantation into mice with severe combined immune deficiency (SCID), these cells can form tumours that phenotypically resemble the patient's original tumour.7,8 These cells are necessary and sufficient to sustain leukemia and are therefore tumorigenic. This notion has subsequently been verified in several solid tumours, including cancers of the breast,9 brain,10 prostate,11 ovary,12 colon,13 and pancreas.14 These cells have been termed cancer stem cells (CSCs) because they possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. A recent study showed that side population (SP) cells, a subpopulation of human NPC cell line CNE-2, display stem cell characteristics in vitro and are able to form tumours in vivo.15 However, whether CSCs are present in human primary NPC remains unclear.
The findings of CSCs are very important not only for our understanding of the biologic behaviours of cancer cells but also for opening new strategies to treating cancers. Growing evidence has shown that CSCs may contribute to some individuals' resistance to cancer therapy. A plausible explanation is that CSCs have not been completely destroyed. Thus, therapeutic strategies that specifically target CSCs should destroy tumours more effectively than current treatments while reducing the risk of relapse and metastasis. Consistent with this notion, a recent study shows that SP cells have a strong tumorigenic ability and are more resistant to chemotherapy and radiotherapy, which may result in NPC recurrence.15 If this hypothesis holds true, CSCs could be the most promising target for new therapies.
In addition, despite the huge growth in the CSC field, the biological behaviours of CSCs remain largely unexplored. Notch signaling defines a fundamental pathway controlling cell fate acquisition during embryonic development.16 New evidence shows that Notch signaling has been found in many solid tumours, plays diverse roles in different malignancies, and affects differentiation, proliferation, survival, cell-cycle progression, angiogenesis, and possibly self-renewal.17,18 Recent studies show that Notch signaling promotes medulloblastoma “stem cell” survival19 and is involved in tumour initiation in pancreatic cancer.20 However, the roles of Notch1 signaling in NPC are less well understood.
In the present study, 32 patients with NPC, including (1) a well-differentiated keratinizing type, (2) a moderately differentiated nonkeratinizing type, and (3) an undiffer-entiated type according to the World Health Organization (WHO) classification, were examined by immunocyto-chemistry.21 We found that embryonic stem (ES) cell markers SOX2 and OCT4 were expressed in a small subpopulation of cells for all three subtypes of NPC. Double immunostaining shows that the ES-immuno-reactive cells coexpressed proliferative markers, suggesting that NPC in humans may contain cancer stem–like cells. In addition, we also found that the Notch1-activated form and Hes1, a downstream target of Notch1 signaling, were predominantly expressed in SOX2- and OCT4-positive cells, indicating the activation of Notch1 signaling in these cells. Our findings suggest that Notch1 signaling–mediated cancer stem–like cells might have a role in the tumorigenesis and progression of NPC in humans.
Materials and Methods
Nasopharyngeal Tumour Tissue Specimens
Human NPC specimens were obtained from the patients undergoing surgical resection at the Department of Otolaryngology, the First Affiliated Hospital, Wenzhou Medical College, between 2001 and 2008. Tumours were diagnosed and classified at the Department of Pathology of Wenzhou Medical College by the attending neuropathologist according to the WHO guidelines.21 Patients ranged in age from 18 to 82 years, with a mean of 46.6 years. Five of them were classified as well-differentiated keratinizing type, 18 specimens as moderately differentiated nonker-atinizing type, and 9 specimens as undifferentiated type. Five normal human nasopharyngeal specimens without clinical and histologic evidence of diseases were obtained and used for controls. The resected tumours were fixed in 10% formaldehyde and embedded in paraffin for histo-pathologic diagnosis. All studies involving patients were conducted under the protocol approved by the Institutional Research Review Board at Marin General Hospital and Wenzhou Medical College, China.
Immunocytochemistry
The specimens embedded in paraffin were cut at 6 μm thickness and sections were deparaffinized with xylene and rehydrated with ethanol following antigen retrieval using antigen unmasking solution (Vector Laboratories, Burlingame, CA) according to the manufacturer's instruction. After blocking peroxidase activity with 1% H2O2, sections were incubated in blocking buffer (2% horse serum, 0.2% Triton X-100, 0.1% bovine serum albumin [BSA] in phosphate-buffered saline [PBS]) for 1 hour at room temperature. After several washes with PBS, sections were incubated in blocking solution (2% goat serum, 0.1% Triton X-100, 1% BSA in PBS) for 1 hour at room temperature. The primary antibodies used were (1) rabbit antihuman Ki-67 (1:50; Zymed Laboratories, South San Francisco, CA); (2) goat antiminichromosome maintenance 2 (MCM2; 1:100; Santa Cruz Biotechnology, Santa Cruz, CA); (3) rabbit polyclonal anti-SOX1 (1:200; Chemicon, Temecula, CA); (4) mouse monoclonal antiproliferating chain nuclear antigen (PCNA; 1:200; Chemicon, Temecula, CA); (5) mouse monoclonal anti-CD133 (1:100; Miltenyi Biotec, Bergisch Gladbach, Germany); (6) mouse monoclonal antihuman-specific nestin (1:200; Chemicon); (7) rabbit polyclonal anti-OCT4 (1:500; Chemicon); (8) rabbit polyclonal anti-SOX2 (1:200; Abcam, Cambridge, UK); (9) goat polyclonal anti-Notch1 (1:100; Santa Cruz Biotechnology); (10) rabbit polyclonal anti-NICD (intra-cellular domain of Notch; 1:500; Abcam;); (11) goat polyclonal anti - Jagged1 (1:100; Santa Cruz Biotechnology); (12) rabbit polyclonal anti-Hes1 (1:500; Chemicon); and (13) mouse monoclonal anti–C-kit (1:100; BD PharMingen, San Diego, CA). Primary antibodies were added in blocking buffer and incubated with sections at 4°C overnight. Sections were then washed with PBS and incubated with biotinylated goat antirabbit or antigoat antibody (1:200) (for polyclonal antibodies) or biotinylated horse antimouse antibody (1:200) (for monoclonal antibodies) for 1 hour at room temperature. Avidin-biotin complex (Vector Elite; Vector Laboratories) and a diaminobenzidine or nickel solution (Vector Laboratories) were used to obtain a visible reaction product. Controls for immunohisto-chemistry included preabsorption and coincubation of the antibodies with the corresponding antigens. Sections were dehydrated, sealed, and coverslipped. A Nikon microscope and a Magnifire digital colour camera were used for examination and photography of the slides, respectively.
Double- or Triple-Label Immunocytochemistry
Double- or triple-label immunocytochemistry was performed as previously described.22,23 The primary antibodies used were, in addition to the list described above, mouse monoclonal anti-Ki-67 antigen (1:50; Novocastra, Newcastle upon Tyne, UK). The secondary antibodies were Alexa Fluor 488-, 594-, or 647-conjugated donkey antimouse, antigoat, or antirabbit IgG (1:200–500; Molecular Probes, Carlsbad, CA). Nuclei were counterstained with DAPI using proLong Gold antifade reagent (Molecular Probes). Fluorescence signals were detected using an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss Ltd, Chester, VA) equipped with a two-photon Chameleon laser (Coherent Inc., Santa Clara, CA). Images were acquired using LSM 510 Imaging Software (Carl Zeiss Ltd). Two-, three-, or four-colour images were scanned using argon, 543 HeNe, 633 HeNe, and Chameleon (750–780 nm for DAPI) lasers. Selected images were viewed at high magnification, and three-dimensional images were constructed using Imars software (iMARS Software Systems, Beverly Hills, CA). Controls included omitting either the primary or secondary antibody or preabsorbing primary antibody.
Cell Counting
The proliferating index was defined as the percentage of Ki-67- or PCNA-immunopositive cells divided by the total number of cells in the evaluated area. For the incidence of stem/progenitor protein expression in the nasopharyngeal tumour, immunopositive cells in the section were evaluated as cell density. Depending on the size of the nasopharyngeal tumour, three to eight viable fields from the area of maximal labeling were chosen for counting. All counts were conducted at a magnification of ×400 by using an eyepiece grid covering an area of 0.0625 mm2. Vascular components and hematogenous cells were excluded from analysis.
Statistical Analysis
Quantitative data were expressed as mean ± SEM from at least three experiments. Analysis of variance and Student t-test were used for statistical analysis, with SPSS for Windows (version 16.0, SPSS Inc, Chicago, IL); p < .05 was regarded as statistically significant.
Results
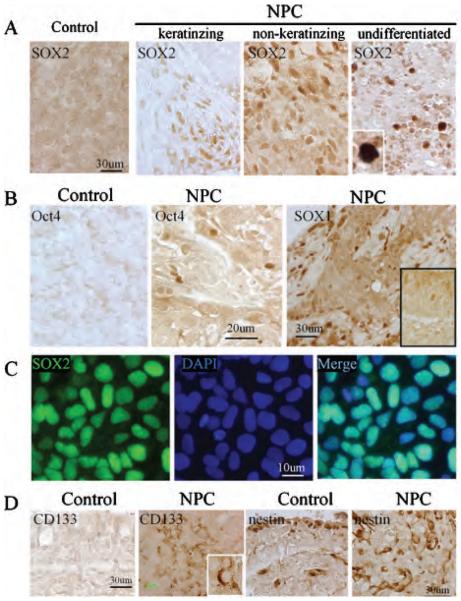
The transcription factors SOX2 and OCT4 are highly expressed in ES cells and are necessary for ES cell pluripotency. To investigate whether human primary NPC contains a subpopulation of tumour cells with features of stem cells, we first performed immunocytochemistry on the NPC sections using the ES cell protein markers SOX2 and OCT4. We found that both SOX2 and OCT4 were selectively expressed in a subpopulation of human primary NPC cells (Figure 1, A and B) but barely in normal controls. To determine the specificity of the antibodies used, human ES cells (H9 cell line) were cultured in the differentiating media, and immunostaining was performed. We confirmed that SOX2 and OCT4 proteins were expressed in the nuclei of human ES cells (Figure 1C). In addition, we also found that a subpopulation of cells from NPC expressed several other stem/progenitor protein markers, including SOX1, nestin, an intermediate filament protein expressed by neural stem cells, and CD133, a novel five-transmembrane segment cell-surface protein originally shown to be a hematopoietic stem cell marker and recently found to be a marker of normal human neural stem cells24 (Figure 1, B and D). However, a number of other neural stem/progenitor protein markers, including doublecortin, TUC4, and NeuroD, were not expressed in human primary NPC (data not shown).
Figure 1.
Expression of embryonic stem cell proteins in human primary nasopharyngeal carcinoma (NPC) A, Expression of SOX2 in human primary NPC and normal control (×20 and ×60 original magnification). B, Expression of OCT4 protein (left and middle panels) and SOX1 (right panel) in human primary NPC and normal control (×20 and ×60 original magnification). C, SOX2 protein (green) was expressed in the nuclei of differentiating human embryonic stem cell (H9). Nuclei were counterstained by DAPI (blue) (×60 original magnification). D, Expression of CD133 and nestin proteins in normal control and human primary NPC cells (×40 original magnification).
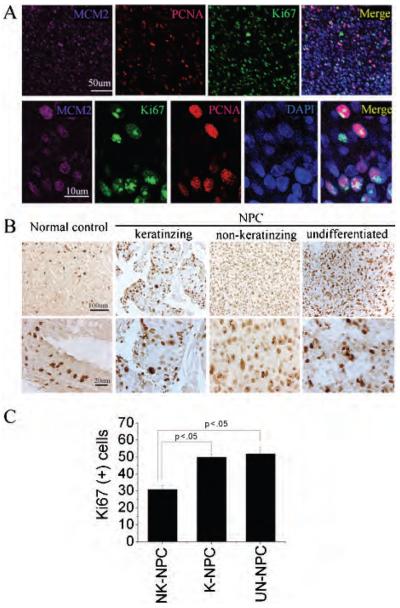
Self-renewal is a hallmark of stem cells and cancer. A stemness program could play an important role in cancer development and progress. Therefore, we performed immunostaining on NPC sections using antibodies against Ki-67 antigen, which binds to nuclear proteins in the G1, S, G2, and M phases of the cell cycle,25 against PCNA and against MCM2. As shown in Figure 2, A–C, we found that the majority of NPC cells were proliferative. The proliferation index in a well-differentiated keratinizing type was significantly different from that of a moderately differentiated nonkeratinizing type and an undifferentiated type (p < .05). However, there was no significant difference between nonkeratinizing types and undifferentiated types (p > .05).
Figure 2.
Proliferation index of different subtypes of human primary nasopharyngeal carcinoma (NPC) A, Triple-immunolabeling was performed using anti-Ki-67 (green), anti-MCM2 (purple), and anti-PCNA (red) in human primary NPC. Nuclei were counterstained by DAPI (blue). Top panel: low magnification; bottom panel: high magnification. B, Sections of different subtypes of primary NPC were immunostained using anti-Ki-67 antibody. Top panel: low magnification; bottom panel: high magnification. C, Ki-67-positive cells in different subtypes of primary NPC were calculated as the percentage of all tumour cells that were Ki-67-immunopositive cells. Data are mean value ± SEM. K-NPC = keratinizing NPC; NK-NPC = nonkeratinizing NPC; UN-NPC = undifferentiated NPC.
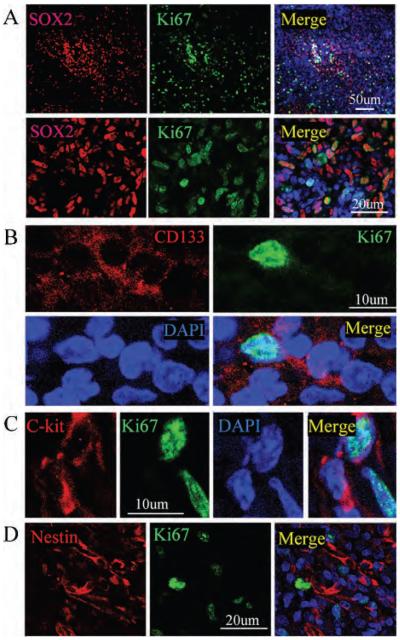
We then asked whether tumour cells immunoreactive for ES cell protein exhibited features associated with cell proliferation. Double immunolabeling was conducted using antibodies against Ki-67 antigen, together with antibodies against the stem cell markers, including SOX2, OCT4, CD133, C-kit, and nestin. Immunopositive cells were scanned by confocal laser scanning microscopy, and z-stack images were reconstructed using Imars software to ensure that both proteins were truly expressed in the same cell. As shown in Figure 3A, a subset of cells that expressed SOX2 were also reactive for Ki-67 antigen, consistent with a proliferative phenotype. However, only a few of the CD133- and nestin-positive cells expressed Ki-67 protein, suggesting that some of them might be retained in the G0 stage of cell cycle (Figure 3, B–D).
Figure 3.
Proliferating capacity of stem/progenitor protein–positive nasopharyngeal carcinoma (NPC) cells A, Double-immunolabeling was performed using anti-Ki-67 (green) and anti-SOX2 (red). Images were recorded using a two-photon confocal laser scanning microscope. Nuclei were counterstained by DAPI (blue). Top panel: low magnification; bottom panel: high magnification. B, Double-immunolabeling was performed using anti-Ki-67 (green) and anti-CD133 (red) on the NPC section. Nuclei were counterstained by DAPI (blue). C, Double-immunolabeling was performed using anti-Ki-67 (green) and anti-C-kit (red) on the NPC section. Nuclei were counterstained by DAPI (blue). D, Double-immunolabeling was performed using anti-Ki-67 (green) and anti-C-kit (red) on the NPC section. Nuclei were counterstained by DAPI (blue).
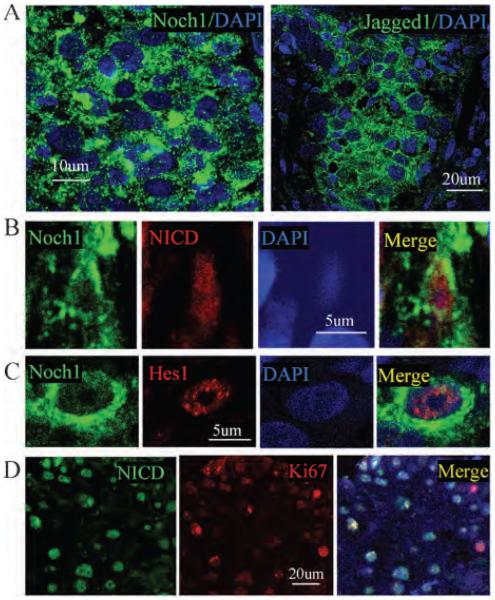
Notch signaling has been suggested to be involved in a wide variety of human cancers. Thus, we next examined whether Notch1 signaling was activated in human primary NPC. We found that expression of Notch1 and Jagged1, a Notch1 ligand, increased in the primary NPC, compared with the normal control and adjacent non-neoplastic tissue (Figure 4A). NICD, an activation form of Notch1 signaling, and Hes1, a downstream Notch1 target, were expressed in Notch1-positive cells (Figure 4, B and C). The specificity of Notch1, NICD, and Hes1 was confirmed by Western blot using recombinant proteins as described in our previous publication.26 Double labeling shows that most NICD-positive cells expressed Ki-67, suggesting that Notch1-activated cells were proliferative (Figure 4D).
Figure 4.
Activation of Notch1 signaling in human primary nasopharyngeal carcinoma (NPC) A, Notch1 (green; left panel) and Jagged1 (green; right panel) proteins were expressed in human primary NPC cells. Nuclei were counterstained by DAPI (blue). B, NICD protein (red) was expressed in the Notch1-positive cells (green) in human primary NPC. C, Hes1 protein (red) was expressed in the Notch1-positive cells (green) in human primary NPC. D, NICD (green) was expressed in the Ki-67-positive cells (red) in human primary NPC. Nuclei were counterstained by DAPI (blue).
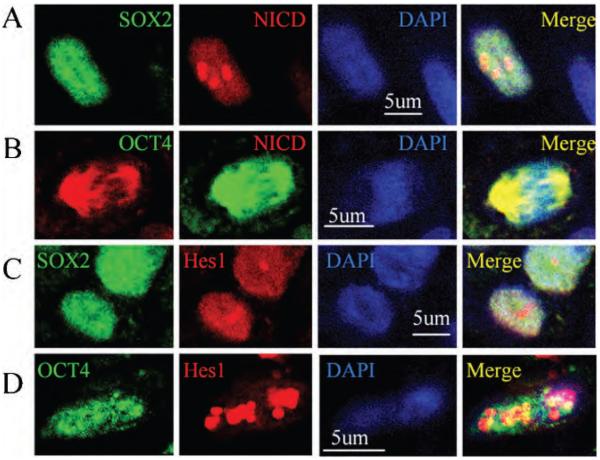
The link between Notch1 signaling and ES cell protein–positive cells in NPC was explored by double immunolabeling. As shown in Figure 5, NICD was expressed in SOX2- and OCT4-positive cells. In addition, Hes1 was also observed in ES cell protein–positive cells, suggesting that Notch1 signaling was activated in these cells in human primary NPC.
Figure 5.
Notch1 signaling was activated in ES cell protein–positive cells in human primary nasopharyngeal carcinoma (NPC) A, Double-immunolabeling was performed using anti-SOX2 (green) and anti-NICD (red) on the NPC section. Nuclei were counterstained by DAPI (blue). B, NICD protein (red) was expressed in OCT4-positive cells (green). Nuclei were counterstained by DAPI (blue). C, SOX2-positive cells (green) expressed Hes1 protein (red). Nuclei were counterstained by DAPI (blue). D, Double-immunolabeling was performed using anti-OCT4 (green) and anti-Hes1 (red) on the NPC section. Nuclei were counterstained by DAPI (blue).
Discussion
In this study, we found that ES cell protein markers SOX2 and OCT4 were expressed in a small subpopulation of cells in human primary NPC. Double immunostaining shows that some of these cells were proliferative. In addition, we also found that Notch1 signaling components were expressed in NPC cells. Confocal images show that a Notch1-activated form and its downstream target were expressed in the SOX2- and OCT4-positive cells, suggesting that Notch1 signaling was activated in these cells. Our findings suggest that NPC may also contain cancer stem–like cells, and Notch1 signaling may be involved in the biological behaviours of these cells in human primary NPC.
OCT4 and SOX2 are two key transcription factors that are highly expressed in ES cells. Studies have shown that they are crucial for maintaining the pluripotent state of ES cells.27,28 ES cells lose the capacity to maintain pluripotency on knockdown of expression of these transcription factors by ribonucleic acid interference.29,30 In combination with Klf4 and c-Myc, their overexpression can induce both mouse and human somatic cells to exhibit the morphology and growth properties of ES cells and express ES cell marker genes, which have thus been designated to be induced pluripotent stem cells.31-33 These findings suggest that these factors are critical regulators for ES cell pluripotency. We confirm that SOX2 and OCT4 are expressed in human ES cells. Importantly, we also find that SOX2 and OCT4 are expressed in a small subpopulation of cells in human primary NPC, consistent with a recent study demonstrating that these transcript factors are expressed in cancer cells.34 With no unique or definitive biochemical and histochemical markers to label stem cells in general, cancer stem cells (CSCs) remain difficult to identify. Given that only a limited amount of biopsy specimens of human NPC are available, we are not able to isolate enough NPC cells for an in vitro self-renewal assay or for transplantation into sublethally irradiated nonobese diabetic SCID mice in vivo. However, SOX2- and OCT4-positive cells coexpress Ki-67, MCM2, and PCNA, suggesting that these cells are proliferative and may have self-renewal capacity, which is a hallmark of stem cells and cancer and stemness program, and could play an important role in cancer. Our data suggest that these dividing ES cell protein–positive cells might be cancer stem–like cells. It remains to be resolved if both of these markers are of functional importance for NPC. Given the role of these factors in normal stem cell proliferation and maintenance, the overexpression of OCT4 and SOX2 could contribute to the pathologic self-renewal characteristics of CSCs in human NPC.
A key goal in cancer research is to identify the mechanism by which CSCs arise and self-renew. Notch signaling is a highly conserved mechanism that regulates specific cell fate decision, controlling diverse events such as cell fate determination, proliferation, apoptosis, and stem cell maintenance during development. Its role in controlling adult stem cell proliferation has now been demonstrated for several cell types, including hematopoietic, neural, and mammary stem cells.35 New evidence indicates that Notch activation plays a role in the onset and progression of many human malignancies.36 Notch signaling was first linked to tumorigenesis through the identification of a recurrent (7; 9) (q34; q34.3) chromosomal translocation involving the human Notch1 gene that is found in a small subset of human pre-T-cell acute lymphoblastic leukemias.37 Growing evidence indicates that Notch signaling is likely to be involved in the pathogenesis of a variety of other human tumors, including neuroblastomas, skin cancer, lung cancer, and prostate cancer.36 Studies show that the pathogenesis could be mediated via gain of function mutation, ligand-mediated activation of the Notch signaling, and down-regulation of the Notch pathway.36
On ligand binding, Notch is proteolytically cleaved by γ-secretase to release the NICD, which subsequently translocates into the nucleus, where it binds to transcription factor CSL (CBF1 in humans, Suppressor of Hairless in Drosophila, LAG in Caenorhabditis elegans), also called RBPJk (recombination binding protein J kappa) in mice.38 The NICD/CSL interaction converts CSL from a transcriptional repressor into a transcriptional activator by displacing the corepressor complex and recruiting coactivators, which, in turn, regulate expression of Notch target genes,16 including members of the basic helix-loop-helix hairy/enhancer of split (Hes) family and the related HRT/Herp (Hes-related repressor protein) transcription factor family. We find that both NICD and Hes1 are observed in cells of NPC, suggesting activation of Notch1 signaling in human primary NPC. More importantly, our data show that the majority of Notch1-activated cells are ES cell protein–positive cells. Consistent with our finding, studies show that Notch signaling plays an important role in controlling the fate of putative breast CSCs39 and medulloblastoma stem cells.40 Given the established role of Notch signaling in tumorigenesis of other solid tumours, our results invite the speculation that Notch1 signaling may also contribute to NPC development and be involved in mechanisms underlying the regulation of biological behaviours of cancer stem–like cells in human primary NPC. Increasing evidence indicates that tumour-initiating (cancer stem) cells may contribute to treatment resistance and relapse. Therefore, effective targeting of these cells may open a new therapeutic approach for cancers including human primary NPC.
Acknowledgements
Financial disclosure of authors: This work was partially supported by Wenzhou Medical College 5010 grant and National Institutes of Health grant AG21980 (to K.J.).
Footnotes
Financial disclosure of reviewers: None reported.
References
- 1.Cho WC. Nasopharyngeal carcinoma: molecular biomarker discovery and progress. Mol Cancer. 2007;6:1. doi: 10.1186/1476-4598-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks JE, Phillips JL, Menck HR. The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer. 1998;83:582–8. doi: 10.1002/(sici)1097-0142(19980801)83:3<582::aid-cncr29>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Stiller CA. International variations in the incidence of childhood carcinomas. Cancer Epidemiol Biomarkers Prev. 1994;3:305–10. [PubMed] [Google Scholar]
- 4.Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261–70. doi: 10.1016/0360-3016(92)90740-9. [DOI] [PubMed] [Google Scholar]
- 5.Sanguineti G, Geara FB, Garden AS, et al. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys. 1997;37:985–96. doi: 10.1016/s0360-3016(97)00104-1. [DOI] [PubMed] [Google Scholar]
- 6.Chien YC, Chen JY, Liu MY, et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345:1877–82. doi: 10.1056/NEJMoa011610. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 8.Blair A, Hogge DE, Ailles LE, et al. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–12. [PubMed] [Google Scholar]
- 9.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 11.Lang SH, Frame FM, Collins AT. Prostate cancer stem cells. J Pathol. 2009;217:299–306. doi: 10.1002/path.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Guo LP, Chen LZ, et al. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–24. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 16.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 17.Zweidler-McKay PA. Notch signaling in pediatric malignancies. Curr Oncol Rep. 2008;10:459–68. doi: 10.1007/s11912-008-0071-2. [DOI] [PubMed] [Google Scholar]
- 18.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007;64:2746–62. doi: 10.1007/s00018-007-7164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 20.Mullendore ME, Koorstra JB, Li YM, et al. Ligand-dependent Notch signaling is involved in tumor initiation and tumor maintenance in pancreatic cancer. Clin Cancer Res. 2009;15:2291–301. doi: 10.1158/1078-0432.CCR-08-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanmugarantnam KSL. Histological typing of tumors of the upper respiratory tract and ear. 2nd ed. Springer-Verlag; New York: 1991. [Google Scholar]
- 22.Jin K, Mao X, Sun Y, et al. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002;110:311–9. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin K, Mao XO, Sun Y, et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002;22:5365–73. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–5. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 26.XiaoMei Wang, XiaoOu Mao, Lin Xie, et al. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J Cereb Blood Flow Metab. 2009 Jun 17; doi: 10.1038/jcbfm.2009.83. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 28.Avilion AA, Nicolis SK, Pevny LH, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–40. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matin MM, Walsh JR, Gokhale PJ, et al. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–68. doi: 10.1634/stemcells.22-5-659. [DOI] [PubMed] [Google Scholar]
- 30.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Loh YH, Agarwal S, Park IH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;28(113):5476–9. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 34.Schoenhals M, Kassambara A, Vos JD, et al. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009;383:157–62. doi: 10.1016/j.bbrc.2009.02.156. [DOI] [PubMed] [Google Scholar]
- 35.Dontu G, Jackson KW, McNicholas E, et al. Role of Notch signaling in cell-fate determination of human mammary stem/ progenitor cells. Breast Cancer Res. 2004;6:R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzo P, Osipo C, Foreman K, et al. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–31. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 37.Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–61. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 38.Kato H, Taniguchi Y, Kurooka H, et al. Involvement of RBP-J in biological functions of mouse Notch1 and its derivatives. Development. 1997;124:4133–41. doi: 10.1242/dev.124.20.4133. [DOI] [PubMed] [Google Scholar]
- 39.Lee CW, Simin K, Liu Q, et al. A functional Notch-survivin gene signature in basal breast cancer. Breast Cancer Res. 2008;10:R97. doi: 10.1186/bcr2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garzia L, Andolfo I, Cusanelli E, et al. MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS ONE. 2009;4:e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]